Abstract
The photocatalytic activity of single transition metal-doped TiO2 nanoparticles is well established. This article reports the synthesis of Fe and Cu co-doped TiO2 nanoparticles with varying Fe and Cu concentrations by the sol–gel method and their photocatalytic activity towards photodegradation of methylene blue under visible light. Nanoparticles were characterized by X-ray diffractometry (XRD), Raman spectroscopy, Transmission electron microscopy (TEM), Scanning electron microscopy (SEM), X-ray photoelectron spectroscopy (XPS), X-ray fluorescence spectroscopy (XRF), and Diffuse reflectance UV–Visible spectroscopy. XRD patterns revealed the existence of both anatase and rutile phases which was confirmed by Raman and TEM analysis. Both XRD and Raman analysis confirmed the successful doping of Fe and Cu without causing any significant lattice distortions. Nanoparticles were aggregated as shown in TEM and SEM images. XPS analysis revealed the presence of the only Ti4+ in pure TiO2 while both Ti4+ and Ti3+ were present in doped TiO2 in addition to Fe3+, Cu+, and Cu2+. XRF analysis showed the presence of only Ti, Fe, and Cu in the co-doped nanoparticles. According to the diffuse reflectance spectroscopic analysis, the visible light sensitivity of TiO2 has increased upon doping with Fe and Cu. Single metal-doped nanoparticles were efficient than the co-doped nanoparticles for the degradation of methylene blue under visible light. Among the single doped nanoparticles, 0.05 Cu/TiO2 showed the highest rate constant (0.0195 min−1) while the maximum activity from the co-doped nanoparticles resulted in 0.05 Cu + 0.05 Fe/TiO2 (0.0098 min−1). The photocatalytic activity was decreased upon increasing the dopant (Fe/Cu) concentration due to the recombination of photogenerated electron-hole pairs, while due to the shielding effect, low photocatalytic activity resulted in co-doped nanoparticles with varying Fe and Cu loadings.

Highlights
-
Cu and Fe co-doped TiO2 nanoparticles with varying dopant concentrations were synthesized by the sol–gel method.
-
Single doped TiO2 nanoparticles are efficient in photodegrading methylene blue than co-doped TiO2 nanoparticles under visible light.
-
Photocatalytic activity of single doped TiO2 nanoparticles is affected by electron-hole pair recombination and that of co-doped TiO2 nanoparticles is influenced by both recombination and shielding effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dyes are abundantly released to normal water reservoirs from various industries including textile, rubber, carpet, food, plastics, and cosmetics, etc. [1,2,3,4]. They cause severe adverse effects such as increase chemical oxygen demand of the effluent and toxicity, reduce light penetration and hence limit photosynthesis. Further, the accumulation of dyes in water damage the aesthetic nature of the environment. Most of the dyes are stable for a long time being stable to light and oxidation and being resistant to aerobic degradation. Therefore, it is mandatory to remove these dyes from the effluents before they discharge to the water reservoirs. Hence, there is a need for a dye removal technique that could be used for larger scales and cost-effective [5]. Various methods are available to remove dyes from wastewater including membrane separation [6], adsorption [7], filtration [8], flotation [9], chemical oxidation [10], reverse osmosis [11] etc. Although these methods have been extensively studied they possess different disadvantages including high initial capital cost, the requirement of different chemicals, ineffective in removing all the pollutants, non-destruction of the pollutants, etc. [12].
Advanced oxidation processes (AOP) are highly active towards the degradation of organic and inorganic compounds that are resistant to conventional treatment methods. AOP produces reactive species, mainly hydroxyl radicals which help in degrading the target molecules to produce CO2, H2O, and mineral acids if the pollutants contain halogens [13]. AOP has gained interest in the treatment of wastewater due to its high efficiency, low toxicity, low cost, and tunable properties that can be modified such as doping, size reduction [14]. Semiconductors are being used as the photocatalysts to generate the reactive species and hence to catalyze the degradation process. Many semiconductors are photocatalytically active, such as titanium dioxide, tungsten oxide, molybdenum oxide, cerium oxide [14]. Among them, TiO2 is considered the most promising candidate due to its high photocatalytic activity, chemical stability, low toxicity, and low cost [15]. However, TiO2 possesses several disadvantages including inefficient exploitation of visible light, low adsorption capacity for hydrophobic contaminants, difficulty in post-treatment recovery, etc. [16]. TiO2 exists in three main polymorphs as rutile (tetragonal), anatase (tetragonal), brookite (rhombohedral). Experimentally observed band gaps (Eg) of the polymorphs vary as Eg (rutile) < Eg (anatase) < Eg (brookite) where Eg of rutile is generally 3.0 eV. Therefore, it is evident that all the polymorphs of TiO2 are active in the UV region being inefficient in visible light harvesting. Further, the rate of recombination of photogenerated species electron-hole pairs is also high in TiO2. Hence, to improve visible light sensitivity many approaches have been implemented. Mainly doping TiO2 with metals and/or non-metals has shown to improve the photocatalytic activity of TiO2 by increasing the visible light sensitivity and impeding the electron-hole pair recombination. Many transition metals such as Fe [17,18,19], Cu [20, 21], Mo [22, 23], V [24, 25], Co [26, 27] non-metals including N [28, 29], S [30, 31], C [32], doped TiO2 have shown promising photocatalytic activity in the visible region.
In the present study, the effect of doping TiO2 with two transition metals, Fe and Cu which get doped via two mechanisms have been studied. Fe3+ is supposed to dope via substitution as the cationic radii of Fe3+ (0.745 Å) and Ti4+ (0.785 Å) are quite similar. But as Cu2+ (0.870 Å) ions are much larger than Ti4+ they may get incorporated into interstitial positions of the lattice. Further, Cu is in the same group of gold and silver in the periodic table and has similar properties due to its electronic configuration and the face centered cubic structure of the atom’s location. Moreover, Cu can exist as copper oxides Cu2O and CuO creating heterojunctions with TiO2. Hence as Cu has different means of enhancing the photocatalytic activity of TiO2, the effect of co-doping Fe and Cu on photocatalytic activity has been studied. Doped nanoparticles were synthesized by the sol–gel synthesis method and their photocatalytic activity was evaluated on the degradation of methylene blue under visible light exposure.
2 Materials and methods
2.1 Materials
Titanium isopropoxide (99%), Fe(NO3)2. 9H2O (99%), Cu(NO3)2.5H2O (98%), ethanol, and nitric acid (70%), methylene blue were procured from Sisco Research Laboratories (Pvt) Ltd, India, and used as such.
2.2 Methods
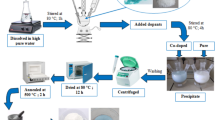
2.2.1 Sol–gel synthesis of TiO2 catalysts
Solution A containing deionized water acidified by nitric acid was added drop-wise to solution B containing ethanol and titanium isopropoxide in a volume ratio of (3:1) and stirred for 24 h. The resultant wet product was dried at 80 °C for about 24 h and calcined at 450 °C for 2 h.
2.2.2 Synthesis of iron and copper doped TiO2 catalysts
Iron and copper were doped in different molar percentages relative to TiO2 as shown in Table 1.
Appropriate amounts of Fe/Cu/Fe and Cu were dissolved in ethanol before TTIP was added to prepare solution B. All the other steps were exactly similar to the synthesis of undoped TiO2.
2.2.3 Photocatalytic activity
The photocatalytic activity of the synthesized catalysts was evaluated towards the degradation of methylene blue using visible light generated by 50 W LED. The light intensity at the sample was maintained at 90,000–100,000. The photocatalytic activity experiments were carried out by taking 200 mg of photocatalyst and 100 ml of 3 mg/L MB solution. The photodegradation studies were done for 2 h excluding the first 30 min during which the samples were kept under dark conditions to reach the adsorption-desorption equilibrium. Samples were constantly shaken during the period of analysis. Samples of 3 ml were withdrawn from the solutions and the absorbance spectrum was collected by using a UV–visible spectrophotometer.
3 Characterization
X-ray diffraction (XRD) analysis was carried out using an Advance Bruker system using CuKα (λ = 0.154 nm) radiation and 2θ varying from 5°–80° at a scan speed of 2°/min. Raman analysis was performed by a Bruker Senterra Raman microscope spectrophotometer. The morphology of the samples was observed by a High-Resolution Transmission Electron Microscope operating at 200 kV (JEOL - JEM–2100) and energy dispersive spectra (EDS) were collected by the same instrument with TEAM EDX software. The sample (1 µl) was mounted on a holey carbon copper grid and allowed to dry at room temperature before TEM analysis. SEM images and EDS spectra were collected by Hitachi SU6600 Analytical Variable Pressure FE-SEM (Field Emission Scanning Electron Microscope) and Oxford Instruments EDX with AZtec software. Samples were mounted onto the sample stub using carbon tapes and the images were taken after gold sputter coating for 15 s. XPS spectra were acquired by Thermo ScientificTM ESCALAB Xi+ X-ray Photoelectron Spectrometer. The chemical composition of the samples was analyzed by X-ray fluorescence (XRF) using a HORIBA Scientific XGT −5200 X-ray analytical microscope equipped with a Rh anode X-ray tube operated at a maximum voltage of 50 kV. Shimadzu 1800 UV/Visible spectrophotometer utilizing a precision Czerny-Turner optical system was used to analyze diffuse reflectance spectra of the prepared powder samples. The measurements were carried out through the range of 190–1100 nm with a bandwidth of 1.0 nm (wavelength accuracy +/−0.1 nm). The absorbance of MB samples was measured by a Shimadzu UV-1990 double beam UV–Visible spectrophotometer. Raman analysis was performed by a Bruker Senterra Raman microscope spectrophotometer.
4 Results and discussion
4.1 XRD analysis
XRD patterns were collected to determine the crystallographic orientation of the synthesized photocatalysts. XRD patterns of the catalysts are shown in Fig. 1a. The main diffraction peaks at 25.3° and 27.4° of the XRD patterns shown in Fig. 1a can be indexed to the (101) plane of anatase and (110) plane of rutile phase, respectively indicating that both anatase and rutile phases are present in the catalysts. Furthermore, no Fe or Cu-related oxides or other impurities can be observed. This signifies that the dopants are successfully doped into the lattice of TiO2 or Fe and Cu ions are uniformly distributed on the surface of TiO2 with quantities too low to be detected by XRD. Figure 1b shows the enlarged XRD peaks of the (101) plane of the anatase phase and the (110) of rutile. The XRD peak positions have shifted towards a lower angle after doping with Fe3+ and Cu2+ slightly. The XRD peaks of crystal planes (101) and (200) in anatase were selected to determine the lattice parameters a, b and c, with Bragg’s law employing tetragonal formula,
Where d is the distance between adjacent lattice planes, a, b, and c are lattice constants and h, k, l are lattice vectors in Miller index notation. Calculated lattice parameters are tabulated in Table 1. Both a (a = b) and c lattice parameters of the doped nanomaterials are different from the pure TiO2. Based on these observations, Fe3+ and Cu2+ have successfully doped into the lattice of TiO2. These two modes of doping of metals into metal oxides as interstitial and substitution doping. The doping mode is determined by the electronegativity and the ionic radius of the doping ions. If the electronegativity and the ionic radius of doping ions match with those of the lattice ions, the dopant can substitute the lattice ions. Electronegativity of Ti4+, Fe3+ and Cu2+ are 1.5, 1.8 and 1.9, respectively. Ionic radius of those are 0.745, 0.785 Å (high spin) and 0.870 Å, respectively [33]. Electronegativity values of Ti4+, Fe3+, and Cu2+ are compatible, however, when ionic radii are considered only the ionic radius of Fe3+ is quite similar to that of Ti4+. Hence Fe3+ dopes to TiO2 lattice substituting Ti4+. However, the ionic radius of Cu2+ is higher than that of Ti4+. Therefore, Cu2+ ions are most likely located in the interstitial positions in the lattice rather than directly in Ti4+ sites due to the relatively large size of the Cu2+ ions [33, 34]. Therefore, the doping mechanism of Fe3+ and Cu2+ could be summarized as substitution and interstitial doping, respectively. The d spacing between the atomic planes of each catalyst was calculated by considering (101) plane of anatase using the following equation,
where λ is the wavelength of the X-ray in nm (0.154 nm for Cu source). No significant influence on the d spacing value upon doping of TiO2 was observed.
a XRD patterns of (a) TiO2, (b) 0.05 Fe/ TiO2, (c) 0.05 Cu/ TiO2, (d) 0.1 Fe/ TiO2, (e) 0.1 Cu/ TiO2, (f) 0.025 Fe + 0.075 Cu/ TiO2, (g) 0.05 Fe + 0.05 Cu/ TiO2, (h) 0.075 Fe + 0.025 Cu/ TiO2, and (i) 0.1 Fe + 0.1 Cu/ TiO2. b Enlarged region of 24°–28.5° showing the (101) plane of anatase phase and the (110) of rutile
The crystallite sizes of the undoped and doped catalysts were calculated by using Scherrer’s equation,
where β is the full width at half maximum (FWHM) in radians. (101) plane of anatase plane was selected for the calculation of crystallite size. As can be seen in the values tabulated in Table 1, no significant trend of the crystallite size was observed on doping TiO2 by Fe3+ and Cu2+ with different doping concentrations.
4.2 Raman analysis
The Raman spectra were acquired to confirm the crystal structure of the catalysts suggested by XRD analysis (Fig. 2). Five characteristic Raman modes of anatase TiO2 with symmetries 144.5 (Eg), 197 (Eg), 396.5 (B1g), 516 (A1g), 637.5 (Eg) cm−1 were observed in the Raman spectrum of undoped TiO2 [35]. Additional peaks at 244, 444 (Eg) and 612 (A1g) cm−1 correspond to a vibrational mode of rutile being consistent with the XRD pattern indicating the presence of both anatase and rutile phases [36]. Similar Raman modes were observed in the Raman spectra of 0.1 Fe/TiO2, 0.1 Cu/TiO2, and 0.1 Fe + 0.1 Cu/TiO2, and additional peaks corresponding to the oxides of Fe and Cu were absent indicating the proper doping of Fe3+ and Cu2+ without creating significant lattice distortions. However, the peak intensity ratio of the main peak at 144.5 cm−1 to the others has shown a reduction when dopants are present compared to the undoped TiO2. Further, peaks at 444 and 612 cm−1 correspond to the rutile phase is clearly visible especially in 0.1 Fe/TiO2, 0.1 Cu/TiO2 and its presence is comparatively less prominent in pure TiO2 and 0.1 Fe + 0.1 Cu/TiO2. Dopants, Fe3+, and Cu2+ should be successfully doped in other catalysts of which the dopant concentrations are lower than 0.1% with no lattice distortions as suggested by the Raman spectroscopic data obtained with the analyzed catalysts.
4.3 TEM analysis
TEM analysis was performed to study morphology at the nanoscale and to confirm the crystallographic orientation of the prepared catalysts resulted from XRD patterns. TEM images of TiO2 are shown in Fig. 3. Bright-field image (Fig. 3a) shows spherical and irregular-shaped nanoparticles and they are aggregated. This could be due to the rapid hydrolysis of titanium isopropoxide catalyzed by diluted nitric acid. Further, the absence of a surfactant during the synthesis may have contributed to particle aggregation. High-resolution TEM image (Fig. 3b) shows the arrangement of the atomic planes and the d spacing calculated from the generated histogram is 0.3598 nm. The obtained d spacing value matches with the d spacing value calculated for (101) plane (0.3515 nm) of the anatase phase. Interplanar spacing calculated from the HRTEM image is different from that calculated from the XRD data. This is because that TEM imaging is a localized effect where a particular location of the sample is selected for imaging at the nanoscale, while at acquiring XRD pattern, a comparatively large area is selected. The selected area diffraction pattern (Fig. 3c) shows a diffused ring pattern. Rings correspond to the (101) and (211) planes of anatase and the (101) plane of rutile could be observed. Phase identification performed by XRD, Raman spectroscopy, and TEM do well agree with each other. Electron map collected (Fig. 3d) show the elemental distribution of nanoparticles consisting of Ti, O, and C where C represents the carbon grid and the electron maps of Ti and O (Fig. 3e and f, respectively) show the individual elemental distribution on nanoparticles. EDS spectrum (Fig. 3g) also supports that the nanoparticles consist only of Ti and O and no other impurities are present.
4.4 SEM analysis
SEM images were collected to study the morphology of the synthesized catalysts at the macroscale. As can be seen in the SEM images are shown in Fig. 4 and basically, nanoparticles have been aggregated during the sol–gel synthesis being consistent with the TEM images as discussed. Incorporation of metal ions during the synthesis have not affected the morphology of the catalysts because the morphology shown by the SEM image of the undoped TiO2 (Fig. 4a) are similar to the SEM images of the metal-doped nanoparticles, 0.05 Fe/TiO2, 0.05 Cu/TiO2, 0.05 Fe + 0.05 Cu/TiO2 (Fig. 4b, c, d, respectively).
4.5 XPS analysis
To analyze the surface chemistry of the synthesized catalysts XPS analysis was performed (Fig. 5). The survey spectrum of pure TiO2 (Fig. 5a) shows the presence of Ti, O, and C. The high resolution spectrum of Ti 2p is shown in Fig. 5a. In this spectrum doublet, Ti 2p3/2 (binding energy 459.8 eV) and Ti2p1/2 (binding energy 465.58 eV) arise from the spin-orbital coupling. These peaks are consistent with the Ti4+ of TiO2 lattice [37]. The high-resolution spectrum of O 1 s of the same sample was deconvoluted into two peaks centered at 531 and 532.85 eV (Fig. 5b). The peak at 531 eV shows the presence of OH group with oxygen at the bridging oxygen site and the peak at 532.85 eV could be attributed to the OH group as a terminal group with oxygen attached to the five-coordinated Ti4+ with an O-Ti4+ covalent bond [38]. The high resolution spectrum of C 1 s (Fig. 5d) is deconvoluted into four peaks. Peaks at 284.8, 286.35, 287.72, and 289.52 eV could be assigned to C–C, C–O, C=O, and O–C=O bonds [39]. The C/Ti ratio of pure TiO2 is 0.13.
The survey spectrum of 0.1 Fe + 0.1 Cu/TiO2 (Fig. 5e) shows the presence of Cu, Ti, O, and C. The high resolution spectrum of Ti of 0.1 Fe + 0.1 Cu/TiO2 is shown in Fig. 5f. 2p3/2 peak was deconvoluted into two peaks centered at 459.8 eV and 458.45 eV and they correspond to the Ti4+ and Ti3+ oxidation states, respectively. The ratio between the Ti4+ to Ti3+ is approximately 2.17:1 calculated by the area under the curves. The high resolution spectrum of Cu (Fig. 5g) also shows the spin-orbital coupling, where, 2p3/2 and 2p1/2 peaks occurred at 932.87 and 952.46 eV, respectively, indicate the presence of Cu+, while the peaks at 936.53 and 956.37 eV, show the presence of Cu2+ [40]. The ratio between the Cu+/Cu2+ obtained from the area under the curves is 3.60. A similar observation was also reported by Akhaven et al. [41]. The high signal-to-noise ratio of the high-resolution spectrum of Fe (Fig. 5e) makes it difficult to identify the peaks. However, 2p3/2 and 2p1/2 have appeared at 711 and 724.57 eV, respectively revealing the presence of Fe3+ [42]. The high resolution spectrum of C 1 s (Fig. 5i) has been deconvoluted to four peaks. Peaks at 284.8, 286.35, 287.65, and 289.02 eV could be attributed to C–C, C–O, C=O, and O–C=O bonds [39]. Further, the Ti-C bond expected to appear at 283.7 eV [39, 43, 44] was not observed revealing that impurity C has not been doped to TiO2 during the synthesis. It has been reported that carbon can form a Ti-C bond when treated the samples at 400 °C by Akhavan et al. [39, 43, 44]. The Cu/Ti and C/Ti ratios of the synthesized 0.1 Fe + 0.1 Cu/TiO2 nanomaterial are 0.09 and 0.056, respectively.
4.6 X-ray fluorescence spectroscopic analysis
XRF analysis was performed on 0.1Cu + 0.1Fe/TiO2 to identify the elemental distribution. Data tabulated in Table 2 were collected from six different spots of the sample. The nanomaterials consist of Ti, Fe, and Cu only. The Fe: Cu ratio at all the analyzed spots is 1:1 showing the homogeneous distribution of the metal ions on the TiO2 matrix.
4.7 Diffuse reflectance UV–Visible spectroscopic analysis
The electronic band structures of the prepared samples were analyzed by UV–Visible absorption spectroscopy (Fig. 6). Compared with the undoped TiO2, doped TiO2 show a higher light harvest performance reflected by the enhancement of light absorbance covering the visible range. To determine the band gap energies of the synthesized materials cutoff wavelengths were determined by drawing a tangent to the absorption curves. The band gap energy is calculated by the following equation,
Calculated band gap values are tabulated in Table 3. The band gap of pure TiO2 (2.98 eV) is lesser than the reported band gap energy for anatase (3.2 eV). As revealed by the XRD patterns, both anatase and rutile phases are present in pure TiO2. Both Ti4+and Ti3+ were identified in the high resolution XPS spectrum of Ti 2p of 0.1 Fe + 0.1 Cu/TiO2. The decrease of the band gap can be due to the localized gap states induced by Ti3+ and to the oxygen vacancies [45,46,47]. Missing an oxygen atom from the surface or bulk of TiO2 results in one or two electrons localized in an oxygen vacancy state. These electrons localized on the oxygen vacancy state form a donor level below the conduction band of TiO2 [46]. In general transition metals like Fe doped TiO2 show, relatively a lower band gap compared to the undoped TiO2 due to the formation of a new energy level below the conduction band of TiO2 due to the doping of Fe3+ and formation of Ti3+. Moreover, transition metals like Cu and rare-earth metals like La, lead to the lattice deformation and the formation of oxygen vacancies, resulting in impurity states in the TiO2 band gap, and hence narrow the band gap [48]. However, calculated band gap energies do not show a clear significant reduction in band gap upon doping increasing dopant (Fe and Cu) concentrations. It is reported that the band gap of semiconductors is affected by many factors including particle size, shape, surface area, etc. The band gap energy increases with decreasing particle size because of the confinement of electrons and holes. Volume to surface area ratio varies with the size and the shape of the materials that alter the number of surface atoms at the nanoscale and cohesive energy. Therefore the band gap energy changes at the nanoscale. When the particle size reaches the nanoscale, the number of overlapping orbitals or energy levels decreases, and the thickness of the band reduces. Then the energy gap between the valence band and the conduction band increases [49]. A clear relationship between the crystallite size calculated by XRD patterns and the band gap energies was not observed. According to the SEM images collected it could be seen that the particle size varies from one sample to the other. Hence, there cannot be a clear trend in the surface area as well. Therefore, as many factors collectively contribute to the band gap energy, resulted in variation cannot be exactly explained.
4.8 Photocatalytic activity
The photocatalytic activity of the prepared photocatalysts was evaluated on the degradation of MB under visible light. MB was selected as the model pollutant as it is extensively used in the industry. The degradation kinetics was computed by the change in MB concentration measured by UV–Visible spectrophotometer as a function of irradiation time (Fig. 7). The rate constants tabulated in Table 4 reveal that the photodegradation efficiency of the 0.05 Cu/TiO2 (0.0195 min−1) is higher than all the nanomaterials followed by 0.1 Cu/TiO2 (0.0120 min−1). As revealed by the XPS analysis, catalysts contain both Cu+ and Cu2+. Cu+ is quite unstable during both the atmospheric and photocatalytic reaction conditions. Therefore, during the photocatalysis existing Cu+ would probably oxidize to Cu2+. Rate constant for the degradation of TiO2 (0.0062 min−1) is greater than that of 0.025 Fe + 0.075 Cu/TiO2 (0.0044 min−1) and similar to that of 0.075 Fe + 0.025 Cu/TiO2 (0.0060 min−1). The photodegradation efficiency of other photocatalysts was greater than that of TiO2 as shown in Table 4. Therefore it is evident that the visible light sensitivity of TiO2 has been enhanced by doping with Fe and Cu. Cu/TiO2 has shown higher photocatalytic activity than Fe/TiO2. Cu contributes to photocatalytic activity in several ways. Cu2+ dope to the interstitial positions of TiO2 due to the difference of the valence state and the cationic radii [50, 51]. Cu can exist in a wide range of accessible oxidation states as Cu0, CuI, and CuII, and hence the active species in TiO2 are Cu, Cu2O, and CuO [34]. Metallic Cu exists in the same group of gold and silver of the periodic table has similar properties due to the electronic configuration and the face centered cubic (FCC) structure. Metallic Cu nanoparticles can activate TiO2 towards visible light due to the localized surface plasmon resonance (LSPR) effect. LSPR is excited when light interacts with the free electrons of the metallic nanostructure, which results in collective excitations that lead to significant improvement of the local electromagnetic fields surrounding the nanoparticles [52]. Once the visible light is absorbed by the Cu nanoparticle modified TiO2, electrons are transferred to the conduction band of TiO2, which creates an electron-deficient metal and rich TiO2, where direct photocatalytic oxidation occurs on the metal surface. However, metallic Cu does not exist under normal atmospheric conditions because it gets oxidized in the presence of oxygen. The presence of metallic Cu was not evident even from the XPS analysis and hence it is clear that the LSPR effect has not contributed to the photocatalytic activity tested in the study. Copper oxides can form heterojunction systems with TiO2. There are several advantages associated with heterojunctions including improvement of the charge carriers’ separation, increase in the lifetime of the charge carrier, and enhancement of the interfacial charge transfer efficiency [53, 54]. Cu2O and CuO are p-type semiconductors with band gap energies of 2.1 and 1.7 eV, respectively [34]. The most probable type of heterostructure for the CuxO/TiO2 system would be probably type II, where photoexcited electrons are transferred from CB of CuxO to the CB of TiO2 and photogenerated holes transferred simultaneously from the VB of TiO2 to VB of CuxO. As a result, photogenerated electrons and holes are separated reducing the recombination and increasing the lifetimes of the charge carriers [55]. The presence of Cu+ at the surface of the catalyst is confirmed by the XPS analysis. Therefore, both Cu2O and CuO could be present in the prepared catalysts. As revealed by the XPS analysis Fe and Cu co-doped TiO2, Ti3+ was present in addition to Ti4+. The Ti3+ defects can form a shallow donor level just below the conduction band which enhances the visible light sensitivity [46]. Under visible light irradiation, induced electrons on an isolated Ti3+ band could be transferred to the surface of CuO nanoclusters efficiently, in addition to the direct charge transfer from the VB of TiO2 improving the photocatalytic activity [56]. Further, photogenerated electrons from Cu2O would be captured by Ti4+ ions in TiO2 and get reduced to Ti3+, increasing the Ti3+ concentration triggering the effect from Ti3+. Whereas, left holes at the VB of Cu2O hamper the charge recombination resulting in an improved photocatalytic activity [57]. Though an enhanced photocatalytic activity was observed with low Cu loading (0.05%), photocatalytic activity decreased with increasing the Cu loading (0.1%). This could be due to the shielding effect caused by the dispersed CuO nanoparticles covering the surface of TiO2 reducing the photon adsorption. Moreover, a higher concentration of CuO could promote the photogenerated electron-hole pair recombination resulting in a decrease in available holes for redox reactions [58]. Fe is considered as a successful dopant because they dope via substitution due to the quite similar cationic radii (Fe–0.745 Å, Ti–0.785 Å) and Fe3+ has a stable half-filled d5 configuration. Rate constant of 0.05 Fe/ TiO2 (0.0097 min−1) is greater than that of 0.1 Fe/ TiO2 (0.088 S−1). With higher Fe loading comparatively low photocatalytic activity was observed due to the recombination of photogenerated electron and hole pairs. The main hypothesis of this study is to determine the effect of co-doping of Fe and Cu on photocatalytic activity. Fe and Cu have been doped in different proportions to study in detail the effect of co-doping on photocatalytic activity. The obtained rate constants for co-doped TiO2 are lesser than single metal-doped TiO2. Among the co-doped TiO2 catalysts, TiO2 doped with equal proportions of Fe and Cu showed comparatively higher photocatalytic activity for the degradation of MB (0.05 Fe + 0.05 Cu/ TiO2 - 0.0098 min−1 and 0.1 Fe + 0.1 Cu/ TiO2 is 0.0077 min−1) and of these two higher Fe and Cu loading showed low activity due to the recombination of photogenerated electron-hole pairs. Catalysts prepared with unequal Fe and Cu proportions showed lower photocatalytic activity. 0.075 Fe + 0.025 Cu/ TiO2 showed a similar activity to the pure TiO2 (0.0060 min−1) while the photocatalytic activity of 0.025 Fe + 0.075 Cu/ TiO2 (0.0044 min−1) was lower than pure TiO2. This could be due to the masking effect of one metal by the other as well as the shielding effect of TiO2 by both metals. Moreover, the lowest photocatalytic activity has resulted with the photocatalyst with higher Cu loading because though CuxO is present as the TiO2 is shielded proper charge migration and separation at the heterojunction would have not taken place.
5 Conclusion
Fe and Cu co-doped TiO2 nanoparticles with varying Fe and Cu were synthesized by the sol–gel method. The crystal structure of TiO2 has not deteriorated by doping of Fe and Cu. The doping of Fe is confirmed to be mainly +3, which provides extra electronic states in the band gap of TiO2. Doped Cu form heterojunction of CuxO/TiO2 that facilitate the charge separation. The absorption edge of TiO2 is shifted to the visible region by doping with Fe and Cu. Single doping of Fe and Cu has significantly improved the photocatalytic activity while co-doping has resulted in comparatively low photocatalytic activity on photodegradation of methylene blue under visible light irradiation. 0.05 Cu/ TiO2 showed the highest photocatalytic activity among the single doped systems while the highest photocatalytic activity of the co-doped systems was obtained from 0.05 Cu + 0.05 Fe/ TiO2. Low photocatalytic activity with higher metal loading at single doping was resulted due to the electron-hole pair recombination while the low activity of co-doping systems has caused by the shielding effect of each dopant and TiO2 by the doped metals.
References
Arami M, Limaee NY, Mahmoodi NM, Tabrizi NS (2005) Removal of dyes from colored textile wastewater by orange peel adsorbent: Equilibrium and kinetic studies. J Colloid Interface Sci 288:371–376. https://doi.org/10.1016/j.jcis.2005.03.020
Robinson T, Chandran B, Nigam P (2002) Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Res 36:2824–2830. https://doi.org/10.1016/S0043-1354(01)00521-8
Ramakrishna KR, Viraraghavan T (1997) Dye removal using low cost adsorbents. Water Sci Technol 189–196. https://doi.org/10.1016/S0273-1223(97)00387-9
Nigam P, Armour G, Banat IM et al. (2000) Physical removal of textile dyes from effluents and solid-state fermentation of dye-adsorbed agricultural residues. Bioresour Technol 72:219–226. https://doi.org/10.1016/S0960-8524(99)00123-6
Mohan D, Singh KP, Singh G, Kumar K (2002) Removal of dyes from wastewater using flyash, a low-cost adsorbent. Ind Eng Chem Res 41:3688–3695. https://doi.org/10.1021/ie010667+
Jirankova H, Mrazek J, Dolecek P, Cakl J (2010) Organic dye removal by combined adsorption-membrane separation process. Desalin Water Treat 20:96–101. https://doi.org/10.5004/dwt.2010.1170
Robinson T, Chandran B, Nigam P (2002) Removal of dyes from a synthetic textile dye effluent by biosorption on apple pomace and wheat straw. Water Res 36:2824–2830. https://doi.org/10.1016/S0043-1354(01)00521-8
Lafi R, Gzara L, Lajimi RH, Hafiane A (2018) Treatment of textile wastewater by a hybrid ultrafiltration/electrodialysis process. Chem Eng Process - Process Intensif 132:105–113. https://doi.org/10.1016/j.cep.2018.08.010
Mohammed AA, Ebrahim SE, Alwared AI (2013) Flotation and sorptive-flotation methods for removal of lead ions from wastewater using SDS as surfactant and barley husk as biosorbent. J Chem https://doi.org/10.1155/2013/413948
Brisset J-L, Benstaali B, Moussa D et al. (2011) Acidity control of plasma-chemical oxidation: applications to dye removal, urban waste abatement and microbial inactivation. Plasma Sources Sci Technol 20:34021. https://doi.org/10.1088/0963-0252/20/3/034021
Abid MF, Zablouk MA, Abid-Alameer AM (2012) Experimental study of dye removal from industrial wastewater by membrane technologies of reverse osmosis and nanofiltration. Iran J Environ Health Sci Eng 9:17. https://doi.org/10.1186/1735-2746-9-17
Crini G, Lichtfouse E (2019) Advantages and disadvantages of techniques used for wastewater treatment. Environ Chem Lett 17:145–155. https://doi.org/10.1007/s10311-018-0785-9
Joseph CG, Li Puma G, Bono A, Krishnaiah D (2009) Sonophotocatalysis in advanced oxidation process: a short review. Ultrason Sonochem 16:583–589. https://doi.org/10.1016/j.ultsonch.2009.02.002
Chan SHS, Yeong Wu T, Juan JC, Teh CY (2011) Recent developments of metal oxide semiconductors as photocatalysts in advanced oxidation processes (AOPs) for treatment of dye waste-water. J Chem Technol Biotechnol 86:1130–1158. https://doi.org/10.1002/jctb.2636
Saien J, Asgari M, Soleymani AR, Taghavinia N (2009) Photocatalytic decomposition of direct red 16 and kinetics analysis in a conic body packed bed reactor with nanostructure titania coated Raschig rings. Chem Eng J 151:295–301. https://doi.org/10.1016/j.cej.2009.03.011
Dong H, Zeng G, Tang L et al. (2015) An overview on limitations of TiO2-based particles for photocatalytic degradation of organic pollutants and the corresponding countermeasures. Water Res 79:128–146. https://doi.org/10.1016/j.watres.2015.04.038
Ismael M (2020) Enhanced photocatalytic hydrogen production and degradation of organic pollutants from Fe (III) doped TiO2 nanoparticles. J Environ Chem Eng 8:103676. https://doi.org/10.1016/j.jece.2020.103676
Cheng G, Liu X, Song X et al. (2020) Visible-light-driven deep oxidation of NO over Fe doped TiO2 catalyst: synergic effect of Fe and oxygen vacancies. Appl Catal B Environ 277:119196. https://doi.org/10.1016/j.apcatb.2020.119196
Akhavan O (2010) Thickness dependent activity of nanostructured TiO2/α-Fe2O3 photocatalyst thin films. Appl Surf Sci 257:1724–1728. https://doi.org/10.1016/j.apsusc.2010.09.005
Yang WT, Lin CJ, Montini T et al. (2021) High-performance and long-term stability of mesoporous Cu-doped TiO2 microsphere for catalytic CO oxidation. J Hazard Mater 403:123630. https://doi.org/10.1016/j.jhazmat.2020.123630
Ikram M, Umar E, Raza A et al. (2020) Dye degradation performance, bactericidal behavior and molecular docking analysis of Cu-doped TiO2 nanoparticles. RSC Adv 10:24215–24233. https://doi.org/10.1039/d0ra04851h
Huang J, Guo X, Wang B et al. (2015) Synthesis and photocatalytic activity of Mo-doped TiO2 nanoparticles. J Spectrosc 2015:681850. https://doi.org/10.1155/2015/681850
Kumaravel V, Rhatigan S, Mathew S et al. (2020) Mo doped TiO2: impact on oxygen vacancies, anatase phase stability and photocatalytic activity. J Phys Mater 3:25008. https://doi.org/10.1088/2515-7639/ab749c
Klosek S, Raftery D (2002) Visible light driven V-doped TiO2 photocatalyst and its photooxidation of ethanol. J Phys Chem B 105:2815–2819. https://doi.org/10.1021/jp004295e
Avansi W, Arenal R, De Mendonça VR et al. (2014) Vanadium-doped TiO2 anatase nanostructures: the role of v in solid solution formation and its effect on the optical properties. CrystEngComm 16:5021–5027. https://doi.org/10.1039/c3ce42356e
Santara B, Pal B, Giri PK (2011) Signature of strong ferromagnetism and optical properties of Co doped TiO2 nanoparticles. J Appl Phys 110:114322. https://doi.org/10.1063/1.3665883
Barakat MA, Schaeffer H, Hayes G, Ismat-Shah S (2005) Photocatalytic degradation of 2-chlorophenol by Co-doped TiO2 nanoparticles. Appl Catal B Environ 57:23–30. https://doi.org/10.1016/j.apcatb.2004.10.001
Fiorenza R, Di Mauro A, Cantarella M et al. (2020) Molecularly imprinted N-doped TiO2 photocatalysts for the selective degradation of o-phenylphenol fungicide from water. Mater Sci Semicond Process 112:105019. https://doi.org/10.1016/j.mssp.2020.105019
Kovalevskiy N, Selishchev D, Svintsitskiy D et al. (2020) Synergistic effect of polychromatic radiation on visible light activity of N-doped TiO2 photocatalyst. Catal Commun 134:105841. https://doi.org/10.1016/j.catcom.2019.105841
Pillai VV, Lonkar SP, Alhassan SM (2020) Template-free, solid-state synthesis of hierarchically macroporous S-doped TiO2 nano-photocatalysts for efficient water remediation. ACS Omega 5:7969–7978. https://doi.org/10.1021/acsomega.9b04409
Chen X, Sun H, Zelekew OA et al. (2020) Biological renewable hemicellulose-template for synthesis of visible light responsive sulfur-doped TiO2 for photocatalytic oxidation of toxic organic and As(III) pollutants. Appl Surf Sci 525:146531. https://doi.org/10.1016/j.apsusc.2020.146531
Zhao D, Zhang X, Sui L et al. (2020) C-doped TiO2 nanoparticles to detect alcohols with different carbon chains and their sensing mechanism analysis. Sens Actuators B Chem 312:127942. https://doi.org/10.1016/j.snb.2020.127942
Choi J, Park H, Hoffmann MR (2010) Effects of single metal-ion doping on the visible-light photoreactivity of TiO2. J Phys Chem C 114:783–792. https://doi.org/10.1021/jp908088x
Janczarek M, Kowalska E (2017) On the origin of enhanced photocatalytic activity of copper-modified titania in the oxidative reaction systems. Catalysts 7:317. https://doi.org/10.3390/catal7110317
Hardcastle FD (2011) Raman spectroscopy of titania (TiO2) nanotubular water-splitting catalysts. J Ark Acad Sci 65:9, https://scholarworks.uark.edu/jaas/vol65/iss1/9
Balachandran U, Eror NG (1982) Raman spectra of titanium dioxide. J Solid State Chem 42:276–282. https://doi.org/10.1016/0022-4596(82)90006-8
Sanjinés R, Tang H, Berger H et al. (1994) Electronic structure of anatase TiO2 oxide. J Appl Phys 75:2945–2951. https://doi.org/10.1063/1.356190
Krishnan P, Liu M, Itty PA et al. (2017) Characterization of photocatalytic TiO2 powder under varied environments using near ambient pressure X-ray photoelectron spectroscopy. Sci Rep. 7:1–11. https://doi.org/10.1038/srep43298
Akhavan O, Abdolahad M, Abdi Y, Mohajerzadeh S (2009) Synthesis of titania/carbon nanotube heterojunction arrays for photoinactivation of E. coli in visible light irradiation. Carbon N. Y 47:3280–3287. https://doi.org/10.1016/j.carbon.2009.07.046
Biesinger MC (2017) Advanced analysis of copper X-ray photoelectron spectra. Surf Interface Anal 49:1325–1334. https://doi.org/10.1002/sia.6239
Akhavan O, Tohidi H, Moshfegh AZ (2009) Synthesis and electrochromic study of sol-gel cuprous oxide nanoparticles accumulated on silica thin film. Thin Solid Films 517:6700–6706. https://doi.org/10.1016/j.tsf.2009.05.016
Yamashita T, Hayes P (2008) Analysis of XPS spectra of Fe2+ and Fe3+ ions in oxide materials. Appl Surf Sci 254:2441–2449. https://doi.org/10.1016/j.apsusc.2007.09.063
Akhavan O, Ghaderi E (2009) Photocatalytic reduction of graphene oxide nanosheets on TiO2 thin film for photoinactivation of bacteria in solar light irradiation. J Phys Chem C 113:20214–20220. https://doi.org/10.1021/jp906325q
Akhavan O, Ghaderi E (2013) Flash photo stimulation of human neural stem cells on graphene/TiO2 heterojunction for differentiation into neurons. Nanoscale 5:10316–10326. https://doi.org/10.1039/c3nr02161k
Liu X, Gao S, Xu H et al. (2013) Green synthetic approach for Ti3+ self-doped TiO2-x nanoparticles with efficient visible light photocatalytic activity. Nanoscale 5:1870–1875. https://doi.org/10.1039/c2nr33563h
Pan X, Yang MQ, Fu X et al. (2013) Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications. Nanoscale 5:3601–3614. https://doi.org/10.1039/C3NR00476G
Kalathil S, Khan MM, Ansari SA et al. (2013) Band gap narrowing of titanium dioxide (TiO2) nanocrystals by electrochemically active biofilms and their visible light activity. Nanoscale 5:6323–6326. https://doi.org/10.1039/c3nr01280h
Zhang Y, Xu X (2020) Machine learning band gaps of doped-TiO2 photocatalysts from structural and morphological parameters. ACS Omega 5:15344–15352. https://doi.org/10.1021/acsomega.0c01438
Singh M, Goyal M, Devlal K (2018) Size and shape effects on the band gap of semiconductor compound nanomaterials. J Taibah Univ Sci 12:470–475. https://doi.org/10.1080/16583655.2018.1473946
Yan H, Zhao T, Li X, Hun C (2015) Synthesis of Cu-doped nano-TiO2 by detonation method. Ceram Int 41:14204–14211. https://doi.org/10.1016/j.ceramint.2015.07.046
Sreekantan S, Zaki SM, Lai CW, Tzu TW (2014) Copper-incorporated titania nanotubes for effective lead ion removal. Mater Sci Semicond Process 26:620–631. https://doi.org/10.1016/j.mssp.2014.05.034
Chan GH, Zhao J, Hicks EM et al. (2007) Plasmonic properties of copper nanoparticles fabricated by nanosphere lithography. Nano Lett 7:1947–1952. https://doi.org/10.1021/nl070648a
Bessekhouad Y, Robert D, Weber JV (2004) Bi2S3/TiO2 and CdS/TiO2 heterojunctions as an available configuration for photocatalytic degradation of organic pollutant. J Photochem Photobio A Chem 163:569–580. https://doi.org/10.1016/j.jphotochem.2004.02.006
Serpone N, Maruthamuthu P, Pichat P et al. (1995) Exploiting the interparticle electron transfer process in the photocatalysed oxidation of phenol, 2-chlorophenol and pentachlorophenol: chemical evidence for electron and hole transfer between coupled semiconductors. J Photochem Photobio A Chem 85:247–255. https://doi.org/10.1016/1010-6030(94)03906-B
Marschall R (2014) Semiconductor composites: strategies for enhancing charge carrier separation to improve photocatalytic activity. Adv Funct Mater 24:2421–2440. https://doi.org/10.1002/adfm.201303214
Liu M, Qiu X, Miyauchi M, Hashimoto K (2011) Cu(II) oxide amorphous nanoclusters grafted Ti3+ self-doped TiO2: An efficient visible light photocatalyst. Chem Mater 23:5282–5286. https://doi.org/10.1021/cm203025b
Xiong L, Yang F, Yan L et al. (2011) Bifunctional photocatalysis of TiO2/Cu2O composite under visible light: Ti3+ in organic pollutant degradation and water splitting. J Phys Chem Solids 72:1104–1109. https://doi.org/10.1016/j.jpcs.2011.06.016
Chiang K, Amal R, Tran T (2002) Photocatalytic degradation of cyanide using titanium dioxide modified with copper oxide. Adv Environ Res 6:471–485. https://doi.org/10.1016/S1093-0191(01)00074-0
Acknowledgements
The authors acknowledge the Sri Lanka Institute of Information Technology for providing the funds. The authors are much grateful to the Sri Lanka Institute of nanotechnology for facilitating the project with the required instruments.
Funding
This project was funded by a research grant awarded by Sri Lanka Institute of Information Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Thambiliyagodage, C., Mirihana, S. Photocatalytic activity of Fe and Cu co-doped TiO2 nanoparticles under visible light. J Sol-Gel Sci Technol 99, 109–121 (2021). https://doi.org/10.1007/s10971-021-05556-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-021-05556-4