Abstract
During the last years, the demand for biofuels has increased significantly. In Brazil, a production of 1 billion liters of biodiesel was produced by the end of 2007, due to its obligatory use in the composition of the diesel for vehicle use. In this production, a hundred thousand tons of glycerol are produced as by-product, for which alternative uses are needed. As glycerol has already been studied by other conventional characterization methods in the past, thermal analysis has been used mostly for characterization of sub ambient temperature properties of glycerol. In this paper, thermogravimetry (TG), derivative thermogravimetry (DTG) and differential thermal analysis (DTA) were used for its thermal characterization above room temperature. Thermal stability was determined from experimental data, which show that even in air, only a very small part of the volatilized glycerol is burned out. A thermogravimetric quantitative method was developed to determinate the water content of glycerol–water mixtures, which also was used to quantify the water impurity in pro-analysis samples of glycerol, showing compatible results with those obtained by Karl Fischer method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The growing demand for energy, on a global scale, is limited in many ways. The restrictions vary in order of magnitude and importance, but are imposed for technical, technological, economic, social, civil, legal, political and environmental reasons. In this scenario, biofuels are gaining space and interest, as they are able to find in a smaller space of time, the answers for these questions. Biodiesel, according to Brazilian regulation [1, 2], is a “biofuel derived from renewable biomass for use in internal combustion engines with compression ignition or, as regulation for generation of another type of energy that can replace partially or totally fuel fossil.” The American Society for Testing and Materials (ASTM), in method ASTM D6751/2002 (Terminology item) establishes that biodiesel is “a fuel composed of mono-alkyl ester-long chain fatty acids, derived from vegetable oils or animal fats, designated B100.” Biodiesel is typically produced by the reaction of vegetable oils or animal fats with alcohol, methanol or ethanol, in the presence of catalysts, to produce mono-alkyl-esters and glycerin, which is removed from the ester [1, 3, 4].
The growing offer of glycerin in the market has been the subject of discussions among those who see it a good occurrence [5] and those who prefer to call it a “tsunami of glycerin”, an allusion to the devastating effects that could cause in the economy [6]. According to Bouças [6], in 2007 the production of glycerol was ten times higher than that offered by Brazilian chemical industries [7], and this would be a worrying factor, because prices tend to drop dangerously. In Brazil, a production of 1 billion liters of biodiesel was produced by the end of 2007 [1], due to the Brazilian 11097/2005 law, which obligates until 2013 the addition of 5% of biodiesel in the composition of the diesel oil used in vehicles [6]. This content in 2008 has to be 2% [1, 2, 4].
Glycerin, glycerol or 1,2,3-propanotriol is a polyalcohol or polyol [8, 9], which has some peculiar characteristics [10–12]: it is very soluble in water, liquid in environmental conditions, colorless, odorless, viscous, very sweet (which derives its name glykys, “sweet” in Greek [13]), and with thousands of uses [5]. It is employed in the industries of paper, cosmetics, food, tobacco, and drugs, in addition to being raw material for hundreds of other products, in industries of explosives, polymers (e.g. as a plasticizer [14]) and biotechnology [5–7, 15]. The three hydroxyls ensure the formation of hydrogen bonds responsible for its high boiling point (290 °C), and infinite solubility in water and ethanol [8, 9, 11], but also make the glycerin a hygroscopic substance [16]. The determination of the water content in liquid systems has been made by different methods, as the Coulometric Karl–Fischer Titration [17] or, specifically in the case of binary systems water–glycerol, through the thermal conductivity method, developed by Yang et al. [18]. According to American Society for Testing and Materials method ASTM E-1064-08, “The precision and accuracy of the coulometric technique decreases for concentrations of water much greater than 2.0% because of the difficulty in measuring the small size of sample required.” Meanwhile, the method of thermal conductivity is satisfactory for all concentrations [18]. Thermal analysis has been used mostly for vegetal oils [19, 20] and characterization of sub ambient temperature properties of glycerol [21–23].
The thermal stability is a very important data when glycerin is thought as a fuel, itself. Some industries are already burning it, as a source of energy, replacing part of the oil [6], but this can lead them to a problem, because heating glycerin can produce acrolein, a very toxic product [12, 24]. In this work, we show that through TG/DTG and DTA analysis, the glycerin thermal behavior above room temperature may be analyzed and its water content may be determined by derivative thermogravimetry.
Experimental
Pro Analysis (P. A.) glycerol from VETEC (minimum 99.5%), and its mixtures with distilled water were prepared, with concentrations of water from 3 to 30%. The analysis were performed in a simultaneous TA Instruments TG-DTA equipment, model SDT 2960, with a 10 °C/min constant heating rate, from 30 to 1,000 °C. By way of comparison, tests on air and nitrogen ambient were performed, both in 100 mL min−1 flow, were done. About 10–20 mg of sample were used in open platinum pans.
Results and discussion
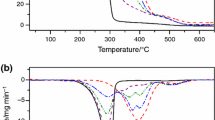
Figure 1 shows the TG/DTG and DTA curves of pure glycerin in air and nitrogen. As can be seen from the TG curve, and more precisely from the DTG, in the operation conditions of the experiments, the mass loss due to vaporization of glycerol starts at 120 °C in both cases. Actually, the onset temperature in the case of airflow is 194 °C with the mass loss ending at 246 °C. In nitrogen flow, the onset temperature is 199 °C, showing a little higher thermal stability, which also causes the end of mass loss to occur at 249 °C. The shape of DTG curve in nitrogen is typical of a kinetic process of mass loss of order zero [25] due to the practically vertical end of the DTG peak. In airflow, from TG and DTA curves, it can be seen that there is an overrun of the temperature, due to partial burning of the sample. When it occurs, the exothermal partial combustion phenomenon gives an extra source of energy into the heating chamber of the furnace, so the heating system turns off. This causes a decrease of the temperature until the system starts heating again, when the programmed temperature is higher than the temperature attained during the cooling caused by the interruption of the heating current.
Figure 2 is a typical TG/DTG curve of the P. A. glycerol, showing details of the beginning of the volatilization process. The nominal concentration of the pro analysis glycerol, according to the manufacturer was “minimum 99.5%”, witch means a value near 0, 5% of water. Indeed, it’s possible to see a little mass decrease between 30 and 100 °C, due to water loss, which is the most volatile, and some initial glycerol vaporization after that, as indicated by the different behavior of the DTG line curvature, at this temperature range. The middle of the straight part of the DTG line, is the considered point, in this graphic at 85.69 °C, related to the descending TG curve, up to where the mass loss is due to the water release, which corresponds to 0.55% of the initial sample mass. This matches with the nominal concentration (0.5%) and, in order to validate the method, samples of the same pro analysis glycerol were analyzed using the KF (Karl Fischer) Metrohm 836ASTM method, and the results (0.60%, 0.62%, and 0.58%) gave the average concentration as 0.60%, an expected value for an hygroscopic substance such as glycerol. As Karl Fischer analyses were performed at the end of the study, 1 year after the thermal analyses have been performed, the little difference observed, may be due to some water absorption from ambient humidity.
Mixtures of glycerol–water were analyzed. Figure 3 shows the TG/DTG curves of the solutions freshly prepared containing 3%, 7%, 10%, 20%, and 30% of water. Using the same method described above, the obtained values were compatible with the nominal ones.
Figure 4 shows the comparison between values obtained by the developed method from TG/DTG curve data, and the nominal ones. It is important to notice that the glycerol used for the preparation of the solutions carried the 0.55% of water previously determined. Thus, the water concentration in the prepared solution has been corrected on this basis.
Conclusions
-
The TG/DTG curves show that the thermal stability of glycerol is quite the same in both atmospheric air and nitrogen.
-
The quantitative method developed by TG and DTG to determinate the water content can be used for glycerol–water mixtures to quantify the water impurity in pro-analysis samples of glycerol.
References
ANP. Brazilian National Agency of Petroleum: Resolution 30, (9/20/2004), 1–3 (in Portuguese).
Brazilian Ministery of Mines and Energy: 11097 Law, Offic. Union Journ. (D.O.U.), January 14th 2005, p. 8 (in Portuguese).
ASTM American Society for Testing and Materials: ASTM D6751-02 specification for biodiesel fuel blend stock (B100) for middle distillate fuels (2002).
Brazilian National Program for Biodiesel Production and Use – The Biodiesel: www.biodiesel.gov.br (August 2008) (in Portuguese).
Glycerol generated in biodiesel production will have new applications: www.biodieselbr.com/noticias/biodiesel/glicerina-gerada-producao-biodiesel-novos-usos-29-03-07.htm (2007) (in Portuguese).
Bouças C. Glycerol from biodiesel floods the market in the country and knocks prices. Valor Journ. p. B7. RJ, Brazil, May 2nd 2007 (in Portuguese).
Monteiro NA Jr. Subtitution strategy of imported diesel by biodiesel and eventual implications. M.Sc. Thesis. Rio de Janeiro: School of Chemistry, UFRJ; 2005. 235 p (in Portuguese).
Morrison R, Boyd R. Organic chemistry. 6th ed. Lisbon, Portugal: Calouste Gulbenkian Foundation; 1978 (in Portuguese).
Allinger N. Organic chemistry. 2nd ed. Rio de Janeiro, Brazil: Livros Técnicos e Científicos; 1979 (in Portuguese).
Oxford University. Safety data for glycerol (11/24/06). http://ptcl.chem.ox.ac.uk/MSDS/GL/glycerol.html. (2008).
Perry RH, Green DW. Perry’s chemical engineers handbook. 7th ed. McGraw-Hill; 1997, p. 2–116.
Quijada-Garrido I, Iglesias-Gonzalez V, Mazon-Arechederra JM, Barrales-Rienda JM. The role played by the interactions of small molecules with chitosan and their transition temperatures. Glass-forming liquids: 1,2,3-Propanetriol (glycerol). Carbohydr Polym. 2007;68(1):173–86.
Ramiz-Galvão BF. Etymological, spelling and prosodic vocabulary of the Portuguese words derived from the Greek language. Rio de Janeiro: Francisco Alves; 1909 (in Portuguese).
Schlemmer D, de Oliveira ER, Araújo Sales MJ. Polystyrene/thermoplastic starch blends with different plasticizers. J Therm Anal Calorim. 2007;87(3):635–8.
Fangrui M, Hanna MA. Biodiesel production: a review. Bioresour Technol. 1999;70:1–15.
Ryabov YE, Hayashi Y, Gutina A, Feldman Y. Features of supercooled glycerol dynamics. Phys Rev B. 2003;67(13):132202/1–/4.
Margolis SA. Sources of systematic bias in the measurement of water by the coulometric and volumetric Karl Fischer methods. J Am Chem Soc Anal Chem. 1997;69(23):4864–71.
Hu L, Yang M, Wang C. Determination of the concentrations of binary mixtures of glycerin and water by a transient method. J Therm Anal Calorim. 1988;53(3):855–9.
Dweck J, Sampaio CMS. Analysis of the thermal decomposition of commercial vegetable oils in air by simultaneous TG/DTA. J Therm Anal Calorim. 2004;75(2):385–91.
Vecchio S, Campanelle L, Nuccilli A, Tomassetti M. Kinetic study of thermal breakdown of triglycerides contained in extra-virgin olive oil. J Therm Anal Calorim. 2008;91(1):51–6.
Hayashi Y, Puzenko A, Balin I, Ryabov YE. Relaxation dynamics in glycerol-water mixtures. 2. Mesoscopic feature in water rich mixtures. J Phys Chem B. 2005;109(18):9174–7.
Hayashi Y, Puzenko A, Feldman Y. Ice nanocrystals in glycerol-water mixtures. J Phys Chem B. 2005;109:16979–81.
Inaba A, Anderson O. Multiple glass transitions and two step crystallization for the binary system of water and glycerol. Thermochim Acta. 2007;461(1–2):44–9.
Remião F. Acrolein. Toxicology and toxicological analysis monograph. Pharmaceutics School, Porto University; 2005.
Kissinger HE. Reaction kinetics in differential thermal analysis. J Am Chem Soc Anal Chem. 1957;29(11):1702–6.
Acknowledgements
Our acknowledgements to the Brazilian Research Council (CNPq), the Chemical School of the Federal University of Rio de Janeiro (UFRJ), and to the Fluminense Federal Institute – campus Macaé.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castelló, M.L., Dweck, J. & Aranda, D.A.G. Thermal stability and water content determination of glycerol by thermogravimetry. J Therm Anal Calorim 97, 627–630 (2009). https://doi.org/10.1007/s10973-009-0070-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-009-0070-z