Abstract
This study successfully synthesized Fe2O3@rGO nanocrystals using a straightforward sol-gel method in a pigskin-gel (gelatin type A) environment. The fabricated nanocomposites include Fe2O3-linked nanosheets in a reduced graphene oxide-like double tetrahedral pyramid (DTP) structure and Fe2O3 nanocrystals resembling Cheetos puffs (CPs). The Fe2O3@rGO material was characterized through a variety of analytical techniques, including X-ray diffraction, field emission scanning electron microscopy, energy-dispersive X-ray spectroscopy, elemental mapping, Fourier transform infrared spectroscopy, ultraviolet-visible spectroscopy, Raman spectroscopy, vibrating sample magnetometer, nitrogen physisorption, and electrochemical impedance spectroscopy. The photocatalytic efficiency of Fe2O3 and Fe2O3@rGO samples in demineralizing rhodamine B in an aqueous solution was thoroughly evaluated. Remarkably, the Fe2O3@rGO composites exhibited significantly enhanced photocatalytic activity and degradation efficiency compared to pure Fe2O3 nanocrystals. The improved performance can be attributed to effective electron transport between the reduced graphene oxide and the semiconductors, effectively reducing electron-hole recombination. Additionally, the Fe2O3@rGO nanocrystals demonstrated excellent magnetic properties, enabling easy separation and recovery after five cycles of reusability testing. These findings highlight the promising potential of this magnetic nano photocatalyst for efficient and sustainable wastewater treatment applications, particularly in the degradation of rhodamine B.
Graphical Abstract

Highlights
-
In the presence of pigskin gel, the synthesis of Fe2O3 nanocrystals (NCs) resembling Cheetos puffs (CPs) were carried out.
-
In the presence of pigskin gel as a reducing agent, Fe2O3 nanocrystals with double tetrahedral pyramidal geometry (DTP) attached to reduced graphene oxide (rGO) nanosheets were obtained.
-
Fe2O3@rGO:DTP Showed improved responsiveness compared to the pure Fe2O3:CPs for the degradation of organic pollutants.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent years, there has been a notable increase in population and industrial development, resulting in the discharge of a wide range of pollutants, including both organic and inorganic chemicals. These pollutants have a significant impact on water resources and pose a considerable threat to the environment [1]. The phenomenon of water contamination has been significantly exacerbated as a result of human activities, particularly in the context of waste-water treatment [2]. Therefore, several techniques have been developed to eliminate contaminants, such as adsorption, coagulation, ion exchange, sedimentation, chemical oxidation, and photocatalysis, among others [3, 4]. The semiconductor heterogeneous photocatalysis method is widely regarded as a very promising and efficient technique for the removal and degradation of organic contaminants from wastewater. This method is particularly advantageous due to its usage of solar energy as a sustainable power source and its environmentally friendly nature [5, 6]. A photoreaction mechanism called photocatalysis uses photocatalysts such as TiO2, ZnO, Fe2O3, etc, to quicken the process when exposed to light. Several phenomena are covered by this topic by accounting for the reduction and oxidation processes [7]. The two types of photocatalysis reactions are homogeneous and heterogeneous. Between the two, heterogeneous photocatalysis reactions show promise due to their fast reaction rates, low energy requirements, and kinder reaction conditions compared to traditional thermal catalysis. They also use sunlight as a sustainable resource [8]. On semiconductor surfaces, a series of oxidation and reduction reactions typically form the basis of the heterogeneous photocatalysis mechanism. In the course of photoirradiation, electrons in the semiconductor’s valence band (VB) are excited into the conduction band (CB) [9]. At the same time, holes are generated on VB. Since radical species are needed for the processes of reduction and oxidation, electron-hole pairs will be used to create them.
Researchers have recently shown increasing interest in Fe2O3 due to its remarkable properties. These include cost-effectiveness, low toxicity at concentrations below 5 mg kg−1, stability in acidic environments, and photocatalytic activity [10]. The desirable valence band edge level, exceptional corrosion stability, and efficient visible light absorption contribute to these traits. The photocatalytic characteristics of Fe2O3 can be attributed to its spinel crystal structure, which is distinguished by a closed cubic packing arrangement of oxide ions. Fe2O3 assumes a cubic crystal structure within the Ia3 space group [11]. It shows excellent potential as an n-type semiconductor material for applications in photocatalysis and photoelectrochemistry due to its wide band gap of 1.9–2.2 eV (corresponding to a wavelength range of 650–560 nm) and its remarkable chemical properties [12]. Historically, Fe2O3 has been used in various applications, such as supercapacitors [13], batteries [14], and gas sensors [15]. Additionally, numerous studies have explored the utilization of Fe2O3 in the area of photocatalysis, and they have demonstrated its favorable performance as a photocatalyst, primarily attributed to its ability to exist in various oxidation states [16, 17]. This characteristic facilitates the generation of oxygen vacancies (OV) in Fe2O3, which are crucial for promoting efficient photocatalytic activity. A high concentration of OV species has been found to benefit the recombination of positive and negative charge carriers in photocatalytic systems, thus enhancing the overall photocatalytic performance [18]. Several studies have been published on the self-modification of Fe2O3 to achieve optimal photocatalytic efficacy. Indeed, Fe2O3 exhibits inadequate photocatalytic performance in its pure form due to the swift recombination of photo-generated charge carriers. Nevertheless, researchers have successfully modified Fe2O3 through various approaches, such as incorporating doping elements, introducing supporting materials, surface functionalization, and loading metals and combining them with other photocatalysts [19,20,21,22,23].
While integrating doping elements and support materials can enhance the overall photocatalytic process, their impact on mitigating the recombination of photo-generated charge carriers remains limited. On the other hand, when Fe2O3 is combined with other semiconductors, a distinct charge carrier mechanism emerges, resulting in a different photogeneration process. This alteration in the charge carrier mechanism reduces the recombination process, consequently boosting the photocatalytic activity of Fe2O3 [24].
Graphene, a carbon allotrope, possesses exceptional properties such as remarkable electron mobility, high surface area, high thermal stability, excellent electrical conductivity, and significant transparency, making it highly regarded as a material [25]. Its structure, composed of a crystalline lattice resembling a honeycomb, consists of singular or multiple graphite layers with sp2 carbon atoms [26]. The incorporation of magnetic materials into graphene has recently gained significant attention due to its unique properties and wide range of applications in sensors [27], optoelectronics [28], antimicrobials [29], and water treatment [30]. Nanoscale magnetic photocatalysts have been a research focus, capable of recovery and reusability through the application of a magnetic field. Among them, nanocomposites comprising reduced graphene oxide and iron oxide have been studied for their notable photocatalytic efficiency under visible light irradiation. These graphene-based composites have shown improved adsorption rates and light absorptivity, leading to more efficient charge transfer [31].
The present investigation focuses on fabricating a photocatalyst of a nanocomposite composed of Fe2O3@rGO. The study explores this composite’s photocatalytic capability by removing Rhodamine B dye (RhB) under visible light exposure. We also demonstrate the use of pig skin gelatin (PSG) as a natural surfactant to enhance the properties of the final product. A rapid and simplified sol-gel approach is used to synthesize Fe2O3 nanoparticles with precise particle-size distribution. These nanoparticles were then applied onto a reduced graphene oxide (rGO) sheet in the presence of PSG, which acted as growth terminators to prevent particle aggregation during calcination. This innovative approach ensures an eco-friendly process and brings about several advantages. Gelatin was crucial in stabilizing the synthesized graphene as a reducing agent. Moreover, the by-products generated during the process were effectively employed for the photocatalytic decomposition of RhB, adding value to the overall methodology. Of particular significance, this study introduces a novel one-pot sol-gel synthesis method, enabling the uniform decoration of rGO with Fe2O3 nanoparticles in a PSG environment. The proposed approach stands out for its efficiency, simplicity, affordability, and eco-friendliness, with the entire process taking place in a single step.
2 Experimental
2.1 Materials
Merck & Co. provided RhB, hydrochloric acid 37%, hydrogen peroxide 30%, potassium permanganate 99.9%, sodium hydroxide 99.99%, and sulfuric acid 98%. Asbury Carbons Inc. (New Jersey, USA) provided graphite flakes. Systerm Chemicals, PAYAMNOUR University of Iran, provided ferric nitrate nonahydrate. Sigma-Aldrich provided PSG for this study. During sample preparation, double distilled water (DDW) was used.
2.2 Graphene oxide fabrication
Graphene oxide (GO) was produced from graphite using Hummer’s method [32, 33]. In an ice bath, 120 mL concentrated H2SO4 was added to 4 g graphite powder, followed by the slow addition of 12 g KMnO4 with vigorous stirring. The mixture was heated to 40 °C for 45 min. The suspension was left to oxidize at room temperature for 30 min after adding 250 mL of DDW. After that, gentle additions of 15 mL H2O2 and 70 mL DDW were made. To achieve a pH range of 4 to 5, the GO was washed three times with a 1 M HCI aqueous solution and then six times with DDW. The washing procedure was carried out utilizing centrifugation-assisted decantation of the supernatant. GO exfoliation occurred during the washing procedure, causing the GO solution to thicken and graphene oxide gel to form (GOG). The concentration of the GOG that resulted was then calculated.
2.3 Preparation of Fe2O3@rGO:DTP nanocomposite
The Fe2O3@rGO:DTP composite was synthesized using the sol-gel technique. Initially, 1 g of PSG was added to 50 ml of DDW at a temperature of 50 °C to form a PSG solution. Fe(NO3)2·9H2O (2.5 g) was dissolved in DDW (30 mL) at room temperature and mixed with 50 mL of a 3% wt./v GO solution. The Fe(III)/GO solution was then combined with the PSG solution and processed at 80 oC in a silicon oil bath for approximately 12 h. The resulting blend was subsequently aged at 80 °C for 12 h, forming a dark red gel.
The Fe2O3@rGO:DTP nanocomposite was acquired via calcination of the gel at a temperature of 400 °C for two hours, with a gradual heating rate of 2 °C per min. After this, Fe2O3@rGO:DTP was dispersed in water and centrifuged at 6000 revolutions per minute, followed by multiple rinses with ethanol and DDW to eliminate any excess ions and PSG. The sample was dried in a vacuum oven at 60 °C for 15 h. The entire process is summarized in Fig. 1.
2.4 Sample preparation for photocatalytic degradation
Photocatalytic degradation of RhB dye under UV irradiation was used to evaluate the photocatalytic activity of the nanocomposite. A 30 ml aqueous solution of RhB dye (10 mg/L) was combined with the photocatalyst (10 mg). A magnetic stirrer was used to agitate the mixture for one hour in the dark until a desorption/adsorption equilibrium was reached. The suspension was then exposed to UV light for 1–6 h (produced by a 400 W high-pressure xenon arc lamp with a 400 nm maximum wave peak, located 30 cm from the photocatalytic reactor), and 2.5 ml of the combined solution was centrifuged to collect the photocatalyst. Through measuring the absorption of the RhB dye in the filtrate at 553 nm, the removal efficiency was studied using UV-vis absorption spectroscopy [34].
2.5 Sample characterization
Several techniques were used to characterize the Fe2O3@rGO:DTP nanocomposite. XRD (X-ray powder diffraction, Philips X’pert X-ray diffractometer with graphite monochromatic CuKα radiation) was used to determine the product’s crystal phase, while FESEM was used to explore its microstructure and morphology (Field emission scanning electron microscopy, MIRA3TESCAN-XMU). Energy-dispersive X-ray analysis (EDXA, SAMX electron microscope) was used to determine the chemical compositions. Raman spectra were obtained using a 514 nm excitation laser wavelength in conjunction with a Renishaw inVia Raman microscope. FTIR spectra were acquired using a System 2000 spectrophotometer (Bruker Tensor, USA), employing the KBr pellet technique. To investigate their optical properties, UV/Vis spectroscopy (Thermo ScientificTM EvolutionTM, USA) was used, as well as electrochemical impedance spectroscopy (EIS) (Potentiostat Ivium technology vertex, the Netherlands). A vibrating sample magnetometer (VSM, model MDK, Iran) was used to evaluate the magnetic characteristics of the samples, while nitrogen physisorption isotherms were obtained using a Belsorp mini II (Microtrac Bel Corp Japan).
3 Results and Discussion
3.1 Characterization of morphology
FESEM investigations were conducted on two types of Fe2O3 nanostructured samples to explore how the presence of GO and PSG (a natural surfactant) affects the sample morphology. The first sample consisted of Fe2O3 resembling Cheetos puffs (CPs) (Fig. 2a–c), while the second sample comprised Fe2O3NSs@rGO (reduced graphene oxide) in the form of double tetrahedral pyramid (DTP) composites, as depicted in Fig. 2d–f.
The absence of PSG resulted in the aggregation of Fe2O3:CPs. The FESEM images of the virgin Fe2O3, shown in Fig. 3a illustrated how the high surface-to-volume ratio of the nanoparticles contributed to particle aggregation, due to their high surface reactivity. To overcome this issue, PSG was introduced, which led to a more tightly controlled particle-size distribution. As shown in Fig. 3b, the FESEM images of as-synthesized Fe2O3:CPs demonstrated the effect of PSG as a natural surfactant, effectively separating and preventing the agglomeration of NCs, making it an ecologically acceptable capping agent.
On the other hand, when GO was introduced into the system during synthesis, the evolving Fe2O3 became fixed on the rGO surface, resulting in an effective loading of rGO nanosheets decorated with Fe2O3:DTP, as observed in Fig. 2d–f. The GO surface appeared to be reduced, with no typical wrinkle-like characteristics. A closer examination revealed that individual Fe2O3:DTP nanoparticles with sizes between 40 and 80 nm were well-separated and scattered across the rGO sheets, and it appears that the Fe2O3 DTPs do not accumulate on the rGO sheets. Nevertheless, the incorporation of rGO appears to expedite the transfer of photo-generated charge carriers to enhance transient photocurrent, as discussed below.
From a mechanistic perspective, we propose that the introduction of negatively charged GO during the sol-gel process facilitated the attraction of Fe(III) cations, which initiated the nucleation and growth of Fe2O3 on the GO’s surface. Consequently, an in-situ charge transfer occurred between the Fe2O3 nuclei and graphene oxide, culminating in the creation of rGO.
Despite the utilization of PSG as a natural surfactant to avoid agglomeration during the 400 °C calcination, it hindered the development of Fe2O3@rGO:DTP. Figure 4 provides a comprehensive understanding of the FESEM images of Fe2O3@rGO:DTP, presenting elemental mapping (EM) and EDXA spectra. The EM analysis demonstrated a consistent nanostructure with a well-balanced spatial distribution of components.
3.2 Crystal structure
Figure 5 illustrates the phase structures of Fe2O3@rGO:DTP composites (containing 3%wt. /v GO) and Fe2O3:CPs in the presence of PSG. Both specimens display X-ray diffraction (XRD) patterns that are similar to that of the control Fe2O3, thereby indicating that the reduction of GO does not impede the emergence of crystalline Fe2O3.
The utilization of rGO serves as a fundamental substrate upon which the nucleation and growth of Fe2O3 can occur. The diffraction peaks are located at 75.50°, 71.99°, 69.64°, 64.23°, 62.65°, 57.81°, 54.27°, 49.51°, 40.91°, 35.68°, 32.21°, and 24.22°, which can be indexed to lattice planes (220), (1010), (208), (300), (214), (018), (116), (024), (113), (110), (104) and (012), respectively. The X-ray diffraction pattern of pure α-Fe2O3 shows a maximum at ~33°, corresponding to the (104) plane, indicating the production of the hematite phase. All observed peaks in the XRD patterns could be assigned to α-Fe2O3 with a cubic configuration and an Fd-3m spatial grouping [35].
In Table 1, we present the crystallite sizes of Fe2O3 in the samples, which were determined using the Scherrer Eq. (1). This analysis was conducted in the absence and presence of rGO (with 3% wt./v GO). The Fe2O3@rGO:DTP composites did not exhibit any significant diffraction peaks associated with GO, due to the small quantity of GO in the nanocomposites.
The Scherrer Eq. (1) is as follows:
where D is the crystallite size, λ is the CuKα-radiation wavelength (λ = 1.54056 Å), k is a constant (k = 0.94), βhkl is the full width at half maximum (FWHM), and θ is the peak position [36]. The higher crystallinity of the pure samples is expressed by the sharp diffraction peaks. By using Eq. (2), the samples’ percentage of crystallinity was determined [37,38,39]:
The calculated % crystallinity of the pure samples was 72%. The X-ray density (ρx) of the pure Fe2O3:CPs was also calculated by using Eq. (3):
Here, “M” corresponds to molecular weight and “V” to the unit cell volume. The X-ray density (ρx) was calculated to be 4.13 gcm−3. A pellet (0.3 g) of the pure Fe2O3:CPs was formed (under the pressure of 1 tons) to calculate their bulk density (ρb) by using Eq. (4):
The bulk density was calculated to be 5.21 gcm−3, which is close to the literature value of 5.27 gcm−3 for α-Fe2O3 [40]. Equation (5) was used to determine the Fe2O3:CPs’ percent porosity using the values for the X-ray density and bulk density [41]:
According to the calculated results, CPs have a 26% porosity.
To gain a better understanding of the effect of annealing temperature on the growth process of Fe2O3, samples of Fe2O3:CPs were heated at 300, 400, 500, and 600 °C. The corresponding XRD patterns are shown in Fig. 6. As expected, increased annealing temperature led to a corresponding increase in the heights of the diffraction peaks. A corresponding decrease in the full-width at half-maximum of the peaks was also observed, consistent with an increase in the Fe2O3 crystallite size. The peak height, full width at half maximum, and average crystallite size of the prepared samples are listed in Table 2.
3.3 Composition of Nanocomposites
The FTIR spectra of pure Fe2O3-based CP nanoparticles, Fe2O3@rGO:DTP, and GO are displayed in Fig. 7. In the GO spectrum, the most prominent peak is attributed to OH stretching vibrations at 3210 cm−1. On the other hand, the C-OH stretching, OH deformation, sp2-hybridized C = C group, and C = O stretching exhibit peaks at 1047, 1194, 1565, and 1731 cm−1, respectively [42].
Notably, the peaks at 1047, 1194, and 1731 cm−1 are absent in the FTIR spectra of the Fe2O3@rGO:DTP composite, indicating that the GO has been successfully reduced to rGO. In the FTIR spectra of Fe2O3-based CP nanoparticles and Fe2O3@rGO:DTP nanocomposites, PSG (pre-heat stabilization graphitization) peaks are significantly diminished. It was observed that annealing the composites at 400 °C reduced GO to rGO and eliminated PSG, corroborating the XRD results.
Additionally, the absorption bands corresponding to Fe-O vibrations at wavenumbers of 400–500 and 500–600 cm−1 are consistent with the successful synthesis of Fe2O3. Noteworthy peaks at 428 and 521 cm−1 are attributed to Fe-O and Fe–O–Fe stretching vibrations in both samples, further confirming the presence of Fe2O3 [43].
Furthermore, the absorption band intensities of alkoxy, carboxy, and carbonyl/carboxy linkages observed in the spectrum of GO were not observed in the nanocomposites, indicating the successful deposition of Fe2O3 nanoparticles on the rGO surface. This finding supports the formation of the Fe2O3@rGO:DTP nanocomposite.
3.4 Optical and Chemical properties
3.4.1 Raman Spectroscopy
Figure 8(a) displays the Raman spectrum of the Fe2O3@rGO:DTP nanocomposite. The graphene formed through the chemical reduction of rGO exhibits a distinct set of peaks. The D-band at approximately 1349 cm−1 arises from the symmetric A1g breathing mode of sp2-hybridized carbon atoms, while the G-band at around 1595 cm−1 results from first-order scattering of E2g phonons. Table 3 summarizes the D-band and G-band positions and their intensity ratios \(\frac{({\rm{Intensity}}\,{\rm{D}}\,{\rm{band}})}{({\rm{Intensity}}\,{\rm{G}}\,{\rm{band}})}\) for both Fe2O3@rGO:DTP and GO composites synthesized using the sol-gel technique. In comparison to pure GO, the Raman spectra of the Fe2O3@rGO:DTP composite reveals shifts in the D and G band maxima to shorter wavenumber, specifically at 1343 and 1584 cm−1, respectively. This shift suggests a reduction in GO levels facilitated by PSG.
In the case of cubic Fe2O3 five Raman active modes are expected, based on Group Theory, for its Fd-3m symmetry [44]. The Raman spectra of Fe2O3 in the range from 320 to 440 cm−1, which includes all five Raman-active modes, are shown in Fig. 8b. The Fe-O vibrations at the octahedral sites (FeO6) correspond to its A1g Raman mode and produced a relatively intense band at 377 cm−1. A lower intensity band at 328 cm−1 was produced by the Fe-O vibrations at the tetrahedral sites (Fe2O3) and is related to the F2g Raman modes of the cubic phases of Fe2O3 [45, 46]. The vibration of Fe-O at the Fe2O3 surface gives rise to the band at 340 cm−1, which corresponds to the Eg mode [47]. The total of all forms of vibrations at the tetrahedral and octahedral sites of the cubic-phased Fe2O3 was assigned to the two F2g bands that were reaming at 405 and 428 cm−1. Raman spectroscopic analysis confirmed the successful fabrication of cubic Fe2O3 and the presence of graphene nanosheets in the Fe2O3@rGO:DTP nanocomposite.
3.4.2 UV-vis spectroscopy for bandgap measurement
Figure 9 displays the UV-vis absorption spectra of Fe2O3@rGO:DTP and Fe2O3:CPs at room temperature. Both composites were dispersed in 0.1% wt. ethanol and the resulting solution were utilized for UV-vis absorption analysis. The spectra reveal distinct absorption peaks within the wavelength range of 350–700 nm for both Fe2O3@rGO:DTP and Fe2O3:CPs. These changes in absorption are attributed to an electron transitioning from the valence band (VB) to the conduction band (CB) (O2p → Fe3d) as a consequence of the inherent bandgap absorption of Fe2O3. However, the Fe2O3@rGO:DTP nanocomposite exhibits a more significant increase in absorbance compared to Fe2O3:CPs, possibly due to the additional absorption contribution from rGO, an enhanced oxide surface charge, and the generation of modified electron-hole pairs during irradiation [48, 49]. Consequently, the presence of rGO in Fe2O3 is believed to enhance light absorption, leading to improved optoelectronic performance, as previously demonstrated [50, 51].
The optical bandgap energy (Eg) of Fe2O3@rGO:DTP and Fe2O3:CPs is calculated using the Tauc Equation:
where values of the exponent n are 1/2, 2, 3/2 and 3 for direct allowed, indirect allowed, direct forbidden and indirect forbidden transitions, respectively, hν is energy in eV and α (cm−1) is the absorption coefficient, calculated using the following relation:
where ρ is theoretical density (gcm-3) (Fe2O3 = 5.2, rGO = 2.9 gcm−3), A is absorbance, L is path length (1 cm), C is concentration in molcm−3 and M is molecular mass of substance (gmol−1) [52,53,54].
The Eg values of the samples were estimated using the standard extrapolation approach, as shown in Fig. 10. This method involves extrapolating the linear regions of the curve and identifying the point where the extended line intersects with the x-axis, indicating the bandgap energy. The calculated optical bandgap energies for the as-obtained Fe2O3 samples are 2.1 and 2.4 eV for Fe2O3@rGO:DTP and Fe2O3:CPs, respectively [55]. Moreover, the optical absorption edge of Fe2O3:CPs is slightly shifted towards longer wavelengths with the introduction of GO into the composite, consistent with a slight reduction in the bandgap of Fe2O3:CPs [56].
3.5 Magnetic properties
At room temperature, a vibrating-sample magnetometer (VSM) was employed to analyze the magnetic properties of the Fe2O3@rGO:DTP and Fe2O3:CPs nanocomposites, as depicted in Fig. 11. Both nanocomposites displayed superparamagnetic behavior with a hysteresis of approximately 150 and 162 Oe and a saturation magnetization (Ms) of about 32 and 58 emu/g−1 at 27 °C (300 K), respectively. This phenomenon was evident from the magnetic hysteresis loop illustrated in Fig. 11. The high Ms values of the nanocomposites enabled efficient recycling of the nanocatalysts through low-energy magnetic separation.
Furthermore, the Fe2O3:CPs NCs nanocomposite exhibited a magnetic saturation of 58 emu/g−1 with a remanent magnetization of 16.49 emu/g−1, while the Fe2O3@rGO:DTP nanocomposite showed a magnetic saturation of 32 emu/g−1 with a remanent magnetization of 7.12 emu/g−1. Both nanocomposites exhibited typical ferromagnetic and super magnetic properties. Compared to their bulk counterparts, the higher Ms value observed in the Fe2O3:CPs NCs nanocomposites can be attributed to surface spin effects, as supported by previous research. It is known that the magnetic characteristics of ferromagnetic materials are influenced by size, surface, and crystalline nature. In this case, the magnetic properties of the nanocomposites were attributed to the specific surface shape and crystal size [57].
The remarkable magnetic saturation of the Fe2O3@rGO:DTP NCs nanocomposite can be linked to shape anisotropy and magnetostatic dipole interactions. Overall, the VSM analysis provided valuable insights into the magnetic behavior of the nanocomposites, making them promising candidates for various applications [58].
3.6 Nitrogen adsorption-desorption analysis
To gain deeper insights into the textural properties of both pure Fe2O3:CPs and Fe2O3@rGO:DTP composites, nitrogen adsorption-desorption studies were conducted. The samples show a characteristic IV isotherm with an H3 hysteresis loop, indicating the presence of a mesoporous structure. The corresponding pore size distributions for Fe2O3:CPs and Fe2O3@rGO:DTP are depicted in the inset of Fig. 12. Both samples exhibit significant mesoporosity, with an average pore size of 8.7 nm for Fe2O3:CPs and 7.8 nm for Fe2O3@rGO:DTP. The corresponding BET specific surface areas are found to be 53 m2g−1 and 126 m2g−1, respectively.
In Fig. 12a, b, the Brunauer-Emmett-Teller specific surface areas (BET), BJH pore volume, and average pore size of pure Fe2O3:CPs and Fe2O3@rGO:DTP with 3% GO are illustrated. The BET surface areas show a steady increase with the addition of rGO. The presence of Fe2O3 on the surface of reduced graphene oxide, nanosheets enhance the total surface area of the latter, creating a significant number of porosity channels in the Fe2O3@rGO:DTP nanocomposite. Since both BET and FESEM presented the nanocomposite sample with a greater surface area, the findings are in excellent agreement with each other.
As a result, the OHads molecules can be easily transported through these channels and adsorbed on the surface of the rGO nanosheets. Additionally, Fe2O3 contributes to the expansion of the active surface area of the photocatalyst, leading to improved performance in RhB degradation for the Fe2O3@rGO:DTP samples [59, 60].
3.7 Electrochemical impedance spectroscopy
Electrochemical impedance spectroscopy (EIS) is an electrochemical technique used to assess the impedance of a system irrespective of the frequency of the alternating current potential. To further validate the electrochemical characteristics of both Fe2O3:CPs and Fe2O3@rGO:DTP composites, EIS experiments were conducted in a 1 M KOH solution, as depicted in Fig. 13. The Nyquist plots for pure Fe2O3:CPs and Fe2O3@rGO:DTP nanocomposites were recorded between 0.1 kHz and 105 Hz with an amplitude of 5 mV. In order to conduct a more comprehensive analysis of the samples’ behavior, the Nyquist plots of the samples were evaluated by fitting using a complex equivalent circuit, as illustrated in the inset of Fig. 13. The fitting circuit consists of four sections: R1, R2, Q, and C, which represent the solution resistance, charge transfer resistance, constant phase element, and double layer capacitance, respectively. The Faradaic charge transfer phenomenon occurring at the interface between the sample and electrolyte gives rise to a semicircular response in the low-frequency region. The diameter of this semicircle corresponds to the electron transfer resistance (R2) between the solution and the coating layer. The value of charge transfer resistance was determined by identifying the point of intersection between the diameter of the semicircle and the low-frequency regions. The inclusion of the Q and R3 components in the fitting procedure was implemented in order to achieve a satisfactory level of agreement between the simulated and experimental data. As evidenced by the data presented in Table 4, the solution resistances of Fe2O3:CPs and the Fe2O3@rGO:DTP modified GCE nanohybrid exhibited a negligible difference (R1). The Fe2O3@rGO:DTP nanohybrid exhibited a reduced R2 value in comparison to pure Fe2O3:CPs, suggesting an enhanced charge-transfer rate for the Fe2O3@rGO:DTP nanohybrid under identical conditions. In addition, the Fe2O3@rGO:DTP nanohybrid exhibited a reduced R2 value in comparison to pure Fe2O3, meaning an enhanced charge-transfer rate for the Fe2O3@rGO:DTP hybrid under identical conditions. As a result, the findings from electrochemical impedance spectroscopy (EIS) demonstrate a clear correlation between the presence of rGO and the enhanced electrical conductivity of the nanohybrid electrode. This, in turn, would be expected to result in improved photocatalytic activity of the sample, facilitating a more rapid rate of interfacial electron transfer [61,62,63].
3.8 Photocatalytic activity
3.8.1 RhB degradation
Figure 14a, b presents the optical absorption spectra of an aqueous solution containing RhB, where 10 mg of Fe2O3:CPs and Fe2O3@rGO:DTP nanocomposites were exposed to UV light for varying time intervals. The decrease in the intensity of the RhB dye absorption peak at 553 nm with increasing irradiation time suggests the catalytic degradation of the dye molecules.
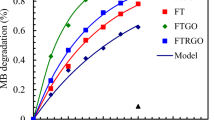
Further investigations were carried out to assess the impact of graphene oxide (GO) on the catalytic activity of Fe2O3:CPs, as shown in Fig. 15. A comparison was made between Fe2O3:CPs and Fe2O3@rGO:DTP under similar conditions. The results revealed that Fe2O3@rGO:DTP achieved 95% degradation of the RhB dye solution, while Fe2O3:CPs only achieved 32%. The results indicate that Fe2O3@rGO: DTP exhibited superior photocatalytic activity.
The larger rGO surfaces facilitated the dispersion of Fe2O3:CPs, leading to increased light absorption and generation of more electron-hole pairs (EHPs) used to degrade the dye molecules. The efficiency of RhB dye degradation was determined using the following Equation:
Here, C0 represents the absorbance of RhB dye in the dark, and Ct represents the absorbance of RhB dye under light irradiation at the time of measurement (in minutes).
The photodegradation rate constant (k) for the RhB dye was obtained from the first-order plot using the following Equation:
C0 denotes the initial absorbance, Ct denotes the absorbance after time t, and k is the first-order rate constant. Figure 16 displays the plots for Fe2O3@rGO:DTP and Fe2O3:CPs nanocomposites, and the rate constants were calculated to be 0.022 and 0.003 min−1, respectively. Table 5 shows a comparison of the current work with similar work reported in earlier studies.
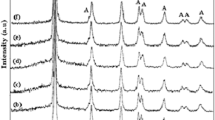
Stability tests were conducted for the photodegradation of RhB dye by Fe2O3@rGO:DTP under UV light, as shown in Fig. 17. The photocatalytic activity was evaluated through five consecutive cycles of 120 min each. The study demonstrated that the photodegradation performance of the Fe2O3@rGO:DTP composite remained stable, indicating its superior performance and indefinite reusability. After five cycles of 600 min treatments, the nanocomposites were analyzed using FTIR, XRD, and FESEM to assess their stability. Figure 18a–c showed no significant difference between the data obtained after the cycles and the initial characterizations conducted before the treatment. Therefore, the nanocomposites maintained their crystalline, chemical, and phase compositions during photocatalysis, enhancing the overall photocatalytic effectiveness of the system under investigation.
3.8.2 Photodegradation using live trapping of active species
The photodegradation of RhB dye in the presence of the Fe2O3@rGO:DTP nanocomposite through live trapping experiments utilizing various scavengers was also explored. The active species involved in the photocatalytic process were identified by employing ammonium oxalate (AO), benzoquinone (BQ), and tert-butyl alcohol (t-BuOH), as illustrated in Fig. 19. Specifically, AO was used to confirm the role of holes (h+); BQ to verify the presence of superoxide anion radicals (\({\dot{O}}_{2}^{-}\)); and T-BuOH to ascertain the involvement of hydroxyl radicals \(\dot{(OH)}\) in the photodegradation process [64].
The RhB dye solution was supplemented with scavengers before the introduction of the Fe2O3@rGO:DTP nanocomposite. The nanocomposite was then subjected to UV light to conduct photocatalytic degradation experiments. The results showed that when treated with AO, the efficiency of organic RhB dye degradation was slightly reduced, indicating a modest role of h+ in the photodegradation process. On the other hand, the presence of BQ and t-BuOH scavengers significantly decreased the photocatalytic activity of the Fe2O3@rGO:DTP nanocomposite, providing evidence that (\({\dot{O}}_{2}^{-}\)) and \(\dot{(OH)},\) are the primary active species responsible for photodegradation, consistent with previous findings.
3.8.3 Mechanism of photocatalysis
Incorporating graphene in the nanocomposite increased photocatalytic activity, attributed to reduced recombination and enhanced absorption rates. When Fe2O3 is exposed to visible light, it absorbs and generates holes and electrons. The holes can react with hydroxide ions in water, forming highly reactive hydroxyl radicals \(\dot{(OH)}.\) These radicals effectively decompose the dye molecules. Additionally, in some instances, the electrons generated may adsorb onto the dissolved oxygen in the dye solution, forming peroxide radicals(\({\dot{O}}_{2}^{-}\)). These radicals(\({\dot{O}}_{2}^{-}\)) and \(\dot{(OH)}\) are crucial in decomposing the RhB.
The incorporation of graphene, as depicted in Fig. 20, significantly reduced the recombination rate during the percolation process. RAMAN and EIS studies confirmed the effective interfacial contact between graphene and Fe2O3. This contact resulted in reduced recombination and increased charge transfer efficiency. Consequently, the nanocomposite exhibited a faster degradation rate compared to pure Fe2O3 [65, 66].
4 Conclusions
In this study, we successfully synthesized Fe2O3@rGO:DTP through a sol-gel process in a PSG environment. The resulting nanocomposite showed the effective decoration and diffusion of a double tetrahedral pyramid (DTP) of iron oxide (Fe2O3) across the surface of rGO sheets, as evidenced by FESEM images. XRD analysis confirmed the presence of the cubic phase in the final product. During annealing, the PSG environment was removed, as shown by FTIR data, resulting in the formation of the desired Fe2O3 nanostructure.
Furthermore, the annealing process in a PSG environment facilitated the conversion of GO to rGO. The photocatalytic activity of Fe2O3@rGO:DTP, was superior to that of Fe2O3:CPs, indicating its higher efficiency in eliminating RhB dye. This finding presents a promising approach for reducing contaminants in sewage.
Notably, incorporating rGO nanosheets in Fe2O3@rGO:DTP significantly enhanced the photocatalytic performance of the Fe2O3@rGO:DTP nanocomposite. The prevention of aggregation in Fe2O3@rGO:DTP caused smaller DTP molecules to form on surfaces, which enhanced the adsorption of RhB dye molecules and inhibited the recombination of electron-hole pairs. Furthermore, the magnetic nature of the nanocomposite would be expected to facilitate its easy removal from water.
In conclusion, this study demonstrates the successful synthesis and promising photocatalytic performance of the Fe2O3@rGO:DTP nanocomposite, highlighting its potential applications in wastewater treatment and contaminant removal.
Data availability
All data generated or analyzed during this study are included in this published article. The raw data retrieved during the current study are available from the corresponding author on reasonable request.
References
Dharmaraja C, Nicholas PE, Ramya P, Premkumar II, Vijayan V, Senthilkumar N (2021) Investigation on photocatalytic activity of ZnS/NiFe2O4 NCs under sunlight irradiation via a novel two-step synthesis approach. Inorg Chem Commun 126:108481
Sangeetha M, Senthil T, Senthilkumar N, Kang M (2022) Solar-light-induced photocatalyst based on Bi–B co-doped TiO2 prepared via co-precipitation method. J Mater Sci: Mater Electron 33(20):16550–16563
Palanivel B, Ayappan C, Jayaraman V, Chidambaram S, Maheswaran R, Mani A (2019) Inverse spinel NiFe2O4 deposited g-C3N4 nanosheet for enhanced visible light photocatalytic activity. Mater Sci Semiconductor Process 100:87–97
Arunkumar M, Veerakumar S, Mohanavel V, Vairamuthu J, Vijayan V, Senthilkumar N (2021) A novel visible light-driven p-type BiFeO3/n-type SnS2 heterojunction photocatalyst for efficient charge separation and enhanced photocatalytic activity. J Clust Sci 32(5):1431–1439
Mark JAM, Venkatachalam A, Pramothkumar A, Senthilkumar N, Jothivenkatachalam K, Prince Jesuraj J (2021) Investigation on structural, optical and photocatalytic activity of CoMn2O4 nanoparticles prepared via simple co-precipitation method. Phys B: Condens Matter 601:412349
Ali KA, Mohanavel V, Gnanavel C, Vijayan V, Senthilkumar N (2021) Structural and optical behavior of SnS2/NiFe2O4 NCs prepared via novel two-step synthesis approach for MB and RhB dye degradation under sun light irradiation. Res Chem Intermediat 47:1941–1954
Sedaghati N, Habibi-Yangjeh A, Pirhashemi M, Vadivel S (2019) Boosted visible-light photocatalytic performance of TiO2-x decorated by BiOI and AgBr nanoparticles. J Photochem Photobiol A: Chem 384:112066
Wang Z, Li C, Domen K (2019) Recent developments in heterogeneous photocatalysts for solar-driven overall water splitting. Chem Soc Rev 48(7):2109–2125. https://doi.org/10.1039/C8CS00542G
Shekofteh-Gohari M, Habibi-Yangjeh A, Abitorabi M, Rouhi A (2018) Magnetically separable nanocomposites based on ZnO and their applications in photocatalytic processes: a review. Crit Rev Environ Sci Technol 48(10-12):806–857
Hitam C, Jalil A (2020) A review on exploration of Fe2O3 photocatalyst towards degradation of dyes and organic contaminants. J Environ Manag 258:110050
Kubániová D, Brázda P, Závěta K, Kmječ T, Klementová M, Kohout J (2019) Identification of ferric oxide polymorphs in nanoparticles prepared by sol-gel method and maximization of ε-Fe2O3 content. J Magn Magn Mater 472:96–103
Sivula K, Le Formal F, Grätzel M (2011) Solar water splitting: progress using hematite (α‐Fe2O3) photoelectrodes. Chem Sus Chem 4(4):432–449
Yang P, Ding Y, Lin Z, Chen Z, Li Y, Qiang P, Ebrahimi M, Mai W, Wong CP, Wang ZL (2014) Low-cost high-performance solid-state asymmetric supercapacitors based on MnO2 nanowires and Fe2O3 nanotubes. Nano Lett 14(2):731–736
Zhu X, Zhu Y, Murali S, Stoller MD, Ruoff RS (2011) Nanostructured reduced graphene oxide/Fe2O3 composite as a high-performance anode material for lithium ion batteries. ACS nano 5(4):3333–3338
Si S, Li C, Wang X, Peng Q, Li Y (2006) Fe2O3/ZnO core–shell nanorods for gas sensors. Sens Actuators B: Chem 119(1):52–56
Keerthana S, Yuvakkumar R, Ravi G, Kumar P, Elshikh MS, Alkhamis HH, Alrefaei AF, Velauthapillai D (2021) A strategy to enhance the photocatalytic efficiency of α-Fe2O3. Chemosphere 270:129498
Khasawneh OFS, Palaniandy P (2021) Removal of organic pollutants from water by Fe2O3/TiO2 based photocatalytic degradation: A review. Environ Technol Innov 21:101230
Yuan Y, Liu Y, Xie X, Wen Y, Song M, He J, Wang Z (2023) 2D defect-engineered Ag-doped γ-Fe2O3/BiVO4: The effect of noble metal doping and oxygen vacancies on exciton-triggering photocatalysis production of singlet oxygen. Chemosphere 322:138176
Li N, He Y-l, Yi Z-z, Gao L, Zhai F-r, Chattopadhyay K (2020) Multiple-metal-doped Fe3O4@ Fe2O3 nanoparticles with enhanced photocatalytic performance for methyl orange degradation under UV/solar light irradiation. Ceram Int 46(11):19038–19045
Mishra M, Chun D-M (2015) α-Fe2O3 as a photocatalytic material: A review. Appl Catal A: Gen 498:126–141
Pal M, Rakshit R, Mandal K (2014) Facile functionalization of Fe2O3 nanoparticles to induce inherent photoluminescence and excellent photocatalytic activity. Appl Phys Lett 104(23):233110-1-233110-5
Yang J, Li D, Zhang Z, Li Q, Wang H (2000) A study of the photocatalytic oxidation of formaldehyde on Pt/Fe2O3/TiO2. J Photochem Photobiol A: Chem 137(2-3):197–202
Xu H, Li H, Wu C, Chu J, Yan Y, Shu H (2008) Preparation, characterization and photocatalytic activity of transition metal-loaded BiVO4. Mater Sci Eng: B 147(1):52–56
Qu Y, Duan X (2013) Progress, challenge and perspective of heterogeneous photocatalysts. Chem Soc Rev 42(7):2568–2580
Tang H, Hessel CM, Wang J, Yang N, Yu R, Zhao H, Wang D (2014) Two-dimensional carbon leading to new photoconversion processes. Chem Soc Rev 43(13):4281–4299
Georgakilas V, Perman JA, Tucek J, Zboril R (2015) Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem Rev 115(11):4744–4822
Hill EW, Vijayaragahvan A, Novoselov K (2011) Graphene sensors. IEEE Sens J 11(12):3161–3170
Wan X, Huang Y, Chen Y (2012) Focusing on energy and optoelectronic applications: a journey for graphene and graphene oxide at large scale. Acc Chem Res 45(4):598–607
Sun W, Wu FG (2018) Two‐Dimensional Materials for Antimicrobial Applications: Graphene Materials and Beyond. Chem– Asian J 13(22):3378–3410
Wang H, Mi X, Li Y, Zhan S (2020) 3D graphene‐based macrostructures for water treatment. Adv Mater 32(3):1806843
Azarang M, Bakhtiyari A, Rakhshani R, Davarpanah AM, Aliahmad M, Farifteh Jahantigh M (2021) Green gelatin-assisted: Synthesis of Co3O4NPs@rGO nanopowder for highly efficient magnetically separable methylene orange dye degradation. Adv Powder Technol https://doi.org/10.1016/j.apt.2020.12.025
Hummers Jr WS, Offeman RE (1958) Preparation of graphitic oxide. J Am Chem Soc 80(6):1339–1339
Chen J, Yao B, Li C, Shi G (2013) An improved Hummers method for eco-friendly synthesis of graphene oxide. Carbon 64:225–229
Azarang M, Shuhaimi A, Yousefi R, Jahromi SP (2015) One-pot sol–gel synthesis of reduced graphene oxide uniformly decorated zinc oxide nanoparticles in starch environment for highly efficient photodegradation of Methylene Blue. RSC Adv 5(28):21888–21896. https://doi.org/10.1039/c4ra16767h
Yanagihara H, Hasegawa M, Kita E, Wakabayashi Y, Sawa H, Siratori K (2006) Iron vacancy ordered γ-Fe2O3 (001) epitaxial films: The crystal structure and electrical resistivity. J Phys Soc Jpn 75(5):054708–054708
Hargreaves J (2016) Some considerations related to the use of the Scherrer equation in powder X-ray diffraction as applied to heterogeneous catalysts. Catal, Struct Reactivity 2(1-4):33–37
Papajani B, Qoku E, Malkaj P, Dilo T (2015) The study of phase compound and the degree of crystallinity of recycled LDPE by X-ray diffractometer and optical microscope. Int J Sci Res 4(2):2228–3239
Ashraf N, Aadil M, Zulfiqar S, Sabeeh H, Khan MA, Shakir I, Agboola PO, Warsi MF (2020) Wafer‐like CoS architectures and their nanocomposites with polypyrrole for electrochemical energy storage applications. ChemistrySelect 5(27):8129–8136
Aadil M, Zulfiqar S, Shahid M, Agboola PO, Al-Khalli NF, Warsi MF, Shakir I (2021) Fabrication of CNTs supported binary nanocomposite with multiple strategies to boost electrochemical activities. Electrochim Acta 383:138332
Kirik N, Krylov A, Boronin A, Koshcheev S, Solovyov L, Rabchevskii E, Shishkina N, Anshits A (2023) The relationship between the structural characteristics of α-Fe2O3 catalysts and their lattice oxygen reactivity regarding hydrogen. Materials 16(12):4466
Aadil M, Zulfiqar S, Sabeeh H, Warsi MF, Shahid M, Alsafari IA, Shakir I (2020) Enhanced electrochemical energy storage properties of carbon coated Co3O4 nanoparticles-reduced graphene oxide ternary nano-hybrids. Ceram Int 46(11):17836–17845
Aunkor M, Mahbubul I, Saidur R, Metselaar H (2016) The green reduction of graphene oxide. Rsc Adv 6(33):27807–27828
Liu P, Wu D, Gao Y, Wang T, Tan Y, Jia D (2018) Reduced graphene oxide-coated mulberry-shaped α-Fe2O3 nanoparticles composite as high performance electrode material for supercapacitors. J Alloy Compd 738:89–96
Yu T, Shen Z, Shi Y, Ding J (2002) Cation migration and magnetic ordering in spinel CoFe2O4 powder: micro-Raman scattering study. J Phys: Condens Matter 14(37):L613
Shim S-H, Duffy TS (2002) Raman spectroscopy of Fe2O3 to 62 GPa. Am Mineralogist 87(2-3):318–326
Martin T, Merlin R, Huffman D, Cardona M (1977) Resonant two magnon Raman scattering in α-Fe2O3. Solid State Commun 22(9):565–567
Aadil M, Zulfiqar S, Warsi MF, Agboola PO, Shakir I, Shahid M, Al-Khalli NF (2021) Mesoporous and macroporous Ag-doped Co3O4 nanosheets and their superior photo-catalytic properties under solar light irradiation. Ceram Int 47(7):9806–9817
Malaidurai M, Bulusu V, Thangavel R Synthesis, characterization, and investigation of ambipolar behaviour on FeVO4 nanocrystals. 2018 3rd International Conference on Microwave and Photonics (ICMAP), 2018. IEEE, pp 1-2
Ching-Prado E Optical Properties of BiFeO3: A Theoretical and Experimental Approach. 2022 8th International Engineering, Sciences and Technology Conference (IESTEC), 2022. IEEE, pp 764–770
Xu T, Zhang L, Cheng H, Zhu Y (2011) Significantly enhanced photocatalytic performance of ZnO via graphene hybridization and the mechanism study. Appl Catal B: Environ 101(3-4):382–387
Azarang M, Shuhaimi A, Yousefi R, Moradi Golsheikh A, Sookhakian M (2014) Synthesis and characterization of ZnO NPs/reduced graphene oxide nanocomposite prepared in gelatin medium as highly efficient photo-degradation of MB. Ceram Int 40(7):10217–10221. https://doi.org/10.1016/j.ceramint.2014.02.109
Farrukh MA, Butt KM, Chong K-K, Chang WS (2019) Photoluminescence emission behavior on the reduced band gap of Fe doping in CeO2-SiO2 nanocomposite and photophysical properties. J Saudi Chem Soc 23(5):561–575
Aslam I, Cao C, Tanveer M, Farooq MH, Tahir M, Khalid S, Khan WS, Idrees F, Rizwan M, Butt FK (2015) A facile one-step fabrication of novel WO3/Fe2 (WO4)3·10.7H2O porous microplates with remarkable photocatalytic activities. Cryst Eng Comm 17(26):4809–4817
Aslam I, Cao C, Tanveer M, Farooq MH, Khan WS, Tahir M, Idrees F, Khalid S (2015) A novel Z-scheme WO3/CdWO4 photocatalyst with enhanced visible-light photocatalytic activity for the degradation of organic pollutants. RSC Adv 5(8):6019–6026
Mani G, Nair PR, Mathew S (2022) Polymeric Carbon Nitride/Iron Oxide Composites: A Novel Class of Catalysts with Reduced Metal Content for Ammonium Perchlorate Thermal Decomposition. ACS omega 7(43):38512–38524
Ravindra N, Ganapathy P, Choi J (2007) Energy gap–refractive index relations in semiconductors–An overview. Infrared Phys Technol 50(1):21–29
Manikandan A, Vijaya JJ, Kennedy LJ (2013) Structural, optical and magnetic properties of porous α-Fe2O3 nanostructures prepared by rapid combustion method. J Nanosci Nanotechnol 13(4):2986–2992
Al-Hakkani MF, Gouda GA, Hassan SH (2021) A review of green methods for phyto-fabrication of hematite (α-Fe2O3) nanoparticles and their characterization, properties, and applications. Heliyon 7(1):1–16
Khalid MU, Katubi KM, Zulfiqar S, Alrowaili Z, Aadil M, Al-Buriahi M, Shahid M, Warsi MF (2023) Boosting the electrochemical activities of MnO2 for next-generation supercapacitor application: Adaptation of multiple approaches. Fuel 343:127946
Rashid M, Hassan W, Aadil M, Somaily H, Mahdi NM, Lataef R, Taki AG, Srithilat K, Baamer DF, Albukhari SM (2023) Solar-light-driven and magnetically recoverable doped nano-ferrite: an ideal photocatalyst for water purification applications. Optical Mater 135:113192
Hernández HH, Reynoso AR, González JT, Morán CG, Hernández JM, Ruiz AM, Hernández JM, Cruz RO (2020) Electrochemical impedance spectroscopy (EIS): A review study of basic aspects of the corrosion mechanism applied to steels. Electrochem Impedance Spectrosc:137–144
Nazik G, Aadil M, Zulfiqar S, Hassan W, Rahman A, Ibrahim SM, Naseem K, Sheikh TA, Akhtar MN (2023) Synthesis of doped metal sulfide nanoparticles and their graphene reinforced nanohybrid for Pb (II) detection. Z für Physikalische Chem 237(8):1257–1285
Mahmood M, Chaudhary K, Shahid M, Shakir I, Agboola PO, Aadil M (2022) Fabrication of MoO3 Nanowires/MXene@ CC hybrid as highly conductive and flexible electrode for next-generation supercapacitors applications. Ceram Int 48(13):19314–19323
Kumar D, Sharma S, Khare N (2021) RGO nanosheets coupled NaNbO3 nanorods based nanocomposite for enhanced photocatalytic and photoelectrochemical water splitting activity. Adv Powder Technol 32(12):4754–4764
Subagyo R, Yudhowijoyo A, Sholeha NA, Hutagalung SS, Prasetyoko D, Birowosuto MD, Arramel A, Jiang J, Kusumawati Y (2023) Recent advances of modification effect in Co3O4-based catalyst towards highly efficient photocatalysis. J Colloid Interface Sci:1550–1590
Gu J, Li S, Wang E, Li Q, Sun G, Xu R, Zhang H (2009) Single-crystalline α-Fe2O3 with hierarchical structures: Controllable synthesis, formation mechanism and photocatalytic properties. J Solid State Chem 182(5):1265–1272
Sookhakian M, Amin YM, Basirun WJ (2013) Hierarchically ordered macro-mesoporous ZnS microsphere with reduced graphene oxide supporter for a highly efficient photodegradation of methylene blue. Appl Surf Sci 283(0):668–677. https://doi.org/10.1016/j.apsusc.2013.06.162
Kumari V, Kaushal S, Singh PP (2022) Green synthesis of a CuO/rGO nanocomposite using a Terminalia arjuna bark extract and its catalytic activity for the purification of water. Mater Adv 3(4):2170–2184
Tamam N, Aadil M, Hassan W, Ejaz SR, Najm ZM, Alsafari IA, Aman S, Trukhanov A, Al-Buriahi M, Boukhris I (2022) Surfactant assisted synthesis of nanostructured Mn-doped CuO: An efficient photocatalyst for environmental remediation. Ceram Int 48(20):29589–29600
Aadil M, Hassan W, Somaily H, Ejaz SR, Abass RR, Jasem H, Hachim SK, Adhab AH, Abood ES, Alsafari IA (2022) Synergistic effect of doping and nanotechnology to fabricate highly efficient photocatalyst for environmental remediation. J Alloy Compd 920:165876
Ishfaq M, Hassan W, Sabir M, Somaily H, Hachim SK, Kadhim ZJ, Lafta HA, Alnassar YS, Rheima AM, Ejaz SR (2022) Wet-chemical synthesis of ZnO/CdO/CeO2 heterostructure: a novel material for environmental remediation application. Ceram Int 48(23):34590–34601
Acknowledgements
University of Sistan and Baluchestan in Zahedan, Iran, has greatly aided and supported Majid Azarang and his research.
Funding
This work was supported by the University of Sistan and Baluchestan (1398- 1399).
Author information
Authors and Affiliations
Contributions
Conceptualization, M.Az. and E.R.; Methodology, M.Az., and M.Al.; Formal analysis, M.Az. and E.Rh.; Investigation, M.Az.; Writing original draft preparation, M.Az..; Writing review and editing, M.Az.; supervision, M.Az. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Rahmanifar, E., Azarang, M. & Aliahmad, M. Efficient photodegradation of Rhodamine B dye assisted by Pigskin-Gel via sustainable synthesis of Fe2O3@rGO nanocrystals with magnetically separable properties. J Sol-Gel Sci Technol 111, 671–688 (2024). https://doi.org/10.1007/s10971-024-06423-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-024-06423-8