Abstract
Syntheses of monodisperse silica spheres (MSS) and monodisperse mesoporous silica spheres (MMSS) were studied focusing on the age of starting solutions, the solvent partitioning, and the surfactant dissolution as unconscious process factors. Aging of clear and transparent starting solutions decreased nucleation and increased the size of MSS, while the aging effect was opposite in the case of MMSS. Alcohols were used as co-solvent for alkoxides and water to dilute them. Partitioning ratio of alcohols was also a factor that changes the sphere size. Even when a surfactant is soluble in water and alcohol to readily form a transparent starting solution, the clusters consisting of solute and solvent molecules or their molecular level dissolution states may vary during aging, and thus could be a factor affecting the nucleation of nanoparticles. Aging of a methanolic solution of tetramethylorthosilicate (TMOS) containing a surfactant (C16TAC; cetyltrimethylammonium chloride) resulted in unfavorable formation of irregular (non-spherical) particles probably due to the interaction between TMOS and a moisture in the surfactant during aging.

Syntheses of monodisperse silica sphere (MSS) and monodisperse mesoporous silica sphere (MMSS) were studied focusing on the age of starting solutions, solvent partitioning, and surfactant dissolution as unconscious process factors. Aging of clear and transparent starting solutions decrease nucleation and increase the sphere size of MSS, while the aging effect is opposite in the case of MMSS. The decrease in the MMSS size due to aging was the largest when the methanol solvent was used only to dissolve water and surfactant and then reacted with a pure alkoxide free from the solvent (10s/0).
Highlights
-
Aging of starting solutions for synthesis of monodisperse silica spheres (MSS) increased the size of MMS.
-
Aging of starting solutions for synthesis of monodisperse mesoporous silica spheres (MMSS) decreased the size of MMSS.
-
The decrease in the MMSS size due to aging was dependent on the solvent partitioning and surfactant dissolution.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Intoroduction
Monodisperse ceramic spheres are of interests in both industrial and scientific senses. The Stöber synthesis of monodisperse silica spheres (MSS) is a great landmark, and has been reproduced, investigated by many researchers, and also successfully applied in practice [1,2,3]. Introducing self-assembly of organic molecules as pore template has also been successful to achieve mesoporous monodisperse silica sphere (MMSS) [4]. In this paper, the authors focus on “unconscious” factors in the process for preparing MSS and MMSS, which have rarely been written in reports and papers so far.
It is general and usual in liquid phase synthesis of ceramic particles to prepare “transparent” starting solutions by dissolving a solute (raw materials) in a solvent at a certain ratio. Then there are three factors to define the starting solution; solutes, solvents, and their ratio (solute concentration). The authors suggest the fourth factor of “aging” which means how long it has passed since the solution was prepared. It may occur that a certain amount of the starting solutions is prepared to conduct a series of synthetic experiment efficiently. Such “stock” solutions were visibly clear and transparent and look available for a long time. In most cases of aqueous system, there seems no significant changes or problems in using stock solutions for a certain period, probably because a water solvent has high dielectric constant and effectively ionize an electrolyte solute. In a sort of non-aqueous system, however, precipitation behavior significantly changes on aging of the starting solutions. One of the authors reported that precipitation of magnesium oxalate in ethanolic solution of oxalic acid awfully delayed on aging but that a difference in the products was not significant as for particle size and crystallinity [5]. It is known that there is a microscopic inhomogeneity of ~ several nanometers in a mixture of water and a water-miscible organic solvents. It is reported that small angle neutron scattering (SANS) can find the existence of microscopic inhomogeneity in a acetonitrile-water mixture as a correlation length of 3–4 nm [6]. Mass spectrometry of a water-ethanol mixture revealed an obvious partitioning in the cluster size formed among molecules due to hydrogen bonding [7,8,9]. Such microscopic inhomogeneity is too small to be detected by naked eyes but would be large enough to concern the nucleation of nanoparticles. The size of inhomogeneity could be dependent on temperature, composition [6,7,8], and probably on aging time [9].
MSS is considered as a suitable target to study the nucleation subject since the particle size can be determined with sufficient accuracy by a simple microscopy. In the MSS synthesis, an alcoholic solution of silicon alkoxide and that of water with ammonia were separately prepared, aged, and then mixed for the synthesis. The sphere size can be controlled by changing usual three factors (solvent, solute, and concentration) as well as temperature etc., whereas the fourth factor “age” is rarely considered. One of the authors previously reported the effect of aging starting solution on the MSS. In short, the aging increased the sphere size of MSS. This occurred even when the alcoholic solution of water with ammonia was aged and mixed with a fresh alkoxide solution [10]. Also, the aging found to enhance the uniformity of sphere size both in microscopy and in a macroscopic estimation of the colloidal crystallinity of the self-assembled layer of the spheres prepared from aged solution [11].
In this paper, reproduction of this MSS topic is briefly mentioned and compared with the synthesis of MMSS, where the new member of organic surfactant is involved. The unconscious factors in the MMSS synthesis is studied as for partitioning of solvent and dissolution of surfactant as well as the aging time.
2 Experimental
2.1 Monodisperse silica sphere (MSS) synthesis
Tetraethylorthosilicate (TEOS, Si(OC2H5)4; Wako chemicals), ammonia, absolute ethanol (99.5 vol%), distilled water were commercially obtained and used without further purification. Two starting solutions were prepared in ethanol as a solvent. One was made by dissolving alkoxide at [TEOS] = 0.2 mol/L, while the other was made by dissolving ammonia water at [H2O] = 15.2 mol/L and [NH3] = 3.6 mol/L. Before mixing for synthesis, each starting solution was tightly sealed using a Teflon tape in a glass container and aged for 0 to 21 days at room temperature in a dark place. The water solution of 50 mL was poured in a short time into the TEOS solution of the same volume under a magnetic stirring at 20 °C for 90 min to synthesize silica spheres at [TEOS] = 0.1 mol/L, [H2O] = 7.6 mol/L, and [NH3] = 1.8 mol/L. After the reaction, the precipitates were centrifuged (4000 rpm × 10 min), washed twice by ethanol, and then dried in a 50 °C oven overnight.
2.2 Mesoporous monodisperse silica sphere (MMSS) synthesis
Raw materials were chosen after Yano et al. [4]. Tetramethylorthosilicate (TMOS, Si(OCH3)4, 99%; Shin-etsu chemicals), distilled water, sodium hydroxide, methanol (99.8%; Wako chemicals), cetyltrimethylammonium chloride (C16TAC; Wako chemicals) were commercially obtained and used without further purification. They were finally mixed at [TMOS] = 0.12 M, [H2O] = 24 M, [NaOH] = 2.5 mM, [C16TAC] = 12 mM in a methanol solvent. The total amount of methanol was fixed at 25 g and partitioned to 10/0, 5/5, or 0/10 for the H2O or the TMOS solutions; i.e, as shown in Table 1, the 10/0 partitioning means that whole methanol of 25 g was used to dissolve water and NaOH for hydrolysis and then reacted with an undiluted TMOS reagent directly; while the 5/5 partitioning means a half of methanol was used to dissolve TMOS before synthesis. In addition, the surfactant of C16TAC was dissolved in either side of H2O or TMOS; the notation 5 s/5 means that it was dissolved in the methanolic solution of water-containing NaOH, whereas 5/5 s means that it was dissolved in the methanolic solution of TMOS. The sample notations are summarized in Table 1. A run “10/0 s” was not available since the surfactant was not soluble in pure TMOS in the absence of methanol. As in the case of MSS, the starting solutions were aged at room temperature for 0 to 30 days before mixing. After the aging, they were kept at 5 °C for 30 min and then reacted at the same temperature for 24 h. The precipitates obtained were centrifugally separated (4000 rpm × 10 min), washed with ethanol 3 times, and then dried in a 50 °C oven overnight. In order to remove the organic template, samples were fired at 550 °C in an electric furnace.
2.3 Characterization
Samples were mainly subjected to scanning electron microscopy (SEM, Hitachi SU3500) to estimate the sphere size and the morphology. Thermal analysis (TG8120, Rigaku Co., Ltd) was done at 10 °C/min from 100 to 800 °C. In order to remove the effect of physical adsorption of moisture, the samples were initially soaked at 100 for 20 min for the weight change to be flat. Nuclear magnetic resonance (1H-NMR, Bruker Ascend 400) was applied to examine the change before and after aging of starting solution.
3 Results and discussion
3.1 Effect of aging starting solutions on sphere size of MSS and MMSS (10s/0)
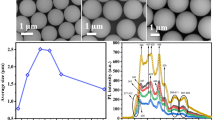
Figure 1 shows the changes in the particle size with aging time. It was carefully checked before mixing the “aged” starting solutions that they are visibly transparent, and that no Tyndall phenomenon are observed using a laser pointer. As previously reported [10], the aging caused a delay in the precipitation and a significant increase in the particle size of MSS. To the contrary, the particle size of MMSS obviously decreased on aging. As the silicon amount in the supernatant was found to be negligibly small, the whole alkoxide can be regarded to be precipitated as spheres different in size. Assuming the density of spheres in each run is the same, the particle size (D) can be considered as a simple function of the number of nucleus (N).
In case of MSS, the ratio Nage/Nfresh = (Dfresh/Dage)3 is calculated to be ~0.5, where Nage and Nfresh are the number of nucleus from aged and fresh solutions, respectively, to grow up to spheres, and Dfresh and Dage are the size of spheres grown from fresh and aged solutions, respectively. This value of ~0.5 means that the nucleus formed in the aged solution decreased about half as much as those in the fresh solution. It is inferred in our hypothesis that heterogeneous nucleation due to clusters among the solutes and the solvent could be less during aging [9, 10]. In case of MMSS, on the other hand, the nucleus increased up to~ three times and thus the particle size decreased probably due to the presence of organic template. One simple hypothesis is that the dissolved surfactant may not be well dispersed just after prepared, even though the solution looks transparent, and may not be effective to nucleate spheres. If the number of nuclei is smaller, then the sphere size would be larger. If the surfactant were more dispersed by aging and could enhance the number of nucleation, the size would be smaller. Further examination is necessary to clarify the detailed feature of such dissolution state in a “complicated” starting solutions which are consist of alkoxide, water, alcohol, and surfactant.
3.2 Effect of solvent partitioning in MMSS
Figure 2 shows the morphology of MMSS prepared from different solvent partitioning. It is obvious that aging (7 d) decreased the particle size in all cases. Normalized decrease ratio (Dage/Dfresh) is plotted in Fig. 3. The largest decrease was found in 10s/0, where the methanol ratio to surfactant and water is the largest, and also the methanol ratio to TMOS is the lowest (zero). Considering the “macroscopic” solubility, the surfactant molecules should be more dissolved in appearance at the 10s/0 run than the others. If the dissolution state of surfactant molecules changes on aging to create more number of nuclei, then the sphere size would be more decreased. At the 5s/5 and the 0s/10 runs, TMOS is dissolved in methanol and more likely to be reacted to reduce the effect of aging on nucleation.
Figure 4 shows the weight loss in thermal analysis. In the samples 10s/0 and 5s/5, where the surfactant is dissolved in the methanol-water mixture, there is a tendency that aging decrease the amount of organic template involved in MMSS before firing or just after synthesized in solution. It is inferred that the surfactant molecules may not be microscopically dissolved in fresh (just after dissolved in solution) and likely to be involved as silica polymerization proceeds. Also, it may be more microscopically dissolved during aging.
Table 2 compares the specific surface area (SSA) and the pore diameter of the MMSS samples after fired at 550 °C. Effect of aging starting solution on SSA is not so clear but the effect of the solvent partitioning is significant. The highest SSA was found in the 0s/10 partitioning where the surfactant would be fully dissolved in water to form fine self-assembly of C16TAC molecules, and thus to form more porosity. The difference in the SSA due to the solvent partitioning may be hopefully explained at the balance of (i) dissolution state of surfactant in methanol-water mixture, and (ii) dissolution state of TMOS in methanol, when their features become clear in future.
3.3 Effect of surfactant dissolution in MMSS
Dissolution of the surfactant in the ethanolic solution of TMOS, concerning the samples 5/5s and 0/10s, was not a good way to synthesize MMSS. Figure 5 shows that aging of starting solution for 5/5s and 0/10s resulted in the unfavorable formation of irregular, non-spherical particles of <100 nm. Thermogravimetric (TG) analysis revealed the surfactant used contained a 3.9% moisture. The irregular particles formation is presumably due to the reaction of TMOS with such moisture during aging even though no visible changes was seen in the starting solution. It also should be noticed that the size of spherical particles did not increase as in the case of 10s/0~0s/10 (Fig. 2). This may suggest that the nucleation of the irregular particles is another factor to determine the nucleation of spheres.
Here it is emphasized again that all of the starting solutions in this work were transparent (no Tyndall phenomenon) after aging. In order to see the subtle changes in the starting solutions, NMR spectra of the TMOS methanolic solution containing the surfactant are shown in Fig. 6. There was a small but obvious difference in the expanded spectrum of the fresh and the aged solution. This must be related to the irregular particles formation and probably due to the interaction between TMOS and the moisture of the surfactant in methanol solvent.
4 Conclusions
The Stöber process of monodisperse (mesoporous) silica spheres (MSS and mainly MMSS) was studied focusing on the age, the solvent partitioning and the surfactant dissolution as the process parameters which are usually unconscious and rarely reported in literatures.
-
(i)
Particle size of MSS increased and that of MMSS decreased with increasing aging time of starting solutions. This is probably due to the number of nucleation decreased in MSS and increased in MMSS during aging.
-
(ii)
The solvent partitioning in the starting solutions is a significant factor to control the particle size even if the concentrations after mixing are fixed. This is probably due to the difference of cluster or the dissolution state in different solvents of water, alcohol, and their mixture.
-
(iii)
It was also influential to the MMSS formation whether the surfactant was dissolved in the alkoxide solution or the water-containing solution. Aging of a solution containing TMOS, C16TAC, and methanol showed a subtle but significant change to form unfavorable irregular particles besides spheres.
It is noted in conclusion that a visible transparent stock solution does not always guarantee a microscopic homogeneity among solute and solvent molecules.
References
Stöber W, Fink A (1968) Controlled growth of monodisperse silica spheres in the micron size range. J Colloid Interface Sci 26:62–69
Ikemoto T, Uematsu K, Mizutani N, Kato M (1985) Synthesis of monodispersed titania fine particles by hydrolysis of Ti(OC2H5)4. J Ceram Soc Jpn 93:261–266
Kojima T, Baba T, Ota K, Yukita C, Inamoto K, Uekawa N (2016) Preparation of porous titania particles by partial dissolution and heat treatment of hydrous titania. J Ceram Soc Jpn 124:1226–1228
Yano K, Fukushima Y (2004) Synthesis of monodispersed mesoporous silica spheres with highly ordered hexagonal regularity using conventional alkyltrimethylammonium halide as a surfactant. J Mater Chem 14(10):1579–1584
Enomoto N, Shiihara J, Hongo T, Nakagawa Z (1999) Aging effect of starting solutions on wet-chemical powder preparation using ethanolic solution of oxalic acid. J Ceram Soc Jpn 107(3):278–281
Takamuku T, Noguchi Y, Nakano M, Matsugami M, Iwase H, Otomo T (2007) Microinhomogeneity for aqueous mixture of water-miscible organic solvents. J Ceram Soc Jpn 115(12):861–866
Nishi N, Koga K, Ohshima C, Yamamoto K, Nagashima U, Nagami K (1988) Molecular association in ethanol-water mixtures studied by mass spectrometric analysis of clusters generated through adiabatic expansion of liquid jets. J Am Chem Soc 110:5246–5255
Yamaguchi K (2003) Cold-spray ionization mass spectrometry: principle and the applications. J Mass Spectromet 38:473–490
Enomoto N, Matsuo M, Inada M, Hayashi K, Hojo J (2020) Aging of starting solutions for nanoparticles synthesis with two different ultrasonication. Ultrason Sonochem 67:105142
Enomoto N, Kumagai A, Hojo J (2005) Aging effect of starting solutions for spherical silica synthesis. J Ceram Soc Jpn 113:340–343
Enomoto N, Takata M, Kamada K, Hojo J, Fudouzi H (2006) Novel processing for improving monodispersity of ceramic spheres and colloidal crystallinity. Sci Tech Adv Mater 7(7):662–666
Acknowledgements
This work was in part supported by JSPS KAKENHI Grant-in-Aid for Scientific Research (C) Grant Number 17K06025. NE organized this work and wrote the manuscript. YN performed sample preparation and data analysis. MI carried out adsorption measurement. All authors approved the final manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Enomoto, N., Nishimura, Y. & Inada, M. Unconscious factors affecting physical characteristics of sol–gel-derived monodispersed silica spheres. J Sol-Gel Sci Technol 104, 512–518 (2022). https://doi.org/10.1007/s10971-022-05917-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05917-7