Abstract
Pure ZnO nanoparticles and ZnO-Al2O3 nanocomposites prepared with Al2O3 content of 10, 20 and 30 atomic ratios were synthetized via Sol–gel method and then calcined for 2 h at different temperature 450, 700 and 900 °C. The structural, morphological and optical properties of the powder as-synthetized are investigated. Photocatalytic activity was assessed using methylene blue degradation. In the present work, the high purity of the nanoparticles was confirmed by EDS spectra. The XRD results show that ZnO powder has a wurtzite structure. The aluminum in composite powders leads to the appearance of the ZnAl2O4 spinel phase and it shows that this phase is appeared in the ZnO-30% at Al (Z30A) nanocomposite from an annealing temperature 450 °C as well as increasing the annealing temperature reduces the crystallite size. The flower-like morphology was well defined in these nanocomposites with an average size in the nanometer range. Photoluminescence reveals the incorporation of aluminum into the as-synthetized nanocomposites compared with XRD results.The optical absorption spectra have been presented the relationship between the band gap, particle size, annealing temperature, Al concentration and Urbach energy. Our result suggests that the annealing temperature 700 °C can be considered as an order-disorder transition temperature. The photo-catalytic application of pure ZnO and their composites nanoparticles shows that the best photocatalytic activity is obtained for the Z30A nanocomposite in the presence of MB dye, under UV light. This sample has the smallest crystal size and a high content of the ZnAl2O4 phase. A small particle size ensures high photoactivity.
Graphical Abstract

Highlights
-
We synthesized pure ZnO and ZnO-Al2O3 nanoparticles using the sol–gel method due to its simplicity.
-
The emergence of the ZnAl2O4 spinel phase in ZnO-Al2O3 nanocomposites results from heat treatment and aluminum content.
-
ZnO-30% Al2O3 nanocomposites, containing a high proportion of the spinel phase, demonstrate the best structural, optical, and morphological properties, leading to enhanced photocatalytic activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Metal oxide nanoparticles, such as titanium dioxide (TiO2), aluminum oxide (Al2O3), and zinc oxide (ZnO), are very useful materials because of their diverse applications in nanoelectronics and photonics [1, 2]. Among the applications pigments, dye solar cells, photo catalysts and sensors [3,4,5]. What’s more, zinc oxide nanoparticles are abundant, inexpensive, easy to prepare and non-toxic. ZnO is a wide-bandgap semiconductor with very interesting optical, electrical, magnetic and chemical properties, which can be easily modified using different synthesis routes, precursors and concentrations of dopants in the materials. Recently, considerable research has focused on ZnO-based nanocomposite films and powders, including ZnO-Al2O3 [6,7,8]. Materials combining ZnO and Al2O3 have proven to be potential candidates for various applications. For instance, ZnAl2O4 spinel can be utilized in photoelectronic devices, optical coatings, stress imaging devices, and electroluminescent displays due to its wide bandgap, high fluorescence capabilities, photocatalytic activity, mechanical strength, chemical, and thermal stability [7, 8]. The goal is to optimize the properties of zinc oxide at the nanoscale, opening avenues for promising new applications. With advancements in current synthesis techniques such as coprecipitation [9], solid-state synthesis [10], thermal decomposition [11], and sol–gel [2, 12], nanomaterials are increasingly captivating and dynamic fields of study. Consequently, methods must be developed to control the morphology, size, and distribution of nanoparticles during their synthesis. In practical applications, zinc oxide (ZnO) has been employed as a photocatalyst for degrading dye compounds under artificial UV light sources as well as under low intensity visible light irradiation [13,14,15]. Photocatalysis is an advanced oxidation technology based on the activation of a semiconductor by light. The photocatalytic activity of zinc oxide nanoparticles can be enhanced and extended into the visible range through doping and the use of carriers. The preparation of high-performance photocatalytic materials has become a major focus of materials science research [16]. The unique interface structure and synergistic effects are utilized to control the photocatalytic properties of these materials. Various materials such as spinels have been explored as photocatalysts and shown to be highly effective [17]. In binary or mixed metal oxides like ZnO-Al2O3, studies have shown that photocatalytic activity becomes effective with the presence of the ZnAl2O4 spinel phase. Additionally, ZnAl2O4 particles contribute to increasing the specific surface area and modifying the morphology due to their mesoporous structure [18,19,20,21]. Aluminum metal theoretically acts as a donor dopant in ZnO, thereby increasing electron density and material conductivity. However, its impact on photocatalytic properties is of interest, particularly when it contributes to forming a phase different from ZnO. This article focuses on synthesizing ZnO-Al2O3 nanocomposite powders using the sol–gel method. Our main objective was to characterize its structural, morphological, chemical, and optical properties under varying calcination temperatures and aluminum atomic concentrations in the nanocomposite. We investigated how these physical characteristics correlate with the photocatalytic activity of ZnO-Al2O3 nanocomposites using methylene blue (MB) dye in water under visible light irradiation.
2 Experimental section
The synthesis of nanoparticles is an important area of scientific interest due to its wide range of applications in fields such as fine chemicals and pharmaceuticals. In this study, pure ZnO powder and ZnO- Al2O3 composites were prepared using the sol–gel method due to its ease of use. Zinc acetate dihydrate (Zn (CH3COO)2-2H2O) was used as the precursor, as its decomposition produces zinc oxide particles. Citric acid (HOC(CO2H) (CH2CO2H)2) acted as a dispersant to keep the particles suspended. The gel was prepared by mixing two solutions: the first solution consisted of dissolving 20 g of zinc acetate dihydrate in 100 ml of deionized water, while the second solution involved dissolving 2 g of citric acid in 100 ml of ethanol. These two solutions were stirred magnetically to achieve homogeneity. The second solution was added dropwise to the first under magnetic stirring. A white precipitate appeared, forming the gel. The gel was then filtered and dried at 80 °C in a scientific oven for 24 h to obtain an aerogel powder. This white ZnO powder was ground and annealed at three temperatures: 450, 700, and 900 °C for two hours each in an RTP oven to crystallize the gel. To obtain nanopowders composed of ZnO-Al2O3, we mixed quantities of commercial Al2O3with the ZnO hydrogel. For atomic ratios of 10%(mZnO = 1.096 g, \({{\rm{m}}_{{\rm{Al}}_{2}{\rm{O}}_{3}}}\) = 0.0686 g), 20% (mZnO = 1.096 g, \({{\rm{m}}_{{\rm{Al}}_{2}{\rm{O}}_{3}}}\) = 0.1373 g) and 30% (mZnO = 1.096 g, \({\rm{m}_{\rm{Al}_{2}{\rm{O}}_{3}}}\) = 0.2059 g) aluminum (samples Z10A, Z20A, and Z30A, respectively), we used a mass m1 of gel mixed with a mass m2 of alumina. The resulting mixture was annealed for 2 h at the same temperatures (450, 700, and 900 °C) to enhance aluminum diffusion in ZnO. This annealing process resulted in the crystallization of the AlxZn1-xO compound.
Pure and nanocomposite powders were analyzed using the PANalytical Empyrean X-ray diffractometer in the diffraction angle range 2θ = 20º–80º with CuKα radiation (λ = 1.5406 Å). Each sample was scanned continuously for 30 minutes at a scan speed of 2°/min. Morphological studies were conducted using a Hitachi S-4800 field emission scanning electron microscope (SEM) equipped with energy dispersive spectroscopy (EDS). Photoluminescence measurements were carried out using a Perkin Elmer LS 55 luminescence spectrometer, with a He-Cd laser emitting UV light at 325 nm as the excitation source. UV-visible absorption spectra of the prepared samples were recorded using a Shimadzu 2700i UV-visible spectrometer. We will demonstrate the photo-catalytic efficiency of pure ZnO nanoparticles and as-synthesized nanocomposites Z10A and Z30A calcined at 450 and 900 ºC. For this purpose, 50 mg of crystalline powder was added to an aqueous solution of methylene blue (organic dye) with a concentration of 10 mg/L. The powder was placed at the bottom of a vessel containing a fixed volume of 50 mL of the dye solution to be studied. The photocatalysis reaction was carried out under a 365 nm UV radiation lamp, illuminating the solution perpendicularly. The lamp was positioned at a constant distance of 10 cm from the dye solution. The solution was irradiated with UV light for 1 hour. The catalyst used in the irradiated solution was separated using a centrifuge. The progress of the reaction was monitored using a UV spectrophotometer (UV 3101PC), periodically recording absorption spectra to track changes in the concentration of the organic dye over time. The concentration of degraded methylene blue was calculated using Beer–Lambert’s law, where absorbance A = εCl. Here, ε represents the molar absorption coefficient [M−1cm−1], l is the optical path length [cm], and C is the molar concentration [M].
3 Results and discussion
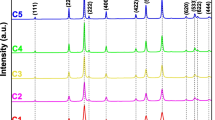
Figure 1 shows the XRD patterns of pure ZnO nanoparticles and ZnO-Al2O3 nanocomposites calcined for 2 h at 450, 700 and 900 °C. The XRD patterns of pure ZnO nanoparticles exhibit diffraction peaks with intensities corresponding to planes such as (100), (002), (101), (102), (110), (103), (200), (112), and (201), indicating well-crystallized samples. The crystal structure was identified by comparison with a database [22], revealing a polycrystalline phase consistent with the hexagonal würtzite structure of ZnO. The XRD patterns of Z10A and Z20A composite nanopowders annealed at 450 °C did not show any peaks associated with secondary crystalline phases, confirming the presence of a single ZnO phase (Fig. 1a). For the Z30A nanocomposite annealed at 450 °C, the XRD pattern exhibits peaks corresponding to the ZnO phase, along with a low-intensity peak around 2θ = 45°. This peak corresponds to the reflection of X-rays by the (040) family of lattice planes, indicative of a phase other than ZnO. Specifically, this peak corresponds to the spinel phase ZnAl2O4 (COD database code 9007028) [23]. The emergence of additional peaks associated with the spinel phase is observed in the nanocomposite powders Z10A, Z20A, and Z30A annealed at 700 and 900 °C, as shown in Fig. 1b, c. In the Z30A composite powder, the majority of peaks correspond to the ZnAl2O4 spinel phase, including peaks at 31.7° (022), 37.5° (131), 45° (040), 49.9° (133), 56.63° (242), 60.4° (151), and 66.42° (044), highlighted with asterisks. It was also observed that with increasing aluminum concentration, the intensity of the ZnO diffraction peaks slightly decreased (Fig. 1). This observation aligns with findings from several recent studies [24, 25]. The crystallization of ZnO decreases with increasing aluminum concentration. As aluminum concentration rises, there is an increased probability of forming the new phase (ZnAl2O4) at the expense of the ZnO phase. It should be noted that several diffraction peaks of the spinel phase appear after the annealing process. Initially, in the gel, the ZnO and Al2O3 phases exist separately. However, after annealing, the spinel phase forms due to aluminum diffusion. The crystallinity of this spinel phase further increases with higher annealing temperatures [20].
The XRD data were used to calculate several structural properties, including the inter-lattice distance and the lattice parameters a and c. The microstructure, characterized by the average crystallite size D, was determined from the XRD diffraction pattern using Scherrer’s formula [26].
Where λ is the incident Cu-Kα radiation wavelength (λ = 1.5406 Å), θ is the diffraction angle and β is the peak broadening after removing the instrumental broadening, \(\beta =\sqrt{{\beta }_{{FWHM}}^{2}-{\beta }_{0}^{2}}\), βFWHM is the full width at half maximum and β0 is the correction factor (0.007 radians).
The interplanar spacing of the diffraction planes dhkl has been identified by Bragg’s law [27].
Where dhkl, θ, n, and λ are the interplanar distances, diffraction angle, line diffraction order, and X-ray wavelength, respectively. The dhkl values were given by the formula:
Where a and c are the two lattice parameters and (hkl) are the Miller indices of the crystallographic planes. The crystal parameters a and c of pure ZnO show a slight increase with increasing calcination temperature, as shown in Table 1. This suggests lattice expansion at higher calcination temperatures.Additionally, we observed that the average crystallite size of ZnO nanoparticles increased from 27.2 nm to 122.2 nm as the calcination temperature was raised from 450 to 900 °C. As the temperature increases, there is an inverse relationship between the width at half-height and the size of the crystallites. Thermal calcination induces the fusion of ZnO. At high temperatures, defects cause diffusion of zinc or oxygen at grain boundaries, resulting in grain coalescence on calcination [28, 29]. Moreover, thermal energy is given to atoms to diffuse and reside at appropriate sites in the crystal lattice, resulting in larger grain sizes with less surface energy. This phenomenon contributes significantly to the abrupt increase in crystallite size at elevated temperatures [30,31,32]. The values of the lattice parameter c for ZnO-Al2O3 composite powders exhibit a slight variation depending on the aluminum content and annealing temperature, as shown in Table 1 These values are slightly lower than those for pure ZnO powder. This difference in the lattice parameter is likely caused by the incorporation of Aluminum atoms at substitutional sites in the powder. Given that the radius of the Al3+ ion (0.054 Å) is smaller than that of the Zn2+ ion (0.074 Å), this substitution leads to a contraction of the lattice [33].
The calculation of the average crystallite size reveals that the presence of aluminum influences the crystalline quality, as indicated by the increase in the width at half-height of the XRD peaks with increasing Al content. This suggests a reduction in the grain size of the powders [34]. Thus, the annealing process enhances the incorporation of aluminum atoms, leading to the formation of the ZnAl2O4 spinel phase and refinement of grain size. In the case of the Z30A nanocomposite powder, the average crystallite size decreases from 30.7 nm at 450 °C to 13.7 nm at 900 °C. For the Z10A and Z20A composite nanopowders, the effect of annealing is reflected in an increase in crystallite size up to 700 °C, followed by a decrease beyond this temperature. This evolution can be explained by the presence of a single ZnO phase below 700 °C, where its grain size increases. At around 700 °C, a transition occurs where a second spinel phase emerges and grows at the expense of the ZnO phase, resulting in reduced defects. Regarding the behavior of the Z30A nanocomposite, the presence of the spinel phase from 450 °C onwards, and its growth at 700 and 900 °C at the expense of ZnO, explains the decrease in the size of ZnO grains and the reduction in defects as the temperature increases.
In addition, the dislocation density (δ) has been calculated to quantify the defects within the crystal structure. The dislocation density (δ) is related to the crystallite size (D) of the samples and can be calculated using the following formula [35]:
This formula establishes an inverse relationship between the dislocation density and the crystallite size, where smaller crystallite sizes correspond to higher dislocation densities.
In pure ZnO it is evident that the dislocation density decreases with increasing temperature, decreasing from from 1.3 × 10−3 to 0.06 × 10−3 (Table 1). Similarly, for the nanocomposites Z10A and Z20A, dislocation density behaves identically to pure ZnO between 450 and 700 °C. This behavior can be attributed to the presence of a single ZnO phase in which aluminum is dissolved. Above 700 °C, however, the appearance of a new ZnAl2O4 phase with a crystallographic structure different from that of ZnO introduces deformations, resulting to an increase in dislocation density. For the Z30A nanocomposite, dislocation density increases with annealing temperature, due to the presence of the ZnAl2O4 spinel phase which exist from 450 °C.
The Williamson–Hall (W-H) plot method was used to separate the effects of microstrain (ε) and crystallite size (D) on peak broadening [36].
The dimensionless shape factor k (chosen as 0.75 for spherical and 0.86 for cylindrical shapes [15]) was used in the Williamson–Hall plot method to analyze peak broadening. In the plots, \({\beta }_{{hkl}}\cos \theta\) versus \(\sin \theta\) forms a straight line for all studies samples, as exemplified by the Z30A nanocomposite Z30A in Fig. 1d. The most crystalline nanoparticles show lower microstrain, whereas the smallest nanoparticle show higher microstrain. Microstrain demonstrates an inverse relationship with crystallite size. The crystallite size was evaluated using both Williamson–Hall and Debye Scherrer methods, yielding close values that exhibit similar trends with respect to annealing temperature and aluminum atomic concentration evolution. Microstrain increases with increasing aluminum concentration. A good correlation was observed between dislocation density and microstrain. The impact of aluminum atomic concentration on average crystallite size, dislocation density and microstrain was conclusively demonstrated for the prepared samples (refer to Table 1).
The lattice parameter of the ZnAl2O4 spinel phase was determined from the XRD results obtained for Z30A composite nanoparticles calcined at 900 °C, where the majority of the XRD peaks correspond to this phase. This phase exhibits a cubic structure with a lattice parameter of approximately 8.09 (Å), as detailed in Table 2. The formation mechanism of the ZnAl2O4 spinel phase is proposed to involve the reaction between Zn(CH3COO)2 and the polycrystalline α-Al2O3 to form ZnAl2O4 in the presence of oxygen via water vapor through the following reaction:
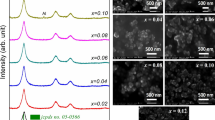
In order to verify the role of aluminum in changing the morphology of ZnO nanoparticles, SEM images and particle size distribution of synthesized powders are shown in Fig. 2, (panel a) of pure ZnO calcined at 900 °C, (panel b) Z30A calcined at 450 °C and (panel c) Z30A calcined at 900 °C. The micrograph clearly indicates that the pure ZnO nanoparticles annealed at 900 °C are homogeneously distributed and have agglomerates of polyhedral and spherical shapes with an average size close to 164 nm (Fig. 3a). The average particle sizes of all samples are determined by counting a sufficient number of particles to ensure accuracy. In addition, the Z30A nanocomposite calcined at 450 °C shows that the morphology of the nanoparticles has been modified. The presence of nanoflowers can be seen (Fig. 2 panel b). The addition of aluminum affects the morphology and average particle size. It is also evident that annealing contributes to microstructural stabilization and reduces the average particle size. The Z30A nanocomposite annealed at 450 °C exhibits an average particle size of around 56 nm, which decreases to 35nm for the same composite annealed at 900 °C. The morphological analysis is in good correlates well with the XRD data. There is a difference between the particle size calculated by XRD and the statistical measurements obtained by SEM. This difference can be attributed to porosity, which is not accounted for in the XRD calculations. SEM measurements are considered closer to reality because they are based on statistical measurement of physical characteristics of the particles (refer to the calculation of crystallite size by Debye Scherrer [19]).
Figure 3 shows the chemical composition of pure ZnO nanopowders and their ZnO-Al2O3 composites. These measurements were conducted using energy dispersive X-ray spectroscopy (EDS). The EDS spectra confirmed the synthesis of the nanoparticles and provide conclusive evidence of the presence of aluminum in the composite powders. The surface analysis of the samples shows the presence of Zn, O, Al and C with no other element or impurity is detected in our synthesized powders. In ZnO-Al2O3 nanocomposites, the substitution of Al at certain Zn sites leads to a decrease in Zn intensity compared to pure ZnO, as depicted in the EDS figures. The annealing process influences the reduction in Carbon concentration, which disappears as CO2 when the annealing temperature is increased (from 20.24% to 7.88% atomic of carbon for the Z30A nanocomposite annealed at 450 and 900 °C respectively).
The PL spectra obtained for pure ZnO calcined at 450 and 900 °C are shown in Fig. 4. The PL spectrum reveals two emission bands. The first band, around 380 nm, is attributed to excitonic emission (ultraviolet emission and also called near-band emission, NBE), involving recombination between an electron near the conduction band (CB) and a hole in the valence band (VB). resulting a photon with energy corresponding to the ZnO band gap [37]. The second is a large band of high intensity comprising two peaks in green and red regions. Gaussian curve deconvolution of the experimental PL peak reveals these peaks are located at wavelengths of 520 and 681 nm, respectively. The green luminescence band is associated to oxygen vacancies, while the red luminescence originates from OH groups on the [38, 39]. It should be noted that the green luminescence is more frequently observed than the red band [40]. The presence of various defects, such as interstitial oxygen and oxygen vacancies enhances the intensity of the green emission band after annealing. Annealing partially eliminates surface impurities. Several studies have already demonstrated that PL intensities trend to increase after annealing under suitable experimental conditions [41]. The peak observed at 608 nm attributed to yellow emission which is generally related to oxygen defect (Oi). This emission can be substituted for the green and red bands through a heat-treatment desorption process of Zn(OH)2 hydroxyl groups [42]. The various emissions observed in PL Gaussian curve deconvolution comprising Near-Band Edge Excitonic Emission (NBE) and deep-level emission (DLE) are summarized in Table 3.
For comparison of the PL spectra of Z10A and Z30A composite nanopowders, Fig. 5a shows a similar appearance as the PL spectra of pure ZnO with a slight shift in the UV emission from 383 to 386 nm for the composites. This shift is attributed to the substitution of Al atoms in the Zn sites [43]. The incorporation of aluminum into the ZnO matrix results in a decrease in the intensity of the PL bands. When aluminum is introduced into the ZnO, the Al ions consume the remaining Zn ions and reduce the concentration of interstitial oxygen. Additionally, Al helps reduce the concentration of oxygen vacancies according to the reaction [44, 45]:
A small shoulder on the right between 760 and 786 nm, which corresponds to a red emission band that is distincly present in ZnO-Al2O3 composite nanopowders. This shoulder could confirm the incorporation of Al atoms (with s smaller radius) into the ZnO lattice [46]. In PL spectra of the same nanocomposite (Z10A and Z30A) annealed at 900 °C, shown in Fig. 5b, the UV emission band is obscured by the band related to defects in the case of Z10A powder, wherea a band-to-band absorption (gap) appears in Z30A powder. This indicates that the defect density and impurities play a significant role in the composites Z10A calcined at 900 °C. the shoulder at 760 nm has disappeared, presumably due to the formation of the spinel phase.
The optical absorption spectra (UV-Visible) of pure ZnO nanoparticles and their composites (ZnO-Al2O3) reveal that in the visible light region (wavelength between 400 and 800 nm), no significant absorption is observed for all the studied samples. However, a strong absorption band appears below 400 nm, attributable to fundamental absorption (band-to-band transition). As the aluminum concentration increases, the intensity decreases and the absorption edge shifts towards the lower wavelengths (Fig. 6).
From the absorption spectra, we determined the optical gap of our powders. The direct optical band gap energy (Eg) of the synthesized samples was calculated using the Tauc plot method [47]. The optical absorption α is expressed as a function of the gap (Eg) according to the following equation:
A is a constant and hν is the photon energy. Band gap graphs were plotted in the linear region near the onset of (αhυ)2 versus hν. The point of intersection with the horizontal axis indicates the gap value, as shown in Fig. 6. It can be seen that the optical gap value for pure ZnO decreases with increasing calcination temperature: 3.13, 3.10 and 3.08 eV for powders calcined at 450, 700 and 900 °C respectively (Table 4). Gap energy is influenced by particle size, carrier concentration and the material`s stress state [48]. Moreover, the annealing process improves crystallinity, increases the average grain size and reduces defects, therby decreasing the band gap energy [49]. At the same annealing temperature, the band gap energy tends to increase with the aluminum concentration compared to pure ZnO. According to quantum band theory, the band gap of a semiconductor increases with decreasing crystal size [50, 51]. The Burstein-Moss effect explains the increase in the gap with the aluminum content in ZnO. Aluminum act as a donor in ZnO and is completely ionized at room temperature, causing electrons moving from aluminum donor level to the lower levels of the conduction band. This has the effect of increasing the value of the gap for electrons coming from the valence band [51,52,53]. Furthermore, the apparition of spinel phase ZnAl2O4 leads to an increase in the band gap energy. For the Z30A nanocomposite the band gap energy increases from 3.11 eV to 3.17 eV for 450 and 900 °C.
The incorporation of impurities and the presence of defects in semiconductors introduce to local elastic fields that affect the band tails near the edge of the band. Urbach’s energy is thought to be a function of structural disorder and follows Urbach’s rule of thumb [54].
It is determined from the inverse of the slope of the linear portion of the curve Lnα as a function of (ℎv).
The results of the calculation of the Urbach Energy are also shown in Table 4. For pure ZnO it can be observed that the two quantities Eu and Eg evolve in opposite directions: Eu increases while Eg decreases with increasing calcination temperature. This clearly confirms the correlation between these two parameters, which are logically linked to the elastic distortion field possibly caused by varying density of dangling bonds in the material. The lowest Urbach Energy is obtained for the Z30A powder nanocomposite annealed at 900 °C which contains both ZnO phases and the ZnAl2O4 spinel phase and has the smallest particle size. For the synthesized powder composites the Urbach energy values follows the same crystallite size behavior observed in the XRD results.
The high value of Urbach Energy is observed at the annealing temperature 700 °C which is considered as an order-disorder transition temperature in the ZnO (Al) single-phase system transitioning to the binary system (ZnO-ZnAl2O4).
Photocatalytic activity testing was conducted on both pure ZnO and nanocomposites (Z10A and Z30A) calcined at 450 and 900 °C. These samples exhibited interesting results in terms of their structural and optical properties. The photocatalytic activities of the samples were evaluated by measuring the degradation of methyl blue (MB) in an aqueous solution under visible irradiation. MB was selected as a model organic pollutant. The photocatalytic degradation of methylene blue is attributed to the formation of hydroxyl radicals (•OH). These radicals are generated by the reaction between adsorbed OH− on the ZnO surface and the holes h+ on the valence band.
Figure 7a shows that the highest photocatalytic activity is obtained for pure ZnO, followed by the Z30A nanocomposite. Pure ZnO calcined at 450 °C has the smallest crystallite size (27.2 nm) compared with the Z10A and Z30A nanocomposites. PL spectra of pure ZnO reveal the presence of defects. Photo-generated electrons are captured by oxygen vacancies and defects in the sample, enhancing the inhibition of photo-generated electrons and holes. This leads to greater photocatalytic degradation of the dye under sunlight [55]. The higher intensity of PL signals leads to greater photocatalytic degradation of organic pollutants [56]. Furthermore, the carbon content plays an important role on the photocatalytic performances. In pure ZnO, the carbon content decreases as the temperature increases from 450 to 900 °C, while photo-catalytic performance decreases.
Comparison of the photocatalytic degradation of MB under visible-light irradiation in the presence of pure ZnO nanoparticles and ZnO-Al2O3 nanocomposites powder (a and b). First-order kinetics of MB dye degradation with time of irradiation using the same nanoparticles calcined at 450 and 900 °C (c and d)
The increase in the atomic concentration of aluminum reveals the presence of the ZnAl2O4spinel phase in Z30A nanocomposite samples calcined at 450 °C (see XRD spectra). It is suggested that the presence of the ZnAl2O4 spinel phase may enhance photocatalytic degradation. However, the situation was inversed for photocatalytic activity in the case of nanoparticles annealed at 900 °C. Figure 7b illustrates that the absorption capacities and photocatalytic activities increase with the content of Al. Nanoparticles containing 30% Al showed the best photodegradation. This sample has the smallest crystalline size (13.7 nm) and a high ZnAl2O4 phase content. Studies have shown that a small particle size, let alone a large specific surface area, ensures high photoactivity [57, 58]. In their studies, Xinjuan Zhang et al. [20] demonstrated that the high concentration of Aluminum in AZO samples present excellent photocatalytic activities and adsorption capacities. Nanoparticles containing 20% Al already show unprecedented absorption capacity, reducing the concentration of Methyl orange (MO) concentration from 200 to 2.7 mg/L, and completely degrading MO within 30 min irradiation. The photo-catalytic activity becomes effective in the presence of the ZnAl2O4 spinel phase. The ZnAl2O4 particles help to change the shape and increase the specific surface area. Their structure is mesoporous [19,20,21]. SEM image shows the correlation between surface area, particles size and photocatalytic performance. The surface effect outweighs the stress effect on the photocatalytic activities. despite the low carbon concentration in this sample, it didn`t hinder improved photocatalytic performance.
The presence of the ZnAl2O4 phase promoted the generation of electron-hole pairs, facilitating photons to be absorbed in both ZnO and ZnAl2O4. In ZnAl2O4, electrons and holes are generated through deep donor and acceptor levels introduced by the defects associated with this phase in ZnO. In contrast, in pure ZnO electron-hole pairs are generated via band-to-band transition [15]. Figure 8 illustrates the process of transferring photogenerated pairs (electron-hole).Electrons located in the conduction band (CB), the lower part, of ZnAl2O4 migrate towards that of ZnO, while holes located in the valence band (VB), the upper part, of ZnAl2O4 remain there. On the other hand, the holes at the top of the VB of ZnO migrate towards that of ZnAl2O4, whereas the electrons at the bottom of the CB of ZnO does not migrate [59]. This process is energetically favorable and the photogenerated pairs can be efficiently separated, which is considered to be the key factor for improving photocatalytic activities in the case of ZnO- ZnAl2O4 nanocomposites.
Diagram of the energy band levels of the semiconductors ZnO and ZnAl2O4 and the procedure for transferring photogenerated electron and hole pairs [59]
The possible mechanism for dye degradation using ZnAl2O4 nanoparticles can be presented as follows [60, 61]:
Determination of the rate of degradation can be very beneficial for evaluation of the color degradation behavior of nanopowders. As shown in Fig. 7c, d, the degradation rate of pure ZnO and nanocomposites (Z10A and Z30A) calcined at 450 and 900 °C is obtained by the first order kinetic equation as follow [62]:
Where C0 is the initial concentration (10 mg/L for MB in the present experiment) and C is the concentration factor after the time illumination of t. K is the rate constant (1/min), which expresses the rate of MB degradation. For the nanopowders crystallized at 450 °C, the MB degradation rate decreases in the following order: 1.8 10−3 min−1 (pure ZnO), 1.2 10−3 min−1 (Z30A) and 0.8 10−3min−1 (Z10A). For the same nanopowders crystallized at 900 °C, the order of kinetic decreases is as follows: 1.9 10−3 min−1 (Z30A), 1.5 10−3 min−1 (pure ZnO) and 0.4 10−3 min−1 (Z10A). There is a good correlation between kinetics and photocatalytic performance. ZnAl2O4 spinel phase contributed to an increase in the number of hydroxyl radicals •OH on the ZnO surface, and therefore to an increase in the kinetics degradation.
4 Conclusions
ZnO nanoparticles and their composites have been successfully prepared using the sol–gel method. The as-synthetized nanoparticles with aluminum atomic concentration of 10%, 2%0 and 30% are annealed at different temperature (450, 700 and 900 °C) to improve their structural and optical properties.
The XRD patterns revealed that the pure ZnO crystallizes in a hexagonal würtzite structure. The presence of aluminum in the composite powders significantly influenced the appearance of the ZnAl2O4 spinel phase and led to a reduction in crystallite size with increasing annealing temperature. The Z30A nanocomposite (ZnO-30% at Al), which contained a high content of ZnAl2O4 phase, exhibited the smallest crystal size of about 13.75 nm. The introduction of ZnAl2O4 spinel phase with a different crystallographic structure compared to ZnO induced deformations that increased the density of dislocations and microstrain in the material. These factors impacted on the morphology of the agglomerates and contributed to the formation of nanoflowers. Photoluminescence analysis showed that optical quality progressively deteriorated with increasing aluminum concentration, while the annealing temperature had the opposite effect. The analysis of UV-Visible optical absorption spectra of the nanoparticles showed the relationship between band gap energy, particle size and annealing temperature. Moreover, the band gap energy tended to increase with aluminum concentration at the same annealing temperature. The calculation of the Urbach energy further supported these findings, confirming that the annealing temperature of 700 °C represents an order-disorder transition temperature from the single-phase ZnO(Al) to the biphasic ZnO-ZnAl2O4 system. The best photocatalytic activity is observed for the biphasic ZnO-ZnAl2O4 system compared with the single-phase ZnO system. Good kinetics across all cases contributed to excellent photocatalytic performances. The effect of annealing and of the atomic concentration of aluminum in ZnO-Al2O3 composite nanopowders shows a new property at the nanometric scale that is of considerable interest for its application as a technology for water depollution and environmental preservation. These nanopowders reveal a good structural quality and spectroscopic and optical aptitude that respond favorably to these aspirations. For practical purposes the best sample contains spinel phase with high doped Aluminum. Looking forward, further studies will expand the investigation of photocatalytic activity by using other organic and inorganic dyes in order to elucidate the different mechanisms involved in photocatalysis.
References
Rekha K, Nirmala M, Nair MG, Anukaliani A (2010) Structural, optical, photocatalytic and antibacterial activity of zinc oxide and manganese doped zinc oxide nanoparticles. Phys B Condens Matter p05:3180–3185. https://doi.org/10.1016/j.physb.2010.04.042
Belabed N, Chari A, Ayadi A, Bousnina K, Kasouit S (2022) Effects of Additives on Anatase to Rutile Phase Transformation of TiO2 Powder Synthetized by Sol–Gel Route. Acta phys Pol A 142:226. https://doi.org/10.12693/APhysPolA.142.226
Thambidurai M, Muthukumarasamy N, Velauthapillai D, Arul NS, Agilan S, Balasundaraprabhu R (2011) Dye-sensitized. ZnO nanorod based photoelectrochemical solar cells with natural dyes extracted from Ixora coccinea, Mulberry and Beetroot. J Mater Sci Mater Electron 22:1662–1666. https://doi.org/10.1007/s10854-011-0342-9
Sarker NH, Barnaby SN, Fath KR, Frayne SH, Nakatsuka N, Banerjee IA (2012) Biomimetic growth of gallic acid–ZnO hybrid assemblies and their applications. J Nanopart Res 14:773. https://doi.org/10.1007/s11051-012-0773-0
De Lacy Costello BPJ, Ewen RJ, Ratcliffe NM, Richards M (2008) Highly sensitive room temperature sensors based on the UV-LED activation of zinc oxide nanoparticles. Sens Actuators B 134:945–952. https://doi.org/10.1016/j.snb.2008.06.055
Elam JW, George SM (2003) Growth of ZnO/Al2O3 Alloy Films Using Atomic Layer Deposition. Tech Chem Mater 15:1020–1028. https://doi.org/10.1021/cm020607+
Romcevic N, Hadzic B, Romcevic M, Paunovic N, Sibera D, Narkiewicz U, Kuryliszyn-Kudelska I, Ristic-Djurovic JL, Dobrowolsk WD (2020) Structural and optical properties of ZnO–Al2O3 nanopowders prepared by chemical methods. J Lumin 224:117273. https://doi.org/10.1016/j.jlumin.2020.117273
Purwaningrum W, Fingky Pristika S, Julinar, Ahmadi A, Said M (2022) Effect of Al Concentration over ZnO-Al2O3 Physicochemical Characteristics and Removal of Remazol Red RB. ASEAN J Chem Eng 22:206–217. https://doi.org/10.22146/ajche.70084
Aghaei M, Sajjadi S, Keihan AH (2020) Sono-coprecipitation synthesis of ZnO/CuO nanophotocatalyst for removal of parathion from wastewater. Environ Sci Pollut Res 27:11541–11553. https://doi.org/10.1007/s11356-020-07680-0
El-Naggar ME, Wassel AR, Shoueir K (2021) Visible-light driven photocatalytic effectiveness for solid-state synthesis of ZnO/natural clay/TiO2 nanoarchitectures towards complete decolorization of methylene blue from aqueous solution. J Photochemistry Photobiol A Chem 15:100425. https://doi.org/10.1016/j.enmm.2020.100425
Hassan AA, Qusay AN (2023) New approach for synthesis of ZnO nanoparticles via thermal decomposition of [Zn(BIAHQ)2].H2O complex: Characterization and photocatalytic degradation of reactive blue 4 dye. AIP Conf Proc 2845:020029. https://doi.org/10.1063/5.0157251
Demircan G, Gurses EF, Aktas B, Yalcin S, Acikgoz A, Ceyhan G, Vehbi Balak M (2023) Sol–gel synthesis of Si-ZnO, Ti-ZnO and Si-Ti-ZnO thin films: Impact of Si and Ti content on structural and optical properties. Mater today Commun 34:105234. https://doi.org/10.1016/j.mtcomm.2022.105234
Zheng Y, Chen C, Zhan Y, Lin X, Zheng Q, Wei K, Zhu J, Zhu Y (2007) Luminescence and Photocatalytic Activity of ZnO Nanocrystals:Correlation between Structure and Property. Inorg chem 46:6675–6682. https://doi.org/10.1021/ic062394m
Xu L, Hu YL, Pelligra C, Chen CH, Jin L, Huang H, Sithambaram S, Aindow M, Joesten R, Suib SL (2009) ZnO with Different Morphologies Synthesized by Solvothermal Methods for Enhanced Photocatalytic Activity. Chem Mater 21:2885–2875. https://doi.org/10.1021/cm900608d
Ferreira NS, Sasaki JM, Silva JR RS, Attah-Baah JM, Macêdo MA (2021) Visible-Light-Responsive Photocatalytic Activity Significantly Enhanced by Active [VZn+VO+] Defects in Self-Assembled ZnO Nanoparticles. Inorg Chem 60:4475–4496. https://doi.org/10.1021/acs.inorgchem.0c03327
Chaves NO, Lima LS, Monteiro MDS, Sobrinho RAL, Ferreira NS, Ramos GQ, da Fonseca Filho HD, Oliveira RMPB, Matos RS (2024) Associating Physical and Photocatalytic Properties of Recyclable and Reusable Blast Furnace Dust Waste. Materials 17(4):818. https://doi.org/10.3390/ma17040818
Rohit B, Madhulika S, Bahadur D (2013) Visible light-driven novel nanocomposite (BiVO4/CuCr2O4) for efficient degradation of organic dye. Dalton Trans 42:6736–6744. https://doi.org/10.1039/C2DT32753H
Iaiche S, Djelloul A (2015) ZnO/ZnAl2O4 Nanocomposite Films Studied by X-Ray Diffraction, FTIR, and X-Ray Photoelectron Spectroscopy. J Spectroscopy 1–9. https://doi.org/10.1155/2015/836859.
Kumar TR, Selvam NCS, Ragupathi C, Kennedy JL, Vijaya JJ (2012) Synthesis, characterization and performance of porous Sr (II)-added ZnAl2O4 nanomaterials for optical and catalytic applications. Powder Technol 2012(224):147–154. https://doi.org/10.1016/j.powtec.2012.02.044
El-Nabarawy T, Attia AA, Alaya MN (1995) Effect of thermal treatment on the structural, textural and catalytic properties of the ZnO-Al2O3 system. Mater Lett 24:319–325. https://doi.org/10.1016/0167-577X(95)00101-8
Zhang X, Chen Y, Zhang S, Qui C (2017) High photocatalytic performance of high concentration Al-doped ZnO nanoparticles. Sep Purif Technol 172:236–241. https://doi.org/10.1016/j.seppur.2016.08.016
Abrahams SC, Bernstein JL (1969) Remeasurement of the Structure of Hexagonal ZnO. Acta Cryst Sec B B25:1233. https://doi.org/10.1107/S0567740869003876
Levy D, Pavese A, Sani A, Pischedda V (2001) Structure and compressibility of synthetic ZnAl2O4 (gahnite) under high-pressure conditions, from synchrotron X-ray powder diffraction. Phys Chem Miner 28:612–618. https://doi.org/10.1007/s002690100194
Satoru Y, Fumiyasu O, Rong H, Isao T, Teruyasu M, Tomoyuki Y (2008) Atomic structures of supersaturated ZnO–Al2O3 solid solution. J Appl Phys 103:014309,1-9. https://doi.org/10.1063/1.2829785
Chen R, Zou C, Yan X, Alyamani A, Gao W (2011) Growth mechanism of ZnO nanostructures in wet-oxidation process. Thin Solid Film 519:1837–1844. https://doi.org/10.1016/j.tsf.2010.10.012
Scherrer P (1912) Bestimmung der inneren Struktur und der Größe von Kolloidteilchen mittels Röntgenstrahlen. In: Kolloidchemie Ein Lehrbuch. Chemische Technologie in Einzeldarstellungen. Springer, Berlin, Heidelberg. pp. 387–409. https://doi.org/10.1007/978-3-662-33915-2_7.
Bragg WH (1915) Bakerian lecture: x-rays and crystal structure. Philos Trans R Soc 215:523–537. https://doi.org/10.1098/rsta.1915.0009
Fang ZB, Yan ZJ, Tan YS, Liu XQ, Wang YY (2005) Influence of post-annealing treatment on the structure properties of ZnO films. Appl Surface Sci 241:303–308. https://doi.org/10.1016/j.apsusc.2004.07.056
Babita B, Kishore DK, Manorama VS (2006) Hydrothermal synthesis of highly crystalline ZnO nanoparticles: A competitive sensor for LPG and EtOH. Sens Actuators B: Chem 119:676–682. https://doi.org/10.1016/j.snb.2006.01.028
Kayani ZN, Saleemi F, Batool I (2015) Effect of calcination temperature on the properties of ZnO nanoparticles. Appl Phys A 119:713–720. https://doi.org/10.1007/s00339-015-9019-1
Ferreira NS, Angélica RS, Marques VB, de Lima CCO, Silva MS (2016) Cassava-starch-assisted sol–gel synthesis of CeO2 nanoparticles. Mater Lett 165:139–142. https://doi.org/10.1016/j.matlet.2015.11.107
Menezes JCA, Ferreira NS, Abraçado LG, Macêdo MA, Nanosci J (2014) Synthesis and Characterization of Nickel Nanoparticles Prepared Using the Aquolif Approach. J Nanosci Nanotechnol 14:5903–5910. https://doi.org/10.1166/jnn.2014.8727
Djelloul A, Aida MS, Bougdira JC (2010) Photoluminescence, FTIR and X-ray diffraction studies on undoped and Al-doped ZnO thin films grown on polycrystalline a-alumina substrates by ultrasonic spray pyrolysis. J Lumin 130:2113–2117. https://doi.org/10.1016/j.jlumin.2010.06.002
Shukla RK, Srivastava A, Srivastava AT, Dubey KC (2006) Growth of transparent conducting nanocrystalline Al doped ZnO thin films by pulsed laser deposition. J Cryst Growth 294:427–431. https://doi.org/10.1016/j.jcrysgro.2006.06.035
Zaman A, Uddin S, Mehboob N, Ali A, Ahmad A, Bashir K (2021) Effect of Zr4+ on the structural and microwave dielectric properties of CaTiO3 ceramics. Ferroelectrics 577(1):143–152. https://doi.org/10.1080/00150193.2021.1916357
Rajesh Kumar B, Hymavathi B (2017) X-ray peak profile analysis of solid-state sintered alumina doped zinc oxide ceramics by Williamson-Hall and size-strain plot methods. J Asian Ceram Soc 5(2):94–103. https://doi.org/10.1016/j.jascer.2017.02.001
Erik Cerrato E, Paganini MC, Giamello E (2020) Photoactivity under visible light of defective ZnO investigated by EPR spectroscopy and photoluminescence. J Photochem Photobiol A Chem 397:112531. https://doi.org/10.1016/j.jphotochem.2020.112531
Stanley R, Jeasingh A, Manisha Vidyavathy S, Kingston Stanley P, Ponmani P, Shekinah ME, Vasanthi J (2012) Excellent Photocatalytic degradation of Methylene Blue, Rhodamine B and Methyl Orange dyes by Ag-ZnO nanocomposite under natural sunlight irradiation. Optik Int J Light Electron Optics, 231. https://doi.org/10.1016/j.ijleo.2021.166518.
Li S, Zhang X, Yan B, Yu T (2009) Growth mechanism and diameter control of well-aligned small-diameter ZnO nanowire arrays synthesized by a catalyst-free thermal evaporation method. Nanotechnology 20:495604. https://doi.org/10.1088/0957-4484/20/49/495604
Wang M, Cheng X, Yang J (2009) Controlled visible photoluminescence of ZnO thin films prepared by RF magnetron sputtering. Appl Phys A 96:783–787. https://doi.org/10.1007/s00339-009-5272-5
Xiong G, Pal U, Serrano JG, Ucer KB, Williams RT (2006) Photoluminesence and FTIR study of ZnO nanoparticles: the impurity and defect perspective. phys stat sol c 3:3577–3581. https://doi.org/10.1002/pssc.200672164
Li D, Leung YH, Djuriši´c AB, Liu ZT, Xie MH, Shi SL, Xu SJ, Chan WK (2004) Different origins of visible luminescence in ZnO nanostructures fabricated by the chemical and evaporation methods. Appl Phys Lett 85:1601–1603. https://doi.org/10.1063/1.1786375
Manouni AE, Manjón FJ, Mollar M, Marí B, Gómez R, López MC, Ramos-Barrado JR (2006) Effect of aluminium doping on zinc oxide thin films grown by spray pyrolysis. Superlattices Microstructures 9:185–192. https://doi.org/10.1016/j.spmi.2005.08.041
Kuo SY, Chen WC, Lai FI, Cheng CP, Kuo HC, Wang SC, Hsieh WF (2006) Effects of doping concentration and annealing temperature on properties of highly-oriented Al-doped ZnO films. J Cryst Growth 287:78–84. https://doi.org/10.1016/j.jcrysgro.2005.10.047
Hyoun WK, Mesfin AK, Hyo SK (2010) Structural Raman, and photoluminescence characteristics of ZnO nanowires coated with Al-doped ZnO shell layers. Curr Appl Phys 10:60–63. https://doi.org/10.1016/j.cap.2009.04.012
Thandavan TMK, Gani SMA, San Wong C, Md Nor R (2015) Enhanced Photoluminescence and Raman Properties of Al-Doped ZnO Nanostructures Prepared Using Thermal Chemical Vapor Deposition of Methanol Assisted with Heated Brass. PLoS One 10(3):e0121756. https://doi.org/10.1371/journal.pone.0121756
Tauc J, Grigorovici R, Vancu A (1966) Optical properties and electronic structure of amorphous germanium. Phys Status Solidi b 15:627–637. https://doi.org/10.1002/pssb.19660150224
Zhu BL, Sun XH, Guo SS, Zhao XZ, Wu J, Wu R, Liu J (2006) Effect of Thickness on the Structure and Properties of ZnO Thin Films Prepared by Pulsed Laser Deposition. Jpn. J Appl Phys 45:7860. https://doi.org/10.1143/JJAP.45.7860
Caglar M, Ilican S, Caglar Y, Yakuphanoglu F (2009) Electrical conductivity and optical properties of ZnO nanostructured thin film. Appl Surf Sci 255:4491–4496. https://doi.org/10.1016/j.apsusc.2008.11.055.
Khan I, Khan S, Nongjai R, Ahmed H, Khan W (2013) Structural and optical properties of gel-combustion synthesized Zr doped ZnO nanoparticles. Opt Mater 5:1189–1193. https://doi.org/10.1016/j.optmat.2013.01.019.
Ahammed N, Samim Hassan MD, Hassan M (2018) Effects of aluminum (Al) incorporation on structural, optical and thermal properties of ZnO nanoparticles. Mater Sci Pol 36(3):419–426. https://doi.org/10.1515/msp-2018-0018
Burstein E (1954) Anomalous optical absorption limit in InSb. Phys Rev 93:632. https://doi.org/10.1103/PhysRev.93.632
Moss TS (1954) The interpretation of the properties of indium antimonide. Proc Phys Soc B 67. https://doi.org/10.1088/0370-1301/67/10/306
Urbach F (1953) The Long-Wavelength Edge of Photographic Sensitivity and of the Electronic Absorption of Solids. Phys Rev 92:1324–1324. https://doi.org/10.1103/PhysRev.92.1324
Nishanthi ST, Iyyapushpam S, Sundarakannan B, Subramanian E, Pathinettam Padiyan D (2014) Inter-relationship between extent of anatase crystalline phase and photocatalytic activity of TiO2 nanotubes prepared by anodization and annealing method. Sep Purif Technol 131:102–107. https://doi.org/10.1016/j.seppur.2014.04.047
Stanleya R, Alphas Jebasingh J, Manisha Vidyavathya S, Kingston Stanley P, Ponmani O, Shekinah ME, Vasanthi J (2021) Excellent Photocatalytic degradation of Methylene Blue, Rhodamine B and Methyl Orange dyes by Ag-ZnO nanocomposite under natural sunlight irradiation. Optik 231:166518. https://doi.org/10.1016/j.ijleo.2021.166518
Calza P, Pelizzetti E, Mogyorósi K, Kun R, Dékány I (2007) Size dependent photocatalytic activity of hydrothermally crystallized titania nanoparticles on poorlyadsorbing phenol in absence and presence of fluoride ion. Appl Catal B Environ 72:314–321. https://doi.org/10.1016/j.apcatb.2006.10.019
Cappelletti G, Bianchi CL, Ardizzone S (2008) Nano-titania assisted photoreduction of Cr(VI) The role of the different TiO2 polymorphs. Appl Catal B: Environ 78:193–201. https://doi.org/10.1016/j.apcatb.2007.09.022
Zhao X, Wang L, Xu X, Lei X, Xu S, Zhang F (2012) Fabrication and Photocatalytic Properties of Novel ZnO/ZnAl2O4 nanocomposite with ZnAl2O4 Dispersed Inside ZnO Network. AIChE J 58:573–582. https://doi.org/10.1002/aic.12597
Chaudharya A, Mohammada A, Mobin SM (2018) Facile synthesis of phase pure ZnAl2O4 nanoparticles for effective photocatalytic degradation of organic dyes. Mater Sci Eng B 227:136–144. https://doi.org/10.1016/j.mseb.2017.10.009
Chen X, Zhang F, Wang Q, Han X, Li X, Liu J, Lin H, Qu F (2015) The synthesis of ZnO/SnO2 porous nanofibers for dye adsorption and degradation. Dalton Trans 44:3034–3042. https://doi.org/10.1039/C4DT03382E
Paul DR, Sharma R, Nehra SP, Sharma A (2019) Effect of calcination temperature, pH and catalyst loading on photodegradation efficiency of urea derived graphitic carbon nitride towards methylene blue dye solution. RSC Adv 9(27):15381–15391. https://doi.org/10.1039/C9RA02201E
Meyer BK, Alves H, Hofmann DM, Kriegseis W, Forster D, Bertram F, Christen J, Hoffmann A, Straßburg M, Dworzak M et al. (2004) Bound exciton and donor-acceptor pair recombination in ZnO. Phys Status Solidi Basic Res 241:231–260. https://doi.org/10.1002/pssb.200301962
Quemener V, Vines L, Monakhov EV, Svensson BG (2012) Evolution of deep electronic states in ZnO during heat treatment in oxygen- and zinc-rich ambient. Appl Phys Lett 100:1–5. https://doi.org/10.1063/1.3693612
Bhaskar R, Lakshmanan AR, Sundarrajan M, Ravishankar T, Jose MT, Lakshminarayan N (2009) Mechanism of green luminescence in ZnO. Indian J Pure Appl Phys 47:772–774. http://nopr.niscpr.res.in/handle/123456789/6193
Lv J, Li C, Chai Z (2019) Defect luminescence and its mediated physical properties in ZnO. J Lumin 208:225–237. https://doi.org/10.1016/j.jlumin.2018.12.050
Galdámez-Martinez A, Santana J, Güell F, Martínez-Alanis PR, Dutt A (2020) Photoluminescence of ZnO Nanowires. A review. Nanomaterials 10(5):857. https://doi.org/10.3390/nano10050857
Alvi NH, ul Hasan K, Nur O, Willander M (2011) The origin of the red emission in n-zno nanotubes/p-gan white light emitting diodes. Nanoscale Res Lett 6:130. https://doi.org/10.1186/1556-276X-6-130
Djuriši´c AB, Leung YH, Tam KH, Hsu YF, Ding L, Ge WK, Zhong YC, Wong KS, Chan WK, Tam HL et al. (2007) Defect emissions in ZnO nanostructures. Nanotechnology 18:095702. https://doi.org/10.1088/0957-4484/18/9/095702
Acknowledgements
We thank Dr. Aissa KEFFOUS, Director of the Centre de Recherche en Technologie des Semi-Conducteurs pour l’Energétique CRTSE; http://crtse.dz/ and the members of the MEEC (TESE3) team for their help and collaboration throughout of this work. We would also like to thank Professor Ali DEMIR, Head of TEMAG Laboratory- Turkey, Ali Kilic and Dr. Melike GÜNGÖR for their help in facilitating the conduction of certain characterization.
Author information
Authors and Affiliations
Contributions
Conceptualization, FB and AA; methodology, AC and AA; validation, AA, AC, LM and FB; formal analysis, AA and AC; investigation, AA, LM and FB; resources, LM and KA; writing—original draft preparation, AA, AC and FB; writing—review and editing, FB, AA, AC, LM and KA; visualization, FB, AA, AC, LM and KA; supervision, AA, AC. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Benmammar, F., Ayadi, A., Maifi, L. et al. Temperature annealing and aluminum atomic concentration effect on structural, morphological and optical properties of ZnO-Al2O3 nanocomposites powders and photocatalytic performances. J Sol-Gel Sci Technol (2024). https://doi.org/10.1007/s10971-024-06514-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10971-024-06514-6