Abstract
In this work, Mo-modified TiO2 microspheres (Mo@TiO2) were rapidly prepared by the spray pyrolysis method. The titania sol solution containing molybdenum salt and citric acid (CA) was nebulized to generate fine droplets, which were continuously transported into a quartz reactor heated at 600 °C by an N2 gas flow. Characterization results revealed that the prepared Mo@TiO2 exhibited enhanced porosity and light adsorption ability. In addition, incorporating Mo species into the anatase TiO2 lattice narrowed the bandgap energy and created oxygen deficiencies, which are beneficial for the photo-reduction performance. The adsorption-photodegradation of tetracycline antibiotics (TE) was systematically optimized by monitoring the Mo loads, catalyst dosage, contaminant concentration, and pH media. The results indicated that the optimal Mo@TiO2 microspheres containing 3 wt% Mo showed the highest removal efficiency of ca. ~90% toward tetracycline antibiotics under UV-light irradiation, surpassing the undoped TiO2 and commercial TiO2 (P25). Finally, after several photooxidation cycles, the fabricated Mo@TiO2 microspheres retained good photocatalytic activity and stability. The findings suggest that the spray pyrolysis-derived Mo-modified TiO2 microspheres can be promising photocatalytic materials for treating antibiotics in water.

Graphical abstract
Highlights
-
Mesoporous Mo-incorporated TiO2 microspheres were effectively prepared via sol-spray pyrolysis.
-
Mo-modified TiO2 showed enhanced light absorption ability and porosity.
-
Mo@TiO2 microspheres exhibited improved photo-reduction efficiency under UV-light irradiation.
-
Mo@TiO2 microspheres had good stability and renewability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The remediation of organic antibiotics has recently drawn considerable attention owing to their toxicity to aquatic organisms, terrestrial animals, and humans [1, 2]. Furthermore, after releasing in the environment, these organic pollutants can produce multi-resistant bacterial strains that can no longer be handled with the recent drugs [3, 4]. Thus, research on eliminating antibiotics from wastewater has attracted much tension for decays [5,6,7]. Among known methodologies, photocatalytic oxidation is attracting extensive attention from researchers [3, 8, 9]. Research indicated that the photodegradation efficiency depends on the development of photocatalytic materials. However, there is still a challenge in developing a highly efficient photocatalyst for the photo-reduction of organic pollutants. Recently, metal-organic frameworks (MOFs)-based semiconductors have been applied to photocatalytically degrade organic contaminants [2, 5, 10]. Nonetheless, the production of MOFs is expensive, which hinders them from practical applications [11,12,13].
The use of metal oxides, such as ZnO [14,15,16], Cu2O [17], Fe3O4 [18], Fe2O3 [19], or TiO2 [1, 3, 20,21,22,23] as photocatalysts for the photo-reduction of organic pollutants has been received much attention for years because they are cost-effective and easily prepared. Among them, anatase TiO2 is a promising material because it has a high surface area and mesoporous structure, providing active sites for adsorption and photocatalytic performances [1, 20, 21, 23, 24]. Although the bare TiO2 exhibited a good photo-reduction ability toward organic contaminants, its practical application is still limited because of its poor light absorption and quick recombination of photo-induced species [9, 25,26,27]. Thus, it needs further to improve the photocatalytic activities and adsorption ability of TiO2. Research has found that the modification of TiO2 by Ag [28], Fe2O3 [29], graphene oxide [30], g-C3N4 [31], chalcogenide [32], or carbon nanotube [33] increased the photocatalytic performance. In addition, Wu et al. [1] discovered that incorporating N into the lattice matrix of TiO2 significantly increased the photooxidation efficiency of TiO2. Erdogan et al. [26] reported that dopant elements such as Mo, Fe, and N decreased the bandgap energy of TiO2, enhancing their photooxidation ability. Recently, Dong et al. [24] found that defective mesoporous TiO2 showed more photocatalytic activity than defect-free TiO2. These findings indicated that introducing suitable dopants into the TiO2 matrix to enhance their photocatalytic activities could be a promising strategy.
Efficient photocatalysts for large-scale water treatment require high catalytic activities, low cost, and facile preparation. So far, TiO2-based photocatalysts have been mainly prepared using batch mode synthesis, which is challenging to scale up for large-scale production. For instance, Phromma et al. [34] prepared photocatalyst TiO2 nanoparticles via the wet ball milling sol-gel method, which was proceeded with many preparation steps. Thi et al. [23] synthesized Mo-doped TiO2 using the solvothermal method. Devi et al. [35] prepared Mn+-doped TiO2 (Mn+: V5+, Mo6+, and Th4+) by the impregnation method, followed by calcination. Stengl et al. prepared Mo-doped anatase by thermal hydrolysis of Mo and Ti peroxo-complexes from aqueous solutions. However, this method required a very long reaction time, and the produced material had low porosity [36]. Recently, spray pyrolysis synthesis has been widely applied to prepare materials in various fields. The spray pyrolysis-derived materials exhibited enhanced surface area and pore volume than the conventional batch synthesis [37,38,39,40]. Notably, spray pyrolysis is easy to scale up for large-scale production because of its continous flow nature.
In this work, Mo-incorporated TiO2 microspheres were quickly prepared by the surfactant-assisted spray pyrolysis method (Scheme 1). Titania sol containing molybdenum salt and citric acid was ultrasonicated to generate fine droplets, which were continuously transported into a quartz reactor by an N2 gas flow and pyrolyzed at the desired temperature. Characterization results revealed that the spray pyrolysis-derived Mo@TiO2 exhibited significantly enhanced surface area and pore volume. In addition, the incorporated Mo species narrowed the bandgap energy of the Mo@TiO2 samples, enhancing their light absorption ability. Furthermore, the Mo-modified TiO2 microspheres contained oxygen deficiencies, which improved their photo-reduction efficiency under UV light illumination.
2 Experimental
2.1 Spry pyrolysis synthesis of Mo@TiO2 microspheres
Mo@TiO2 microspheres were synthesized by the spray pyrolysis of the titania sol following our work [6, 37]. Briefly, a calculated amount of titanium tetraisopropoxide [Ti (OC3H7)4, 97%, Aldrich] was dissolved in isopropanol at room temperature and in a water-free atmosphere. To the resultant solution, deionized water was added slowly under stirring conditions for 3 h. Next, the resultant white precipitates were dispersed in the desired amount of deionized water, then peptized with 1 M HNO3 to obtain a stable sol. For the spray pyrolysis process, 400 ml titania sol was added with the desired amount of citric acid and ammonium heptamolybdate [(NH4)6Mo7O24⋅4H2O, Aldrich] under stirring conditions. Herein, citric acid acts as a surfactant that enhances the sample’s porosity and the metal dopant dispersion. The spry pyrolysis procedure is briefly described in Scheme 1. The obtained product was then calcined at 450 °C for 3 h with a 2 °C/min heating rate in the air to eliminate the carbonaceous material certainly. The final samples are named Mo@TiO2-x, in which x is the weight percentage of Mo (x = 1.0, 3.0, and 5.0 wt%).
2.2 Characterizations
The textural properties of the prepared samples were determined using N2 porosimetry (ASAP 2020, Micromeritics Instrument Co., USA) at 77 K. Before analysis, the samples were activated at 150 °C under vacuum conditions for 8 h. The specific surface area was determined by a multipoint Brunauer–Emmet–Teller (BET) method using the adsorption data in the relative pressure (P/P0) range of 0.05–0.25. The Barrett–Joyner–Halender (BJH) method with cylindrical pore size estimated from the Kelvin equation was used in the data processing. The crystallographic structures of the samples were analyzed utilizing powder X-ray diffraction (XRD) using an X-ray diffractometer (MAC-18XHF, Rigaku, Japan) equipped with a CuKα radiation source (λ = 1.54 Å) and operated at a scanning rate of 5o/min from 20o to 80o. The morphologies of the prepared materials were obtained via field-emission scanning electron microscopy (FE-SEM; Leo-Supra 55, Carl Zeiss STM, Germany). The FT-IR absorption analysis was conducted with a Bruker model Tensor 27 in the range of 4000–400 cm−1 using KBr powders. An X-ray photoelectron spectrometer (XPS, Thermo Scientific K-Alpha spectrometer) was employed to determine the chemical state of elements. The binding energies were corrected by setting the binding energy of the adventitious carbon (C 1 s) at 284.6 eV. Inductively coupled plasma-optical emission spectrometry (ICP-OES) analyses were carried on with an OPTIMA 8300 (Perkin-Elmer, USA). The photoluminescence (PL) spectra of the samples were analyzed employing an FLS920 fluorescence spectrometer. The optical properties of the samples were analyzed by UV–vis diffuse reflectance spectrophotometer (DRS; UV-2600, Shimadzu, Japan).
2.3 Adsorption and photodegradation experiments
The removal experiments of tetracycline antibiotics (C22H24N2O8, Aldrich, 99%) was conducted using a photochemical reaction cell assembled with a 300-W Xe-arc lamp. Before use, all samples were degassed at 150 °C under vacuum conditions for 8 h. For each run, 0.15 g of degassed catalyst was added to the TE solution and magnetically stirred for 90 min in the dark. After exposure to UV light irradiation with continuous stirring, the reaction mixture was withdrawn and centrifuged to recover the catalyst; meanwhile, the TE content in the resultant solution was measured employing a UV-visible spectrophotometer (Optizen POP, Mecasys, Korea). The TE removal experiment was also conducted using commercial TiO2 (P25) for comparison.
3 Results and discussion
3.1 Characterizations
Multi-grams of mesoporous microspheres Mo-doped TiO2 were quickly obtained by spray pyrolysis. FE-SEM images of the samples produced by the spray pyrolysis method show that all samples exist in uniform spheres with a particle diameter of 0.5–1.5 μm [Fig. 1(a–d)]. EDS dot maps analyses showed that Mo species were finely dispersed throughout the microspheres [Fig. 1(e–h)]. Moreover, the ICP-OES results revealed that the Mo content increased with the Mo/Ti molar ratio, providing direct evidence of controllable loadings of Mo during the spray pyrolysis (Table 1).
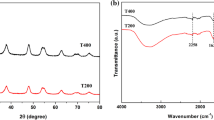
The crystallographic structure of the Mo-modified TiO2 microspheres was examined by XRD analyses, as described in Fig. 2(a). The XRD pattern of the bare TiO2 exhibited diffraction peaks at 2θ = 25.6°, 38.4°, 48.4°, 63.4°, and 76° (JCPDS card No. 21-1272), featuring the anatase-type TiO2 [37]. As shown, the produced Mo-incorporated TiO2 samples exhibited the same XRD patterns as the bare TiO2, suggesting that the incorporation of metal species did not influence the phase structure evolution of the TiO2 host. Furthermore, there were no characteristic peaks of molybdenum oxides witnessed on XRD patterns of the Mo@TiO2 samples. This suggests that the incorporated Mo species could be amorphous or well-dispersed within the anatase crystal matrix and beyond the detection limit of XRD [37, 41]. Noticeably, the peaks of TiO2 slightly shifted right, and the shift magnitude increased with the Mo load [the enlarged Fig. 2(a)]. This implies that Mo species were successfully incorporated into the TiO2 lattice and caused lattice strains. It should be mentioned that the ionic radius of Mo6+ (~0.62 Å) is quite close to that of Ti4+ (~0.68 Å). Thus, the Ti4+ in the TiO2 lattice can be substituted by the guest Mo6+ ones, which results in a lattice strain within the TiO2 host [23, 42]. The functional groups of the SP-derived TiO2 and Mo@TiO2 microspheres were analyzed via FT-IR spectrum, as demonstrated in Fig. 2(b). The spectrum of the bare TiO2 microspheres exhibits the vibration modes ~3430 cm−1 and ~1640 cm−1, which are ascribable to the adsorbed water molecules. The predominant band at the lower wavenumber of 620 cm−1 resulted from the Ti-O-Ti vibration [43]. These bands were slightly moved toward the higher wavenumber on the FT-IR spectra of the Mo-incorporated TiO2 samples. In addition, the FT-IR spectrum of the Mo@TiO2-5 sample containing 5 wt% of Mo showed the characteristic vibration peaks of 638, 825, and 915 cm−1, which shifted compared to those of the bulk MoO3. These shifts could be caused by the newly formed Ti-O-Mo or Mo-Ti-O bonds or the oxygen deficiencies within the TiO2 crystal [26]. The surface structure of the fabricated TiO2 and Mo@TiO2 microspheres was also probed by Raman spectroscopy, as shown in Fig. 2(c). The bare TiO2 shows the Raman bands at ~138, 388, 508, and 632 cm−1, which were the characteristics of the anatase TiO2 [21]. These bands shifted and broadened on the spectra of the Mo-modified TiO2 microspheres. The findings confirmed the distortion and defects of TiO2 lattice due to the incorporation of Mo species [21]. N2 sorption and pore size distribution of the TiO2 and Mo-modified TiO2 microspheres were analyzed, and the results are shown in Fig. 2(d). The prepared Mo@TiO2 samples exhibited enhanced N2 adsorption capacity than the pristine TiO2. In addition, the pore size of the produced Mo@TiO2 samples increased with the Mo loads [the inset of Fig. 2(d)]. This is possible because incorporating Mo dopants created new pore structures within Mo@TiO2. The same phenomenon was previously reported in the literature [37, 44]. From Table 1, the bare TiO2 microsphere had surface area and pore volume was 119 m2/g and 0.18 cm3/g, respectively, which were remarkably higher than the commercial titania. For the Mo@TiO2 sample, increasing the Mo load from 1.0 wt% to 5.0 wt%, the surface are ranged from 128 m2/g to 152 m2/g, respectively; meanwhile, the pore volume ranged from 0.21 cm3/g to 0.32 cm3/g, respectively.
The valence states of the elements were analyzed by XPS, and the results are presented in Fig. 3(a–d). The survey scan result of the Mo-doped TiO2 sample revealed the presence of Mo species [Fig. 3(a)]. The Ti 2p core-level spectrum of the pristine TiO2 shows a peak at 459. 0 eV and 464.6 eV, ascribed to the Ti 2p3/2 and Ti 2p1/2 binding energies, respectively [45] [Fig. 3(b)]. As shown, the incorporation of Mo into the TiO2 matrix resulted in binding energy shifts of the Ti 2p levels. In addition, the spray-derived Mo@TiO2 microspheres exhibited broader and higher binding energy peaks of Ti 2p than the pristine TiO2 sample. These suggest the crystal structure of TiO2 anatase could be disordered owing to the Mo guest species. The O 1 s core-level XPS spectrum of the bare TiO2 exhibited a dominant biding energy peak around 530.3 eV, which is ascribable to the oxygen in the TiO2 lattice [46]. This peak slightly moved on the spectra of the Mo@TiO2 catalysts. Besides the oxygen in the titania lattice, the O 1 s spectrum also revealed the presence of adsorbed oxygen (~ 532.1 eV) and adsorbed water and hydroxyl groups (~533.4 eV) [45,46,47] [Fig. 3(c)]. It is worth mentioning that the concentration of adsorbed oxygen on the Mo-doped TiO2 catalyst was greatly higher than that on the undoped TiO2. The findings implied that the Mo@TiO2 samples contained the surface deficiencies of oxygen, which enhanced their adsorption of oxygen [24, 47, 48]. The Mo 3d core spectra revealed that Mo6+ and Mo5+ co-existed within the Mo-incorporated TiO2 microspheres [Fig. 3(d)]. Specifically, the Mo6+ state was characterized by the energy peak at ~232.6 eV (Mo 3d5/2) and ~235.7 eV (Mo 3d3/2) [37, 49]; meanwhile, the existence of Mo5+ species has been proven by the energy peak at ~231.3 eV (Mo 3d5/2) and ~234.6 eV (Mo 3d3/2) [37, 49]. As shown in Fig. 3(d), Mo6+ was partially reduced to Mo5+, which is undoubtedly evident in the oxygen-deficient states within the TiO2 lattice [26, 47]. These defects are beneficial for adsorbing oxygen onto the catalyst surface, enhancing the photo-reduction efficiency.
The optical properties of the synthesized TiO2 and Mo@TiO2 were investigated via UV-vis diffuse reflection spectroscopy. As demonstrated in Fig. 4(a), the bare TiO2 predominantly absorbed light in the ultraviolet region. However, the absorption edges of the Mo-incorporated TiO2 microspheres obviously shifted towards the visible-light region. This suggests that the introduced Mo species could form MoO3-x oxides that are effectively responsive to visible light [50, 51]. Furthermore, the obtained result is consistent with the presence of Mo5+ species in the XPS analyses. That is to say, the modification of the TiO2 anatase by Mo dopants extended the catalysts’ absorption edge to the visible light region. The bandgap energy values of the prepared samples were estimated using Tauc’s plots, as presented in Fig. 4(b). The pristine TiO2 anatase had a bandgap energy value of ~3.18 eV, which is consistent with the previous report [47]. However, introducing 1.0 to 5.0 wt% of Mo decreased the bandgap value of anatase TiO2 from ~3.08 to ~2.93 eV, respectively Fig. 4(b). Research has found that incorporating Mo6+ ions within the TiO2 lattice narrowed the bandgap structure because the orbital energy of Mo 4d is quite close to that of Ti 3d [47, 52]. In addition, Mo-doping causes crystal defects inside the host (e.g., oxygen vacancies), which greatly enhance the catalyst’s light absorption ability and suppress the recombination of the photo-induced electrons and holes [26, 47]. The findings in the present work revealed that Mo species were finely incorporated into the TiO2 microspheres under spray pyrolysis conditions. Furthermore, the incorporated Mo dopants enhanced the light absorption ability and created oxygen deficiencies in the fabricated Mo@TiO2 microspheres, which are favorable for the photo-reduction performance.
The photoluminescence spectra of the prepared Mo@TiO2 microspheres were analyzed, and the results are depicted in Fig. 5. All catalysts showed a broad peak located around 405 nm, which is caused by the recombination of the photo-generated holes and electrons. As expected, the prepared Mo@TiO2 catalysts exhibited reduced PL peak intensities compared to the pristine TiO2. This suggests that the incorporated Mo species suppressed the recombination of photo-generated holes and electrons during light irradiation. These results were consistent with the above findings that Mo-doping disturbed the Ti-O bonds and created the oxygen vacancies in the TiO2 lattice. These vacancies reportedly aid in trapping and localizing the photo-induced species during light illumination [24, 45].
3.2 Adsorption-photocatalytic degradation of antibiotics
The adsorption-photo-reduction experiments were conducted over the prepared TiO2, Mo@TiO2, and P25. Figure 6(a) shows the UV-vis absorption spectrum of tetracycline as a function of sampling time. Figure 6(b) shows the calculated removal efficiency of TE over the commercial TiO2 and the spray pyrolysis-derived Mo@TiO2 microspheres. At the pre-adsorption stage, the spray pyrolysis TiO2 microspheres exhibited considerably greater adsorption ability toward TE than the commercial one. This is possible because the prepared TiO2 microspheres had higher surface area and pore volume than the commercial titania. Furthermore, spray pyrolysis-derived Mo@TiO2 exhibited increased adsorption ability toward tetracycline compared to the bare anatase. Specifically, the removal yield of TE by the adsorption over the prepared microspheres TiO2, Mo@TiO2-1, Mo@TiO2-3, and Mo@TiO2-5 were ~15, 21, 26%, and 29%, respectively [Fig. 6(b)], which were positively correlated with their porosities as mentioned above.
The photodegradation of tetracycline antibiotics was performed under UV light irradiation. As shown in Fig. 6(b), the commercial TiO2 exhibited a modest TE removal efficiency of ca. ~40%, and that value for the undoped TiO2 microspheres was approximately 62%. As expected, the Mo-incorporated TiO2 catalysts exhibited improved photo-reduction efficiency than unmodified TiO2 and exceeded the commercial catalyst. After 160 min UV-light illumination, the total removal efficiency of TE over the prepared Mo@TiO2-1, Mo@TiO2-3, and Mo@TiO2-5 was approximately 67, 90, and 79%, respectively. Such improved photooxidation ability of the Mo@TiO2 microsphere can be attributable to the synergistic effect of introducing Mo species into the TiO2 by spray pyrolysis. As discussed above, the prepared Mo@TiO2 contained surface oxygen vacancies, which increased the light absorption and accelerated the separation of the photo-excited charge carriers. Furthermore, the incorporated Mo species created more pore spaces that facilitated the accumulation of organic pollutants onto the catalyst surface. The obtained results indicated that the photo-reduction efficiency depended on the Mo content introduced into the TiO2 host, and the catalyst containing 3.0 wt% Mo showed the most excellent photocatalytic efficiency under the investigated conditions. Table 2 compares the TE removal efficiency on various photocatalysts. It can be seen that the spray pyrolysis-derived Mo@TiO2 catalyst exhibited a comparable removal efficiency compared to some previous samples (see Table 2). Notably, the Mo@TiO2 microspheres were fabricated by a rapid, facile, and scalable route, thereby making them a promising material for industrial applications.
Reaction kinetic analyses were performed further to reveal the photocatalytic activity of all catalytic samples [Fig. 6(c)]. As presented, the photo-reduction rate of the produced catalysts follows the order: Mo@TiO2−3 (0.0114 min−1) >Mo@TiO2-5 (0.00744 min−1) >Mo@TiO2-1 (0.00638 min−1) >TiO2 (0.00478 min−1) >P25 (0.00238 min−1). Accordingly, the highest photodegradation rate of ~0.0114 min−1 was achieved over the prepared Mo@TiO2-3 catalyst, which was approximately 2.4 and 4.8 times greater than the pristine TiO2 microspheres and P25, respectively. Furthermore, the results confirmed that the spray pyrolysis-derived Mo@TiO2 microspheres effectively degraded the antibiotics under the investigated conditions.
3.3 Effects of experimental conditions
The effects of experimental factors, including catalyst dosage, pollutant concentration, and pH media on the TE removal efficiency, were systematically surveyed. As demonstrated in Fig. 7(a), the photocatalytic yield gradually increased from 55 to 92% with an increase in the catalyst dosage from 40 to 160 mg L−1, which was attributed to the increasing active sites for adsorption and photooxidation processes. However, further increasing the catalyst dosage to 220 mg L−1, the photocatalytic efficiency diminished to 83%. This phenomenon can be explained by the shielding effect of the light irradiation or self-consumption of the newly formed reactive species by a high catalyst load [46]. In addition, the initial concentration of the antibiotics strongly impacted the photocatalytic performance [Fig. 7(b)]. The prepared Mo@TiO2 microspheres exhibited optimal photocatalytic performance at the TE concentration of 60 mg L−1. Nonetheless, the removal efficiency diminished by increasing the antibiotic concentration from 120 to 180 mg L−1. This could be because a high concentration of contaminants in the solution could clog the pore spaces of the catalyst. Figure 7(c) depicts the effect of pH media on the removal yield of antibiotics. As revealed, the antibiotics removal efficiency was facilitated in acidic conditions (pH < 6.5) but slowed down in base media (pH > 6.5). The experimental results can be explained via the surface interaction between the catalyst and organic molecules. Indeed, the surface of tetracycline is always negatively charged in the pH range of 2–12; meanwhile, for the synthesized Mo@TiO2 microspheres, their surfaces were positively charged in acidic media but negatively charged in base media [46]. Here, the surface hydroxyl groups of TiO2 were involved in the acid-base chemistry of Mo@TiO2 microspheres, which the following equations:
Accordingly, low pH media is favorable for the adsorption of antibiotic molecules onto the catalyst surface because of their electrostatic interaction. As a high amount of organic pollutants accumulated on the catalyst, the photo-reduction of organic pollutants was facilitated.
3.4 Reusability and stability tests
Stability and reusability are crucial factors that evaluate the prepared materials. Here, the spray-pyrolysis-derived Mo@TiO2 microspheres were reused several times. After use, the Mo@TiO2-3.0 catalyst was regenerated by washing in deionized water, then drying at 115 °C. As presented in Fig. 8(a), the removal efficiency of TE has unchanged after the first two cycles. After five cycles, the total removal yield reached approximately 78% compared to that of the as-prepared catalyst. This decrease suggests that some intermediate products could strongly bind to the catalyst’s active sites, which require higher energy to be desorbed. The morphology and XRD pattern of the used catalyst were examined, and the results are presented in Fig. 8(b) and (c), respectively. Accordingly, the prepared Mo@TiO2 microspheres maintained their morphology and crystal structure after several cycles. These findings implied that the spray pyrolysis Mo-modified TiO2 microspheres had good renewability and stability.
4 Conclusions
Mo-modified anatase TiO2 microspheres were rapidly fabricated by combining sol-gel and spray pyrolysis approaches. Increasing the Mo content onto Mo@TiO2 samples from 1.0 wt% to 5 wt%, the surface area increased from 128 m2/g to 152 m2/g, and the pore volume increased from 0.21 cm3/g to 0.32 cm3/g, respectively. Under spray pyrolysis conditions, Mo species were effectively incorporated into the lattice of TiO2, reducing their bandgap energy values. Furthermore, the incorporated Mo dopants created more oxygen deficiencies, which enhanced the photo-reduction ability of the Mo@TiO2 microspheres compared to the bare TiO2 and P25. The produced Mo@TiO2 microspheres loaded 3 wt% of Mo showed the highest tetracycline removal efficiency of ca. ~90% after 160 min UV-light illumination. Moreover, the photocatalytic efficiency on the prepared Mo@TiO2 microspheres was retained after several cyclic experiments. These results indicated that spray pyrolysis-derived Mo@TiO2 materials could be promising for adsorption-photocatalytic water remediation.
Data availability
The data is true and reliable.
References
Wu S, Hu H, Lin Y, Zhang J, Hu YH (2020) Visible light photocatalytic degradation of tetracycline over TiO2. Chem Eng J 382:122842. https://doi.org/10.1016/j.cej.2019.122842
Vo TK, Kim J (2021) Facile synthesis of magnetic framework composite MgFe2O4@UiO-66(Zr) and its applications in the adsorption–photocatalytic degradation of tetracycline. Environ Sci Pollut Res 28(48):68261–68275. https://doi.org/10.1007/s11356-021-15423-y
Zhu X-D, Wang Y-J, Sun R-J, Zhou D-M (2013) Photocatalytic degradation of tetracycline in aqueous solution by nanosized TiO2. Chemosphere 92(8):925–932. https://doi.org/10.1016/j.chemosphere.2013.02.066
Addamo M, Augugliaro V, Paola AD, García-López E, Loddo V, Marcì G, Palmisano L (2005) Removal of drugs in aqueous systems by photoassisted degradation. J Appl Electrochem 35(7):765–774. https://doi.org/10.1007/s10800-005-1630-y
Wang Q, Gao Q, Al-Enizi AM, Nafady A, Ma S (2020) Recent advances in MOF-based photocatalysis: environmental remediation under visible light. Inorg Chem Front 7(2):300–339. https://doi.org/10.1039/C9QI01120J
The Ky V (2022) Spray pyrolysis synthesis of mesoporous γ-AlOOH and γ-Al2O3 microspheres and their properties for Cr (VI) adsorption. Int J Environ Anal Chem:1–21. https://doi.org/10.1080/03067319.2021.2023512
Lei X, Li X, Ruan Z, Zhang T, Pan F, Li Q, Xia D, Fu J (2018) Adsorption-photocatalytic degradation of dye pollutant in water by graphite oxide grafted titanate nanotubes. J Mol Liq 266:122–131. https://doi.org/10.1016/j.molliq.2018.06.053
Ong CB, Ng LY, Mohammad AW (2018) A review of ZnO nanoparticles as solar photocatalysts: Synthesis, mechanisms and applications. Renew Sust Energ Rev 81:536–551. https://doi.org/10.1016/j.rser.2017.08.020
Li G, Lian Z, Wang W, Zhang D, Li H (2016) Nanotube-confinement induced size-controllable g-C3N4 quantum dots modified single-crystalline TiO2 nanotube arrays for stable synergetic photoelectrocatalysis. Nano Energy 19:446–454. https://doi.org/10.1016/j.nanoen.2015.10.011
Xu J, Xu J, Jiang S, Cao Y, Xu K, Zhang Q, Wang L (2020) Facile synthesis of a novel Ag3PO4/MIL-100(Fe) Z-scheme photocatalyst for enhancing tetracycline degradation under visible light. Environ Sci Pollut Res 27(30):37839–37851. https://doi.org/10.1007/s11356-020-09903-w
Le VN, Vo TK, Yoo KS, Kim J (2021) Enhanced CO2 adsorption performance on amino-defective UiO-66 with 4-amino benzoic acid as the defective linker. Sep Purif Technol 274:119079. https://doi.org/10.1016/j.seppur.2021.119079
Tran NT, Vo TK, Kim J, Othman MR (2021) Esoteric CO adsorption by CuCl-NiCl2 embedded microporous MIL-101 (Cr). Colloids Surf A Physicochem Eng 615:126242. https://doi.org/10.1016/j.colsurfa.2021.126242
Vo TK, Kim J-H, Kwon HT, Kim J (2019) Cost-effective and eco-friendly synthesis of MIL-101(Cr) from waste hexavalent chromium and its application for carbon monoxide separation. J Ind Eng Chem 80:345–351. https://doi.org/10.1016/j.jiec.2019.08.013
Mirzaeifard Z, Shariatinia Z, Jourshabani M, Rezaei Darvishi SM (2020) ZnO photocatalyst revisited: effective photocatalytic degradation of emerging contaminants using S-Doped ZnO nanoparticles under visible light radiation. Ind Eng Chem Res 59(36):15894–15911. https://doi.org/10.1021/acs.iecr.0c03192
Meng J, Chen Q, Lu J, Liu H (2019) Z-Scheme photocatalytic CO2 reduction on a heterostructure of oxygen-defective ZnO/reduced graphene oxide/UiO-66-NH2 under visible light. ACS Appl Mater Interfaces 11(1):550–562. https://doi.org/10.1021/acsami.8b14282
Akhavan O, Mehrabian M, Mirabbaszadeh K, Azimirad R (2009) Hydrothermal synthesis of ZnO nanorod arrays for photocatalytic inactivation of bacteria. J Phys D: Appl Phys 42(22):225305. https://doi.org/10.1088/0022-3727/42/22/225305
Koiki BA, Arotiba OA (2020) Cu2O as an emerging semiconductor in photocatalytic and photoelectrocatalytic treatment of water contaminated with organic substances: a review. RSC Adv 10(60):36514–36525. https://doi.org/10.1039/D0RA06858F
Mishra P, Patnaik S, Parida K (2019) An overview of recent progress on noble metal modified magnetic Fe3O4 for photocatalytic pollutant degradation and H2 evolution. Catal Sci Technol 9(4):916–941. https://doi.org/10.1039/C8CY02462F
Jana TK, Pal A, Mandal AK, Sarwar S, Chakrabarti P, Chatterjee K (2017) Photocatalytic and antibacterial performance of α-Fe2O3 Nanostructures. ChemistrySelect 2(10):3068–3077. https://doi.org/10.1002/slct.201700294
Ohko Y, Ando I, Niwa C, Tatsuma T, Yamamura T, Nakashima T, Kubota Y, Fujishima A (2001) Degradation of Bisphenol A in water by TiO2 photocatalyst. Environ Sci Technol 35(11):2365–2368. https://doi.org/10.1021/es001757t
Li J, Zhang M, Guan Z, Li Q, He C, Yang J (2017) Synergistic effect of surface and bulk single-electron-trapped oxygen vacancy of TiO2 in the photocatalytic reduction of CO2. Appl Catal B 206:300–307. https://doi.org/10.1016/j.apcatb.2017.01.025
Akhavan O, Ghaderi E, Rahimi K (2012) Adverse effects of graphene incorporated in TiO2 photocatalyst on minuscule animals under solar light irradiation. J Mater Chem 22(43):23260–23266. https://doi.org/10.1039/C2JM35228A
Thi TV, Rai AK, Gim J, Kim S, Kim J (2014) Effect of Mo6+ doping on electrochemical performance of anatase TiO2 as a high performance anode material for secondary lithium-ion batteries. J Alloy Compd 598:16–22. https://doi.org/10.1016/j.jallcom.2014.02.019
Dong J, Hu C, Qi W, An X, Liu H, Qu J (2020) Defect-enhanced photocatalytic removal of dimethylarsinic acid over mixed-phase mesoporous TiO2. Res J Environ Sci 91:35–42. https://doi.org/10.1016/j.jes.2019.12.013
Xia T, Zhang W, Wang Z, Zhang Y, Song X, Murowchick J, Battaglia V, Liu G, Chen X (2014) Amorphous carbon-coated TiO2 nanocrystals for improved lithium-ion battery and photocatalytic performance. Nano Energy 6:109–118. https://doi.org/10.1016/j.nanoen.2014.03.012
Erdogan N, Park J, Ozturk A (2016) Synthesis and enhanced photocatalytic activity of molybdenum, iron, and nitrogen triple-doped titania nanopowders. Ceram Int 42(15):16766–16774. https://doi.org/10.1016/j.ceramint.2016.07.158
Shayegan Z, Lee C-S, Haghighat F (2018) TiO2 photocatalyst for removal of volatile organic compounds in gas phase – A review. Chem Eng J 334:2408–2439. https://doi.org/10.1016/j.cej.2017.09.153
Akhavan O (2009) Lasting antibacterial activities of Ag–TiO2/Ag/a-TiO2 nanocomposite thin film photocatalysts under solar light irradiation. J Colloid Interface Sci 336(1):117–124. https://doi.org/10.1016/j.jcis.2009.03.018
Akhavan O (2010) Thickness dependent activity of nanostructured TiO2/α-Fe2O3 photocatalyst thin films. Appl Surf Sci 257(5):1724–1728. https://doi.org/10.1016/j.apsusc.2010.09.005
Akhavan O, Ghaderi E (2009) Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J Phys Chem C 113(47):20214–20220. https://doi.org/10.1021/jp906325q
Yu X, Fan X, An L, Liu G, Li Z, Liu J, Hu P (2018) Mesocrystalline Ti3+TiO2 hybridized g-C3N4 for efficient visible-light photocatalysis. Carbon 128:21–30. https://doi.org/10.1016/j.carbon.2017.11.078
Ganguly P, Mathew S, Clarizia L, Kumar RS, Akande A, Hinder SJ, Breen A, Pillai SC (2020) Ternary metal chalcogenide heterostructure (AgInS2–TiO2) nanocomposites for visible light photocatalytic applications. ACS Omega 5(1):406–421. https://doi.org/10.1021/acsomega.9b02907
Akhavan O, Abdolahad M, Abdi Y, Mohajerzadeh S (2009) Synthesis of titania/carbon nanotube heterojunction arrays for photoinactivation of E. coli in visible light irradiation. Carbon 47(14):3280–3287. https://doi.org/10.1016/j.carbon.2009.07.046
Phromma S, Wutikhun T, Kasamechonchung P, Eksangsri T, Sapcharoenkun C (2020) Effect of calcination temperature on photocatalytic activity of synthesized TiO2 nanoparticles via wet ball milling sol-gel method. Appl Sci 10(3):993
Devi LG, Murthy BN, Kumar SG (2009) Photocatalytic activity of V5+, Mo6+ and Th4+ doped polycrystalline TiO2 for the degradation of chlorpyrifos under UV/solar light. J Mol Catal A Chem 308(1):174–181. https://doi.org/10.1016/j.molcata.2009.04.007
Štengl V, Bakardjieva S (2010) Molybdenum-doped anatase and its extraordinary photocatalytic activity in the degradation of Orange II in the UV and vis regions. J Phys Chem C 114(45):19308–19317. https://doi.org/10.1021/jp104271q
Vo TK, Kim W-S, Kim S-S, Yoo KS, Kim J (2018) Facile synthesis of Mo/Al2O3–TiO2 catalysts using spray pyrolysis and their catalytic activity for hydrodeoxygenation. Energy Convers Manag 158:92–102. https://doi.org/10.1016/j.enconman.2017.12.049
Vo TK, Le VN, Quang DT, Kim J (2021) Facile synthesis of spray pyrolysis-derived CuCl/γ-Al2O3 microspheres and their properties for CO adsorption and CO/CO2 separation. Microporous Mesoporous Mater 321:111132. https://doi.org/10.1016/j.micromeso.2021.111132
Ji J, Im K, An H, Yoo SJ, Chung Y, Kim J, Kwon Y (2022) Spray pyrolysis-assisted synthesis of hollow cobalt nitrogen-doped carbon catalyst for the performance enhancement of membraneless fuel cells. Int J Energy Res 46(2):760–773. https://doi.org/10.1002/er.7200
Han S, Yoo KS, Kim D, Kim J, Othman MR (2021) Metal-silica spherical particles development by spray pyrolysis: Effect of metal species on surface area and toluene adsorption. J Anal Appl Pyrolysis 156:105049. https://doi.org/10.1016/j.jaap.2021.105049
Phan D-P, Vo TK, Le VN, Kim J, Lee EY (2020) Spray pyrolysis synthesis of bimetallic NiMo/Al2O3–TiO2 catalyst for hydrodeoxygenation of guaiacol: Effects of bimetallic composition and reduction temperature. J Ind Eng Chem 83:351–358. https://doi.org/10.1016/j.jiec.2019.12.008
Zhang J, Xi J, Ji Z (2012) Mo+N Codoped TiO2 sheets with dominant {001} facets for enhancing visible-light photocatalytic activity. J Mater Chem 22(34):17700–17708. https://doi.org/10.1039/C2JM32391E
Majeed J, Nayak C, Jha SN, Bhattacharyya K, Bhattacharyya D, Tripathi AK (2015) Correlation of Mo dopant and photocatalytic properties of Mo incorporated TiO2: an EXAFS and photocatalytic study. RSC Adv 5(110):90932–90940. https://doi.org/10.1039/C5RA14613E
Ly HV, Im K, Go Y, Galiwango E, Kim S-S, Kim J, Choi JH, Woo HC (2016) Spray pyrolysis synthesis of γ-Al2O3 supported metal and metal phosphide catalysts and their activity in the hydrodeoxygenation of a bio-oil model compound. Energy Convers Manag 127:545–553. https://doi.org/10.1016/j.enconman.2016.09.020
Li J, Zhou H, Zhuo H, Wei Z, Zhuang G, Zhong X, Deng S, Li X, Wang J (2018) Oxygen vacancies on TiO2 promoted the activity and stability of supported Pd nanoparticles for the oxygen reduction reaction. J Mater Chem A 6(5):2264–2272. https://doi.org/10.1039/C7TA09831F
Vo TK, (2022) Spray pyrolysis synthesis and UV-driven photocatalytic activity of mesoporous Al2O3@TiO2 microspheres. Environ Sci Pollut Res. https://doi.org/10.1007/s11356-022-18865-0
Wang S, Bai LN, Sun HM, Jiang Q, Lian JS (2013) Structure and photocatalytic property of Mo-doped TiO2 nanoparticles. Powder Technol 244:9–15. https://doi.org/10.1016/j.powtec.2013.03.054
Deng H, Wang Y, Zhang X, Kou X, Chen B, Zhu C (2019) Photodegradation under natural indoor weak light assisted adsorption of X-3B on TiO2/Al2O3 nanocomposite. Chem Eng J 372:99–106. https://doi.org/10.1016/j.cej.2019.04.079
Kondekar NP, Boebinger MG, Woods EV, McDowell MT (2017) In Situ XPS investigation of transformations at crystallographically oriented MoS2 interfaces. ACS Appl Mater Interfaces 9(37):32394–32404. https://doi.org/10.1021/acsami.7b10230
Saadati M, Akhavan O, Fazli H (2021) Single-layer MoS2-MoO3-x heterojunction nanosheets with simultaneous photoluminescence and co-photocatalytic features. Catalysts 11(12):1445
Zhang Y, Yu X, Liu H, Lian X, Shang B, Zhan Y, Fan T, Chen Z, Yi X (2021) Controllable synthesis of the defect-enriched MoO3−x nanosheets as an effective visible-light photocatalyst for the degradation of organic dyes. Environ Sci Nano 8(7):2049–2058. https://doi.org/10.1039/D1EN00210D
Gai Y, Li J, Li S-S, Xia J-B, Wei S-H(2009) Design of narrow-gap TiO2: a passivated codoping approach for enhanced photoelectrochemical activity Phys Rev Lett 102(3):036402. https://doi.org/10.1103/PhysRevLett.102.036402
Semeraro P, Bettini S, Sawalha S, Pal S, Licciulli A, Marzo F, Lovergine N, Valli L, Giancane G (2020) Photocatalytic degradation of tetracycline by ZnO/γ-Fe(2)O(3) paramagnetic nanocomposite material. Nanomaterials 10(8):1458. https://doi.org/10.3390/nano10081458
Oluwole AO, Olatunji OS (2022) Photocatalytic degradation of tetracycline in aqueous systems under visible light irridiation using needle-like SnO2 nanoparticles anchored on exfoliated g-C3N4. Environ Sci Eur 34(1):5. https://doi.org/10.1186/s12302-021-00588-7
Safari GH, Hoseini M, Seyedsalehi M, Kamani H, Jaafari J, Mahvi AH (2015) Photocatalytic degradation of tetracycline using nanosized titanium dioxide in aqueous solution. Int J Environ Sci Technol 12(2):603–616. https://doi.org/10.1007/s13762-014-0706-9
Acknowledgements
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.05-2020.32. This work is also supported by the Industrial University of Ho Chi Minh City, Vietnam. The authors are also thankful to Professor Jinsoo Kim at Chemical Engineering Department, Kyung Hee University, Korea, for supporting this work.
Authors contributions
The Ky Vo: Conceptualization, investigation, formal analysis, writing manuscript, visualization, editing &r reviewing.
Funding
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 104.05-2020.32
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares no competing interests.
Consent to participate
All authors agree to participate in the editing of the paper.
Consent to publish
All authors agree to publish this manuscript in your journal.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Vo, T.K. Mo-modified TiO2 mesoporous microspheres prepared by spray pyrolysis for adsorption-photocatalytic water remediation. J Sol-Gel Sci Technol 103, 853–864 (2022). https://doi.org/10.1007/s10971-022-05902-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-022-05902-0