Abstract
Titania nanotubes (TNTs) with high length–diameter ratio are incorporated into reduced graphene oxide (rGO) aerogel to modify the porous structure of the obtained rGO/TNTs aerogel for efficient methylene blue (MB) adsorption. A hydrothermal method along with freeze drying is applied for the preparation of the rGO/TNTs composite aerogel. The uniformly dispersed TNTs in rGO matrix can effectively support the network structure and hinder the π–π bonding interaction between graphene sheets, thus resulting in a three-dimensional mesoporous network with high specific surface area. The rGO/TNTs aerogel shows favorable adsorption property toward MB and the maximum adsorption capacity can be 380.7 mg g−1 with the adsorption efficiency of 92.2%. Besides, the adsorption kinetic analysis reveals that a dispersion process is found to be the rate-limiting step when the adsorbent dosage is low. With the increase of adsorbent content, chemical sorption such as the π–π interaction or the reaction of the polar functional groups between MB and rGO–TNTs-C aerogel becomes the rate-limiting step.

Highlights
-
TNTs are successfully employed for the pore structure regulation of rGO aerogel.
-
The rGO/TNTs-C aerogel shows a maximum adsorption capacity of 380.7 mg g−1 towards MB.
-
Diffusion of MB to the active sites of rGO aerogel is the rate-limiting step with low adsorbent dosage.
-
Chemical sorption tends to be the rate-limiting step with the increase of adsorbent dosage.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Dyes are widely used in pharmaceuticals, pesticides, textiles, and varnishes. However, the dyeing effluents discharge into water main cause many problems to the ecological environment and human health [1, 2]. Therefore, it is of great importance to remove these contaminants safely and effectively [3]. So far, a variety of technologies have been proposed to solve this problem, such as chemical precipitation [4], ultrafiltration [5], evaporation [6], ion exchange [7], solvent extraction [8], electrochemical treatment [9], photocatalysis [10], and adsorption [11,12,13]. Among these methods, adsorption has been considered as an efficient and economical process due to its low cost, simplicity, high efficiency, and wide-ranging availability [14, 15]. Thus, it is quite meaningful to design highly active adsorbents for effective dye adsorption. Recently, many studies have been reported for dye adsorption especially toward methylene blue (MB) [16,17,18,19,20].
Graphene, a honeycomb-arranged sp2 carbon, displays high surface area, chemical stability, unique electronic and mechanical properties, and excellent adsorptivity [21,22,23,24,25], which is a promising adsorbent for water treatment. Compared with the powder graphene, graphene aerogel (GA) with well-defined interconnected porous networks in the form of block is more convenient for the adsorption of dyes in aqueous solution in consideration of the separation after adsorption, which can avoid the secondary pollution in water treatment. Wang et al. [26] fabricated a 3D reduced graphene oxide (rGO) aerogel and found that the adsorption capacity of GA varies from 115 to 1260 mg g−1 for different dyes. However, due to the uncontrollable agglomeration between the two-dimensional graphene sheets in the process of assembling into three-dimensional network, there is still a certain gap between the actual specific surface area and the theoretical value [27, 28]. Thus, appropriate modifications are urgently required to improve the comprehensive properties.
Titania nanotubes (TNTs) were first synthesized by Hoyer in 1996 via an electrochemical deposition in a porous aluminum oxide mold [29]. Then, it has been extensively studied due to the high specific surface area [30,31,32,33,34,35], good ion-exchangeable ability [36], and remarkable rapid and long-distance electron-transport capability [37]. Niu et al. found that TNTs show good adsorption property for the removal of organic molecules and radioactive toxic metal ions [38]. Taking these advantages into consideration, TNTs are expected to be a promising nanofiller for the modification of rGO aerogel and introducing more active sites toward dye adsorption.
In this paper, TNTs are employed to regulate the 3D network structure of the rGO aerogel through a simple hydrothermal self-assembly method. The incorporation of TNTs can effectively hinder the strong hydrogen bonds and π–π interaction between the molecules and thus inhibit the uncontrollable agglomeration of graphene sheets, resulting in rGO/TNTs aerogel with high specific surface area and favorable MB adsorption property. The specific adsorption kinetics are also analyzed in detail, which can provide some ideas for other adsorption studies. Our results highlight the potential of TNTs to modify the structure of rGO aerogel and thus enhance the dye adsorption property, which provide new insight for the design of high-performance composites.
2 Experimental
2.1 Chemicals and materials
Graphite was purchased from Heilongjiang Aoyu Graphite Group Co., Ltd. Potassium permanganate (KMnO4, 99%) was purchased from Tianjin Chemical Plant. Concentrated H2SO4, HCl, and cetyltrimethylammonium bromide (CTAB) were purchased from Sinopharm Group Chemical Reagent Co., Ltd. MB was purchased from Tianjin Fengchuan Chemical Reagent Science Co., Ltd. Hydrogen peroxide (H2O2), sodium nitrate (NaNO3, 99%), tertiary butyl alcohol (C4H10O), titanium dioxide (TiO2), sodium hydroxide (NaOH), and distilled water were purchased from Tianjin Jiangtian Unified Technology Co., Ltd. The reagents used in this experiment do not need further purification.
2.2 Preparation of GO
GO was chemically synthesized following the modified Hummers method [39]. Overall, 10 g of natural graphite and 7.5 g of NaNO3 were placed in a flask and 621 g of H2SO4 was added with stir in an ice water bath. 45 g of KMnO4 was gradually added over about 1 h and the system was then reacted at 40 °C for 2 h. The obtained solution was diluted with 80 mL deionized water and kept at 90 °C for 1 h. After cooling down, 50 mL deionized water and a small amount of H2O2 solution were added and stood for 12 h. Then the suspension was washed with 5% HCl and deionized water to neutral, thus stable GO solution was obtained.
2.3 Preparation of TNTs
0.31 g of TiO2 was added to 50 mL NaOH solution and reacted at a certain temperature with different stirring rate for 48 h in a Teflon-sealed autoclave. After cooling down, the sample was washed with deionized water and ethanol to remove impurities, and then dried at 72 °C for 6 h. The specific experimental parameters are shown in Table 1.
2.4 Preparation of rGO/TNTs aerogel
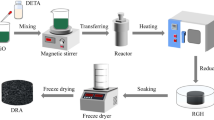
Overall, 2 mg of TNTs was added into 10 mL GO suspension (5 mg mL−1) and ultrasonicated to form a uniform dispersion. In order to promote the dispersion process, surfactant CTAB was added. The mixture was then transformed into a Teflon-sealed autoclave and reacted at 180 °C for 6 h. The obtained wet gel was then exchanged by tert-butanol during aging and followed by freeze drying. The rGO/TNTs aerogel was then obtained and denoted as rGO/TNTs-C. The sample without addition of CTAB was named rGO/TNTs. rGO aerogel was also prepared in the same condition without incorporation of TNTs. Fig. 1 schematically illustrates the preparation process.
2.5 Sample characterization
Fourier transform infrared (FT-IR) spectra of TNTs and rGO/TNTs aerogel were recorded by a FT-IR spectrometer (Nicolet 380) with KBr pellets in the wave number range of 4000–400 cm−1. The phase compositions of the samples were analyzed by X-ray diffractometer (XRD, D8 Advance, Bruker AXS, Germany) with Cu Kα radiation at a scan rate of 0.02° s−1. The morphologies of the samples were observed by field emission scanning electron microscope (SEM, JSM-7800F). Transmission electron microscope (TEM) was carried out on a JEOL JEM-2100F electron microscope at an acceleration voltage of 200 kV. The specific surface area and porosity of the aerogels were characterized by the pore structure analyzer (3H-2000PS1) and the samples were outgassed at 180 °C for 3 h before tests. Specific surface area and pore size distribution of the samples were calculated according to the Brunauer–Emmett–Teller (BET) method and Barrett–Joyner–Halenda method, respectively.
2.6 Adsorption experiments
The adsorption of MB from aqueous solution was conducted by batch adsorption experiments at 298 K. In order to avoid the interference caused by photocatalysis, the adsorption process was carried out in the darkness. The concentration of MB was detected by UV–vis spectrophotometer (TU-1900) at λmax of 665 nm. The amount of MB adsorbed at time t (qt, mg g−1) and at equilibrium (qe, mg g−1) and the removal efficiency (%) were calculated by the following equations, respectively:
where C0, Ct, and Ce are initial, t time and equilibrium concentrations of MB (mg L−1), respectively. m is the weight of the adsorbent (g) and V is the volume of MB solution (L).
3 Results and discussion
3.1 Composition and phase analysis
Figure 2a shows the FT-IR spectra of GO, TNTs, and rGO/TNTs aerogel. The absorption peaks at 3420 and 1080 cm−1 are assigned to the stretching vibration and bending vibration of –OH groups in GO and the absorption band at 1740 cm−1 is assigned to the stretching vibration of C=O groups, which indicate that there are numerous hydroxyl and carbonyl functional groups on the obtained GO. The formation of TNT is certified by the presence of the absorption peaks at 916 and 470 cm−1, which are assigned to the Ti–O stretching vibration in OTi (OH)2 molecule [40, 41] and the vibration of Ti–O in TiO6 octahedrons [42, 43]. The absorption peak at 470 cm−1 can also be found in the rGO/TNTs aerogel, verifying the successful incorporation of TNTs. The decrease of the peak at 3400 cm−1 indicates that GO is reduced in the rGO/TNTs aerogel [26] during the hydrothermal process. The crystalline structures of GO, TNTs, and rGO/TNTs aerogels are studied by XRD. It can be seen from Fig. 2b that the diffraction peak at 12° is attributed to the (0 0 2) plane of GO and the interlayer spacing is calculated to be 0.73 nm by the Bragg equation [26]. The XRD spectrum of TNTs is consistent with the standard spectrum of monoclinic Na2Ti3O7 (PDF 72–0148) and the peak at 10° is the typical diffraction peak of the layered titanate [44]. The peak at 25° in rGO/TNTs aerogel is assigned to carbon which further verifies the reduction of GO.
3.2 Morphology analysis
Figure 3 shows the morphology of TNTs obtained under different preparation conditions. It can be seen that at low NaOH concentration (Fig. 3a, b), the products show poor morphology due to the incomplete reaction. With the increase of the NaOH concentration to 10 M, TNTs with a certain aspect ratio is initially formed, but there are still a lot of flakes. The reaction temperature shows no significant effect on the morphology of the product. It is worth noting that the obtained TNTs manifest a high aspect ratio with few impurities at faster stirring rate. This may be due to the fact that TiO2 and NaOH solution can sufficiently contact with each other at high stirring speed, leading to a complete reaction. Meanwhile, the strong shear force under high stirring speed benefits for the formation of nanotubes. The optimal reaction conditions for the preparation of TNTs are 10 M NaOH at 150 °C with a stirring rate of 860 rpm.
In order to explore the influence of the addition of TNTs on the structure of the composite aerogel, the morphology of rGO, rGO/TNTs, and rGO/TNTs-C aerogels were investigated, respectively. As shown in Fig. 4, the original rGO aerogel has an irregular 3D porous structure with a large pore size. The addition of TNTs significantly reduces the pore size of the aerogel, which may result from the hydrogen bonding between TNTs and rGO aerogel. The obtained rGO/TNTs aerogel still shows an irregular 3D porous structure, but the TNTs in the aerogel are partially agglomerated. The rGO/TNTs-C aerogel obtained after CTAB modification manifests a more uniform pore structure, and TNTs are homogeneously distributed in the aerogel matrix with strong interfacial bonding (Fig. 4c). The coexistence and uniform distribution of Ti, C, Na, and O elements in rGO/TNTs-C aerogel are validated by elemental mapping analysis, which further verifies the successful doping of TNTs. Thus, it can be safely concluded that the incorporation of TNTs shows obvious effect on the structure of rGO aerogel. The oxygenic groups, especially at the edges and wrinkles of the rGO aerogel can effectively interacted with the hydroxy groups in TNTs, thus leading to the regulation of the porous structure. Figure 5 shows the TEM images of the rGO/TNTs-C aerogel. It can be seen that the prepared TNTs are uniformly dispersed in the aerogel matrix. The lattice fringes observed with the interplanar spacing of 0.347 nm are assigned to the (1 1 0) plane of TNTs.
3.3 Pore structure analysis
Figure 6 shows the N2 adsorption–desorption isotherms and the corresponding pore size distributions of rGO, rGO–TNTs, and rGO–TNTs-C aerogels. It can be seen that all the isotherms of the aerogels basically belong to type IV as defined by IUPAC, which is the characteristic of mesoporous materials [45]. Besides, they are in accordance with the type H3 hysteresis loops, indicating the slit-shaped pores in the composites [46], and their specific surface areas are 183.2, 425.9, and 536.2 m2 g−1, respectively. The specific porous characteristics of the aerogels are shown in Table 2. rGO aerogel shows the lowest specific surface area, which is caused by the uncontrollable agglomeration between the two-dimensional graphene sheets in the process of assembling into three-dimensional network. After the addition of TNTs, the specific surface area remarkably increases and shows further improvement with the modification of CTAB. This may be due to the fact that suitable incorporation of TNTs benefits for supporting of the network structure, and can effectively hinder the π–π bonding interaction between graphene sheets, thereby prohibiting their aggregation. The modification of CTAB ensures the uniform distribution of TNTs in the matrix and further optimizes the pore structure.
3.4 Adsorption property
The adsorption properties of TNTs, rGO, and rGO/TNTs-C aerogels toward MB are systematically studied. It can be seen from Fig. 7 that pure TNTs show poor adsorption property to MB. For rGO aerogel, although the adsorption rate is slow, the adsorption capacity at equilibrium is relatively high. The rGO/TNTs-C aerogel exhibits the best adsorption property with a faster adsorption rate. Their adsorption capacities are 198.6, 359.4, and 380.7 mg g−1 with the removal efficiency of 49.7%, 89.8%, and 92.2%, respectively. The results indicate that the incorporation of TNTs can effectively improve the adsorption property of rGO aerogel through regulation of the porous structure. rGO/TNTs-C aerogel with high specific surface area can provide more active sites and increase the contact area between MB and the adsorbent, thereby promoting the adsorption process. Meanwhile, with the increase of adsorbent dosage, the adsorption rate of rGO/TNTs-C aerogel obviously increases and the removal efficiency also increases from 92.2 to 98.0%. This can be attributed to the increase of the adsorbent specific surface area and availability of more adsorption sites [47].
3.5 Adsorption kinetics
In order to identify the mechanism and the potential rate-controlling steps involved in the adsorption process, two kinetic models, pseudo first order and pseudo second order, are adopted to simulate and analyze the adsorption kinetics of the rGO/TNTs aerogels. The equation for pseudo first-order kinetics was introduced initially by Lagergren [48]. It is generally used in the form proposed by Ho and McKay [49]
in which k1 (min−1) is the pseudo first-order rate constant and t (min) is the time.
The equation for pseudo second-order kinetics [50, 51] is generally employed in the form proposed by Ho and McKay [49]
in which k2 (g mg −1 min−1) is the pseudo second-order rate constant.
Figure 8 shows the linear fitting plots of pseudo first-order and pseudo second-order kinetic models for adsorption of MB by TNTs, rGO, and rGO–TNTs-C aerogels with the adsorbent dosage of 1 mg and rGO–TNTs-C aerogel with dosage of 10 mg. The parameters qe and k1 for the pseudo first-order kinetic model (Eq. 4) could be obtained from the intercept and slope of the plots of ln(qe − qt) versus t. It can be seen that the correlation coefficient of TNTs, rGO, and rGO–TNTs-C aerogels with 1 mg is 0.9785, 0.9947, and 0.9930, which suggests that the adsorption of MB fairly follows the pseudo first-order kinetic model. Besides, the calculated qe1 is also consistent with the experimental value qexp, further verifies that it is suitable for rGO and rGO–TNTs-C aerogels. The results also manifest that the adsorption of MB is a dispersion controlled process in this system. For the pseudo second-order kinetic model (Eq. 5), the qe2 and k2 can be obtained from the slope and intercept of the plots of t/qt versus t. It can be seen that the correlation coefficient is relatively low and the calculated qe2 also shows a big variation from the experimental value qexp, indicating that pseudo second-order kinetic model is not suitable for this system and chemisorption is not the rate-controlling step. So, when the adsorbent dosage is relatively low, a dispersion process is found to be the rate-limiting step [49]. With the increase of the rGO–TNTs-C aerogel content, the adsorption rate has been obviously improved and the adsorption kinetics have also changed. It can be seen that both of the kinetic models fit well and the pseudo second-order kinetic model is better than the pseudo first-order kinetic model, which manifests that chemical sorption gradually becomes a rate-controlling step with the increase of adsorbent dosage. Thus, it can be safely concluded that when the adsorbent dosage is low, the adsorption process of MB is mainly controlled by the dispersion of MB molecules to the active sites of the aerogels. With the increase of the adsorbent content, chemical sorption or chemisorption involving valency forces through sharing or exchange of electrons such as the π–π chemical adsorption or the reaction of the polar functional groups between MB and rGO–TNTs-C aerogel becomes the rate-limiting step. The kinetic parameters obtained from these models are given in Table 3.
4 Conclusions
In summary, TNTs are successfully incorporated into rGO aerogel to regulate the pore structure of the aerogel, resulting in rGO/TNTs aerogel with high specific surface area and favorable MB adsorption property. The effects of TNTs on the morphology, pore structure, and adsorption properties of rGO/TNTs aerogel are studied and the main conclusions are summarized as follows:
(1) The doping of TNTs can effectively prevent the agglomeration between graphene sheets in the process of assembling into three-dimensional network, thus resulting in a mesoporous rGO/TNTs aerogel with high specific surface area. The modification by CTAB ensures the uniform distribution of TNTs in the matrix and further optimizes the pore structure.
(2) rGO/TNTs-C aerogel shows the best adsorption property toward MB and the maximum adsorption capacity can be 380.7 mg g−1 with a quite high removal efficiency of 92.2%. Besides, the adsorption kinetic analysis indicates that the dispersion of MB molecules to the active sites of the aerogel is the rate-limiting step when the adsorbent dosage is low. With the increase of adsorbent content, chemical sorption or chemisorption involving valency forces through sharing or exchange of electrons such as the π–π chemical adsorption or the reaction of the polar functional groups between MB and rGO–TNTs aerogel becomes the rate-limiting step. In brief, the rGO–TNTs aerogel with favorable adsorption property shows great potential in water treatment.
References
Acemioglu B (2005) Batch kinetic study of sorption of methylene blue by perlite. Chem Eng J 106(1):73–81
Al-Degs YS, Khraisheh MAM, Allen SJ, Ahmad MN (2009) Adsorption characteristics of reactive dyes in columns of activated carbon. J Hazard Mater 165(1–3):944–949
Robinson T, McMullan G, Marchant R, Nigam P (2001) Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour Technol 77(3):247–255
Sedighi F, Esmaeili-Zare M, Sobhani-Nasab A, Behpour M (2018) Synthesis and characterization of CuWO4 nanoparticle and CuWO4/NiO nanocomposite using co-precipitation method; application in photodegradation of organic dye in water. J Mater Sci Mater Electron 29(16):13737–13745
Vinodhini PA, Sudha PN (2016) Removal of heavy metal chromium from tannery effluent using ultrafiltration membrane. Text Cloth Sustain 2(1):5
Lim SH, Rhee SW (2011) Drip and evaporation method for dye-TiO2 nano junction formation in dye sensitized solar cells. RSC Adv 1(3):518–522
Padmavathi R, Minnoli M, Sangeetha D (2014) Removal of heavy metal ions from waste water using anion exchange polymer membranes. Int J Plast Technol 18(1):88–99
Pandit P, Basu S (2004) Dye and solvent recovery in solvent extraction using reverse micelles for the removal of ionic dyes. Ind Eng Chem Res 43(24):7861–7864
Sala M, Lopez-Grimau V, Gutierrez-Bouzan C (2016) Photoassisted electrochemical treatment of azo and phtalocyanine reactive dyes in the presence of surfactants. Materials 9(3):12
Wang CC, Li J-R, Lv XL, Zhang YQ, Guo G (2014) Photocatalytic organic pollutants degradation in metal–organic frameworks. Energy Environ Sci 7(9):2831–2867
Lee JY, Chen CH, Cheng S, Li HY (2016) Adsorption of Pb(II) and Cu(II) metal ions on functionalized large-pore mesoporous silica. Int J Environ Sci Technol 13(1):65–76
Li J, Gong JL, Zeng GM, Zhang P, Song BA, Cao WC et al. (2018) Zirconium-based metal organic frameworks loaded on polyurethane foam membrane for simultaneous removal of dyes with different charges. J Colloid Interf Sci 527:267–279
Ghaffar A, Zhang L, Zhu X, Chen B (2018) Porous PVDF/GO nanofibrous membranes for selective separation and recycling of charged organic dyes from water. Environ Sci Technol 52(7):4265–4274
Rafatullah M, Sulaiman O, Hashim R, Ahmad A (2010) Adsorption of methylene blue on low-cost adsorbents: a review. J Hazard Mater 177(1–3):70–80
Sharma YC, Uma, Sinha ASK, Upadhyay SN (2010) Characterization and adsorption studies of Cocos nucifera L. activated carbon for the removal of methylene blue from aqueous solutions. J Chem Eng Data 55(8):2662–2667
Hu N, Chen C, Tan J, Wang W, Wang C, Fan H et al. (2020) Enhanced adsorption of methylene blue triggered by the phase transition of thermoresponsive polymers in hybrid interpenetrating polymer network hydrogels. ACS Appl Polym Mater 2(8):3674–3684
Wahlstrom N, Steinhagen S, Toth G, Pavia H, Edlund U (2020) Ulvan dialdehyde-gelatin hydrogels for removal of heavy metals and methylene blue from aqueous solution. Carbohydr Polym 249:116841
Mishra SP, Patra AR, Das S (2020) Methylene blue and malachite green removal from aqueous solution using waste activated carbon. Biointerface Res Appl Chem 11(1):7410–7421
Deng W, Tang S, Zhou X, Liu Y, Liu S, Luo J (2020) Honeycomb-like structure-tunable chitosan-based porous carbon microspheres for methylene blue efficient removal. Carbohydr Polym 247:116736
Feng M, You W, Wu Z, Chen Q, Zhan H (2013) Mildly alkaline preparation and methylene blue adsorption capacity of hierarchical flower-like sodium titanate. ACS Appl Mater Interfaces 5(23):12654–12662
Geim AK, Novoselov KS (2007) The rise of graphene. Nat Mater 6(3):183–191
Zhang YB, Tan YW, Stormer HL, Kim P (2005) Experimental observation of the quantum Hall effect and Berry’s phase in graphene. Nature 438(7065):201–204
Fal’ko VI, Geim AK (2007) Graphene: emerging matter in two dimensions. Eur Phys J Spec Top 148:1–3
Kopelevich Y, Esquinazi P (2007) Graphene physics in graphite. Adv Mater 19(24):4559–4563
Nair RR, Blake P, Grigorenko AN, Novoselov KS, Booth TJ, Stauber T et al (2008) Fine structure constant defines visual transparency of graphene Science 320(5881):1308
Wang JL, Shi ZX, Fan JC, Ge Y, Yin J, Hu GX (2012) Self-assembly of graphene into three-dimensional structures promoted by natural phenolic acids. J Mater Chem 22(42):22459–22466
Wang RH, Xu CH, Sun J, Gao L (2014) Three-dimensional Fe2O3 nanocubes/nitrogen-doped graphene aerogels: nucleation mechanism and lithium storage properties. Sci Rep. 4:7
Qian YQ, Ismail IM, Stein A (2014) Ultralight, high-surface-area, multifunctional graphene-based aerogels from self-assembly of graphene oxide and resol. Carbon 68:221–231
Hoyer P (1996) Formation of a titanium dioxide nanotube array. Langmuir 12(6):1411–1413
Bavykin DV, Parmon VN, Lapkin AA, Walsh FC (2004) The effect of hydrothermal conditions on the mesoporous structure of TiO2 nanotubes. J Mater Chem 14(22):3370–3377
Yu JG, Yu HG (2006) Facile synthesis and characterization of novel nanocomposites of titanate nanotubes and rutile nanocrystals. Mater Chem Phys 100(2–3):507–512
Yu JG, Yu HG, Cheng B, Trapalis C (2006) Effects of calcination temperature on the microstructures and photocatalytic activity of titanate nanotubes. J Mol Catal A-Chem 249(1–2):135–142
Chen XB, Cao S, Weng XL, Wang HQ, Wu ZB (2012) Effects of morphology and structure of titanate supports on the performance of ceria in selective catalytic reduction of NO. Catal Commun 26:178–182
Mozia S, Borowiak-Palen E, Przepiorski J, Grzmil B, Tsumura T, Toyoda M et al. (2010) Physico-chemical properties and possible photocatalytic applications of titanate nanotubes synthesized via hydrothermal method. J Phys Chem Solids 71(3):263–272
Song FJ, Zhao YX, Zhong Q (2013) Adsorption of carbon dioxide on amine-modified TiO2 nanotubes. J Environ Sci 25(3):554–560
Kim M, Hwang SH, Lim SK, Kim S (2012) Effects of ion exchange and calcinations on the structure and photocatalytic activity of hydrothermally prepared titanate nanotubes. Cryst Res Technol 47(11):1190–1194
Rajeshwar K, Osugi ME, Chanmanee W, Chenthamarakshan CR, Zanoni MVB, Kajitvichyanukul P et al. (2008) Heterogeneous photocatalytic treatment of organic dyes in air and aqueous media. J Photochem Photobiol C-Photochem Rev 9(4):171–192
Niu HY, Wang JM, Shi YL, Cai YQ, Wei FS (2009) Adsorption behavior of arsenic onto protonated titanate nanotubes prepared via hydrothermal method. Microporous Mesoporous Mater 122(1–3):28–35
Hirata M, Gotou T, Horiuchi S, Fujiwara M, Ohba M (2004) Thin-film particles of graphite oxide 1: high-yield synthesis and flexibility of the particles. Carbon 42(14):2929–2937
Shao LM, Zhang LN, Chen MH, Lu H, Zhou MF (2001) Reactions of titanium oxides with water molecules. a matrix isolation FTIR and density functional study. Chem Phys Lett 343(1–2):178–184
Chen YC, Lo SL, Kuo J (2010) Pb(II) adsorption capacity and behavior of titanate nanotubes made by microwave hydrothermal method. Colloids Surf A 361(1–3):126–131
Wang W, Zhang JW, Huang HZ, Wu ZS, Zhang ZJ (2008) Surface-modification and characterization of H-titanate nanotube. Colloids Surf A 317(1–3):270–276
Wang H, Wang HL, Jiang WF, Li ZQ (2009) Photocatalytic degradation of 2,4-dinitrophenol (DNP) by multi-walled carbon nanotubes (MWCNTs)/TiO2 composite in aqueous solution under solar irradiation. Water Res 43(1):204–210
Liu F, Jin YJ, Liao HB, Cai L, Tong MP, Hou YL (2013) Facile self-assembly synthesis of titanate/Fe3O4 nanocomposites for the efficient removal of Pb2+ from aqueous systems. J Mater Chem A 1(3):805–813
Tu TH, Cam PTN, Huy LVT, Phong MT, Nam HM, Hieu NH (2019) Synthesis and application of graphene oxide aerogel as an adsorbent for removal of dyes from water. Mater Lett 238:134–137
Sing KSW, Everett DH, Haul RAW, Moscou L, Pierotti RA, Rouquerol J et al. (1985) Reporting physisorption data for gassolid systems with special reference to the determination of surface area and porosity (Recommendations 1984). Pure Appl Chem 57:603–619
Royer B, Cardoso NF, Lima EC, Vaghetti JCP, Simon NM, Calvete T et al. (2009) Applications of Brazilian pine-fruit shell in natural and carbonized forms as adsorbents to removal of methylene blue from aqueous solutions—kinetic and equilibrium study. J Hazard Mater 164(2–3):1213–1222
Lagergren SY (1898) Zur Theorie der sogenannten Adsorption gelöster Stoffe. K sven vetenskapsakad handl 24:1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34(5):451–465
Blanchard G, Maunaye M, Martin G (1984) Removal of heavy metals from waters by means of natural zeolites. Water Res 18(12):1501–1507
Gosset T, Trancart JL, Thévenot DR (1986) Batch metal removal by peat. Kinet Thermodyn Water Res 20(1):21–26
Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 51772202).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, H., Gao, B., Yuan, W. et al. Modification of graphene aerogel with titania nanotubes for efficient methylene blue adsorption kinetics. J Sol-Gel Sci Technol 97, 271–280 (2021). https://doi.org/10.1007/s10971-020-05449-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-020-05449-y