Abstract
Synthesizing multifunctional films to apply to the glasses for optical and advanced engineering applications, especially for concentrating solar power application, is a severe challenge. Herein, we report an anti-reflection SiO2 thin films with super-hydrophobic property. The SiO2 thin films are successfully synthesized on the soda lime glass by sol-gel spin-coating method, using tetraethylorthosilicate as a precursor and octadecyltrichlorosilane as a modification agent. The properties of films were characterized by fourier transform infrared spectra, field emission scanning electron microscopy, UV–VIS–NIR spectrophotometer and water contact angles apparatus. The results indicate that anti-reflection SiO2 thin films have excellent visible light transmittance ranging from 97.8 to 103.4% with treatment time in tetraethylorthosilicate solution increasing from 1 min to 3 h. Moreover, such film exhibits super-hydrophobic property with water contact angles of 150.6° when treatment time is 3 h, owning to a hierarchical structure of the SiO2 nanoparticles (~50 nm) and microscale dendritic aggregates. Fortunately, anti-reflection octadecyltrichlorosilane-treated SiO2 films with super-hydrophobic property display a promising application in various fields, especially in concentrating solar power for reducing specular reflectance efficiency.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Glasses are widely applied to various fields and offer many desirable properties, including optical clarity and overall visual appearance. The reduction in light reflection from the surface of a glass substrate may be desirable for routine applications, such as building glasses, windscreens, video display panels, flat-panel displays, photodetectors, infrared sensors and lenses as well as for advanced engineering applications [1,2,3,4]. Therefore, coating of an anti-reflection layer emerges as required. Righeira [5] had designed novel multifunctional coatings that present anti-reflection, scratch, and abrasion resistance properties. That was achieved by a coating structure with a composite top layer comprising at least one type of metal oxide (ZrO2 or TiO2) or silane compound with low-refractive-index SiO2 layer. However, since the enhancement of transmittance is selective toward specific wavelengths, the coating exhibits a high transmittance only in a narrow wavelength range [6]. As such, broadband anti-reflection coatings are highly desired to achieve an optimal performance. Lei [7] revealed a visible wavelength-independent anti-reflection coating generated from assembled SiO2 particles modified with tetraethoxysilane. The resulted coating surface morphology resembled moth-eye-like nanostructure that exhibited visible wavelength-independent transmittance enhancement for substrate.
Apart from anti-reflection, the wettability is also a very important property of glass surfaces, which is governed by both the chemical composition and geometrical microstructure of the surfaces [8]. The two necessary conditions of the preparation of super-hydrophobic solid surface included low energy surface and special micro surface roughness [9, 10]. In particular, many researches had been done to investigate the super-hydrophobic biological surfaces [11, 12], especially the surface of lotus leaves [13]. The leaf of the Lotus plant achieved super-hydrophobic property using a hierarchical structure with roughness on both the microscale and nanoscale. Daniel [14] had presented a hierarchically structured surfaces with Lotus-effect properties using micro-sized and nano-sized hydrophobic silica particles and a simple spray method. The above results expounded that roughness increase due to existence of the micro-structure and nano-structure on the surface, which will further be conducive to the super-hydrophobic property. Besides these physical approaches, the modification of rough surfaces is another way to promote super-hydrophobic behavior [15,16,17,18]. Li [19] found that oleic acid-modified SiO2 nanoparticles were capable of dispersing through esterification. In study of Jeong [20], hydrophobic porous silica has been prepared by surface modification of TEOS (tetraethylorthosilicate) wet gel with 6 and 12 vol.% of trimethylchlorosilane. The results indicated that modified dried gels had a surface area of 950–1000 m2/g (average pore size 120 Å), compared to the non-modified surface which had a surface area of 690 m2/g (average pore size 36 Å).
Much has been reported for modification agent. It is well-known that fluorine is effective for lowering the surface free energy chemically because fluorine has a small atomic radius and the biggest electronegativity among atoms, so it forms a stable covalent bond with carbon. Takashi [21] found that the dynamic contact angle (CA) of water on its surface was 119°, which corresponds to a surface free energy of 6.7 mJ/m2. This value was considered to be the lowest surface free energy of any solid, based on the hexagonal closed alignment of –CF3 groups on the surface. Xiu [22] proposed that after treating the film surface with a fluoroalkylsilane, the surface became super-hydrophobic with a CA near 170° and a CA hysteresis < 10°. Nevertheless, fluoride modification agent is too expensive to apply to various fields. Guo [23] described a super-hydrophobic silica film prepared by means of Sol–gel and self-assembly techniques, with a very high water CA (155–157°) and a small sliding angle (3–5°) after being modified with perfluorooctyltrichloromethoxysilane. This result could be ascribed to dramatically decrease of surface energy due to the existence of chloride on film surface, which presented similar electron-shell structure and electronegativity.
In the present study, we present anti-reflection OTS-treated SiO2 thin films with super-hydrophobic property. Although some researchers have investigated the super-hydrophobic SiO2 films modified with OTS, there are four highlights in this article as follow: (1) comparing to the current studies, we have given a better understanding of the formation of SiOH groups on SiO2 films surface and the schematic of covalent bond formation between the OTS monolayer and the SiO2 thin films, (2) Comparing to the published researches about transmittance (93.4–99.5%), these films present higher transmittance (97.8–103.4%) in visible region (400–800 nm), (3) when treatment time is 3 h, these films not only exhibit super-hydrophobic property with WCAs of 150.6° but also show excellent anti-reflection effect with transmittance of 97.8–100.9% in visible region and (4) these anti-reflection OTS-treated SiO2 films with super-hydrophobic property present a promising application in various fields, especially in concentrating solar power (CSP) for reducing specular reflectance efficiency.
2 Experimental
2.1 Materials
In this work, TEOS (C8H20O4Si, 93.2–93.6%), absolute ethanol (C2H5OH, purity >99.7%), ammonium hydroxide (NH3·H2O, 36–38 wt%) and octadecyltrichlorosilane (OTS, C18H37Cl3Si, purity > 95.0%) were used as the initial materials, supplied by Sinopharm Chemical Reagent Co. Ltd., China. Besides, soda lime silicate glasses (25 × 25 × 1 mm) were cut to use as substrates.
2.2 Preparation of OTS-SiO2 composite thin films
SiO2 thin films were successfully synthesized on soda lime glass substrates by sol–gel spin coating method shown in Fig. 1. Firstly, the gel was obtained by mixing absolute ethanol, ammonium hydroxide and TEOS, corresponding to the volume of 35, 2 and 2 mL, respectively. And then, the gel was spin-coated on the substrate once with a screw rotation rate of 750 rpm for 15 s and subsequently at 5000 rpm for 30 s. After being calcined at 200 °C for 60 min, SiO2 thin films were gained. Finally, after being immersed in 20 mmol/L OTS solution for 0, 1/60, 3 and 15 h, respectively, and subsequently curing at 150 °C for 30 min, SiO2 porous thin films were successfully modified with OTS.
2.3 Characterization
The Fourier transform infrared spectra of SiO2 films before and after being modified with OTS for various time were recorded on a Fourier transform infrared spectrometer (FTIR, Nicolet6700, USA) in the frequency range from 4000 to 400 cm−1. The surface morphology of SiO2 films were observed by field emission scanning electron microscopy (FESEM, Zeiss Ultra Plus, Germany). The optical transmittance of SiO2 films deposited on soda lime glass substrate were examined by a SHIMADZU UV-2550 spectrophotometer, from 300 to 800 nm. In particular, the transmittance of soda lime glass substrate is used as the baseline. The water contact angles (WCAs) of SiO2 thin films were measured at ambient temperature by an automatic CA measure device (Dataphysics OCA35, Germany). Water droplets were generated automatically, with a volume of 5 μL. Typically, the average value of measurements at five different positions of the SiO2 thin films’ surface was adopted as the value of the WCAs.
3 Results and discussion
3.1 FTIR analysis
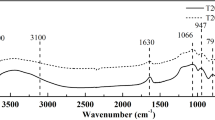
Figure 2a–d are the FTIR spectrums of SiO2 films after being modified with OTS for 0, 1/60, 3 and 15 h, respectively. Vibrational assignments and their corresponding frequencies are listed in Table 1 [24, 25]. Two main bands at 2851 cm−1 and 2921 cm−1 are clearly observed in the high frequency region [26] in Fig. 2b–d. These are assigned to the symmetric mode and asymmetric mode of –CH2 of the OTS alkyl chains, separately. The peak frequencies for the ν s(CH2) and ν a(CH2) modes provide insight into alkyl chain packing in terms of crystallinelike or liquidlike structure. The peak frequency for the ν a(CH2) mode of an all-trans extended alkyl chain in a crystalline environment is reported to be ca. 2915–2921 cm−1, and that for the ν s(CH2) mode is reported to be ca. 2846–2851 cm−1 [27, 28]. So the peak frequencies (2851 and 2921 cm−1) observed for OTS are crystallinelike structure, suggesting that alkyl chains of OTS possess intermediate conformational order. Besides, the FTIR spectrums of all samples show absorption bands at 807 cm−1 and 1080 cm−1 in the high-frequency region (Fig. 2a–d) corresponding to symmetric vibration and asymmetric vibration of Si–O–Si, respectively [29]. This result indicates the formation of a cross-linked siloxane structure. Meantime, comparing to sample modified with OTS for 1 min, sample modified with OTS for 3 h presents more intense bands at both 2851 cm−1 and 2921 cm−1, as shown in Fig. 2b, c. This result can be attributed to the fact that with increasing treatment time, siloxane bond formation has occurred not only between the OTS and SiO2 surface silanols but also between at least a fraction of the head groups of the OTS molecules. However, in Fig. 2c, d, bands at both 2851 cm−1 and 2921 cm−1 are greatly similar, suggesting that SiO2 films have been absolutely modified by OTS with modification time of 3 h. Therefore, when treatment time with OTS is over 3 h, the samples will present a similar bands at both 2851 cm−1 and 2921 cm−1, because siloxane bond formation has occurred absolutely not only between the OTS and SiO2 surface silanols but also the OTS molecules.
3.2 Surface microstructure
The SEM surface images of SiO2 films after being modified with OTS for 0, 1/60, 3 and 15 h are shown in Fig. 3a–d, respectively. In Fig. 3a, SiO2 films present a porous three-dimensional network structure without modifying with OTS. The network structure is formed by a series of microscale dendritic aggregates comprised SiO2 nanoparticles. In particular, SiO2 nanoparticles present a spheroidal structure with mean diameter of 50 nm. Xue [30] revealed that super-hydrophobic silica film was prepared by means of acid/base two step sol–gel and self-assembly techniques with tetraethoxysilane (TEOS) as precursor. The SiO2 films, comprising SiO2 nanoparticles (30–120 nm), had a high porosity with average pore size of 100–600 nm. And the porous structure was also formed by a series of dendritic aggregates comprised SiO2 nanoparticles. This result is similar to that in this paper, due to the same preparation technique. In Fig. 3b, the dimension of dendritic aggregates of SiO2 thin films increase after being modified with OTS for 1 min. Comparing to sample without modification, the mean diameter of SiO2 nanoparticles after being modified with OTS for 1 min remains the same. Significant difference in surface structure is shown in Fig. 3a, b, due to the hydration of OTS and reaction between OTS and SiO2 thin films surface. In Fig. 3c, the porosity of SiO2 thin films with treatment time of 3 h decline, indicating that a self-assembled monolayer has nearly formed on the surface of SiO2 thin films. This result is in accordance with that in Fig. 2c. Figure 3d presents the surface microstructure of the SiO2 thin films modified with OTS for 15 h. It’s obvious that mean diameter of SiO2 nanoparticles remains the same but the dimensions of dendritic aggregates increase. The surface structure is more compact comparing with the sample while treatment time is 3 h, due to reaction not only between OTS and SiO2 thin films surface but also the between head groups of the OTS molecules [28]. Moreover, the relationship between the relative amount of pores and the pore size for SiO2 thin films modified with OTS for various reaction time, ranging from 0 to 15 h, is shown in Fig. 4. Without modifying with OTS, the pore size is mainly 35–55 nm (Fig. 4a). However, an obvious decline of the pore size can be seen in Fig. 4c (25–40 nm) and Fig. 4d (20–35 nm). Besides, the mean pore size of samples treated with OTS for 0, 1/60, 3 and 15 h decreases, corresponding to 44.63, 42.54, 34.14 and 27.93 nm, respectively.
In particular, the formation of SiOH groups on SiO2 films surface and the schematic of covalent bond formation between the OTS monolayer and the SiO2 thin films are demonstrated in Fig. 5a, b, respectively. As shown in Fig. 5a, the hydration chemistry of on high surface area silica (nanoparticles of SiO2) occurs to form SiOH groups. Particularly, the silica surface can be dehydrated and rehydrated reversibly until a temperature of about 400 °C is reached, after which rehydration becomes extremely slow [31]. Meantime, hydrogen bonds occurs between the SiOH groups on the silica surface. Besides, physical absorption reaction happens between surface silanols and the water. Figure 5b shows the reactions between the OTS and SiO2 surface. Firstly, hydration of OTS molecule takes place to form three silanol groups, because it contains three chlorine atoms, which can be hydrolyzed by trace water in solution [31]. Secondly, the dehydration reaction occurs between silanol group of OTS and SiO2 films surface silanols, resulting in covalent bond formation between the monolayer and the SiO2 film surface. Thirdly, OTS molecules crosslink to form polymeric species during film curing at 150 °C. Vapor-phase water can penetrate even a tightly packed, fully covered OTS surface, despite the hydrophobicity observed macroscopically. Water which penetrates the outer alkyl surface binds at the interfacial region to silanol groups attached to both the silica surface and the OTS molecules. Curing at 150 °C, which resulted in cross linking of organosilane molecules and covalent bond formation to the silica surface, decreases the number of silanol groups available for water adsorption. Thus, uncured OTS monolayers actually increase interfacial moisture adsorption relative to the unsilanized surface. After curing, water absorption at the OTS/silica interface is greatly decreased, since the number of silanols present at the interface is reduced [32].
The formation of SiOH groups on SiO2 films surface (a) and the schematic of covalent bond formation between the OTS monolayer and the SiO2 thin films (b). Hydrolysis of the chloride group by trace amounts of water in solution to silanol is followed by condensation with surface silanols, resulting in covalent bond formation between the monolayer and the substrate. Besides, OTS molecules can also cross-link to form polymeric species during film curing
3.3 Transmittance
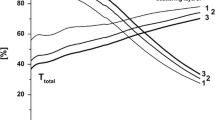
Figure 6 presents the optical transmittance of uncoated glass and SiO2 films after being modified with OTS for 0, 1/60, 3 and 15 h. In particular, the transmittance of soda lime glass substrate is used as the baseline. As presented in Fig. 6a, the uncoated glasses have a high visible light (400–800 nm) transmittance, ranging from 88.4 to 90.3%. Fortunately, glass substrate with SiO2 thin films without modifying with OTS exhibits a higher transmittance within visible region, ranging from 101.3 to 103.4% (Fig. 6b). The measurement optical transmittance exceeds 100%, indicating that the refractive indices of SiO2 films is smaller than that of soda lime glass substrate. In this case, the SiO2 thin films present excellent anti-reflection effect [33]. Anti-reflection films reduce the intensity of reflection and increase the quality of optical lens systems [5].
The basic principle of optical coatings can easily be understood as follow [34]. The reflected light from the air-film and film-substrate interfaces must interfere destructively to maximize the light transmission into the transparent substrate (Fig. 7). In this case, the light amplitudes reflected at both interfaces must be equal, that is,
where with n o, n f, and n s are the refractive indices of air, film, and substrate respectively. Besides, the optical path length must be chosen for the reflected wave to interfere destructively, namely, the film thickness must be 1/4 of a reference wavelength in the optical medium. In this experiment, the refractive indices of glass substrates n s is 1.52. It is obvious that higher transmittance can be achieved when the refractive indices of films is closer to 1.23, according to Eq. (1). The prepared SiO2 thin films present a porous structure (Fig. 3) due to the fact that the films are made up of a series of dendritic aggregates comprised SiO2 nanoparticles synthesized via sol–gel spin-coating method. Such an appropriate porous structure is contributed to reduce the reflection and scattering to induce anti-reflection effect [35, 36]. The refractive indices of SiO2 thin films n f is close to 1.23, that is, the SiO2 thin films present anti-reflection property [34].
From Fig. 6b, c we know that transmittance of SiO2 films after being modified with OTS for 1 min is lower than that of the sample without OTS treatment, varying from 99.8 to 102.6%. Besides, comparing to the sample modified with OTS for 1 min, transmittance of SiO2 films after being modified with OTS for 3 h decrease, but it is still so high, ranging from 97.8 to 100.9% (Fig. 6d). Nevertheless, transmittance of the sample modified with OTS for 15 h decline rapidly (Fig. 6e). In particular, in visible region (400–630 nm), transmittance is lower than that of the uncoated glass, as shown in Fig. 6a, e. Naganuma [37] had investigated the effect of glass particle size on the light transmittance of epoxy matrix composites, and found that reasonably controlling the glass particle size (porosity) can improve the light transmittance. In this paper, the transmittance decreases by increasing reaction time. This is because anti-reflection effect of SiO2 thin films gradually disappears resulting from the decline of porosity as presented in Fig. 3. After immersing in OTS solution, the self-assembled process occurs. The SiO2 nanoparticles reunite to form dendritic aggregates and the aggregates gradually grow up. So the SiO2 thin films become more compact by increasing reaction time, further resulting in decrease of porosity. Besides, when reaction time is too long, a monolayer of OTS would be formed on the surface of SiO2 films (shown in Fig. 3d), resulting in increasing the thinness of the films. In this case, absorbance of visible light increases, leading to the decline of transmittance [6, 7].
3.4 Hydrophobic properties of SiO2 films modified with OTS
The wettability [38] is omnipresent in nature and also plays a crucial role in various industrial processes. Importantly, the wettability depended on the surface chemical composition and roughness. Usually, the CA is used to assess the wettability. In this paper, the surface wettability of SiO2 thin films after being modified with OTS for various time was investigated by measuring the WCAs on the surface of the thin films. Water droplets were generated automatically, with a volume of 5 μL. Typically, the average value of measurements at five different positions of the SiO2 thin films surface was adopted as the value of the WCAs. The optical images of water spreading on the surface of SiO2 thin films after OTS treatment for 0, 1, 30 and 180 min are shown in Fig. 8a–d, respectively. In Fig. 8a, WCA is 9.7°, indicating that SiO2 film without OTS treatment presents a nice hydrophilic property. Nevertheless, in Fig. 8b, c, the samples exhibit hydrophobic property with WCAs of 117.1° and 131.6° while being treating for 1 and 30 min, respectively. In addition, when treatment time is 3 h, SiO2 film shows super-hydrophobic property with WCAs of 150.6°.What’s more, supplementary experiments have been done to investigate the effect of treatment time in OTS solution on WCAs, and the results are presented in Fig. 9. It is obvious that the more the treatment time, the larger WCAs. In particular, WCAs of the SiO2 film rapidly increases and is close to the limiting value after ~3 h of immersion time. The final WCA of SiO2 film is about 154.3°. This result in turn suggested that the OTS molecules have nearly reacted with the SiO2 films surface when treatment time is 3 h.
According to the formation mechanism of the alkylsiloxane monolayers, the OTS molecules in the organic solvent can be gradually adsorbed onto a water layer present on SiO2. Following physisorption, the trichlorosilane head groups are hydrolyzed to form trisilanols. Jae [39] proved that the silanols existed in a highly mobile hydrogen-bonded state. This led to important in-plane reorganizations of OTS molecules, thereby forming a uniform densely packed molecular island at the early stage of monolayer formation on the SiO2 substrate. Silanol head groups of this island then became grafted to the SiO2 substrate by irreversible cross linking to one another and covalent grafting to the substrate surface [40,41,42].
4 Conclusions
Anti-reflection SiO2 thin films with super-hydrophobic property were successfully synthesized on the soda lime glass by sol–gel spin-coating method, using TEOS as precursor and OTS as modification agent.
-
1)
Siloxane bond formation has occurred not only between the OTS and SiO2 surface silanols but also between at least a fraction of the head groups of the OTS molecules, to from a cross-linked siloxane structure.
-
2)
The SiO2 films display a three-dimensional network structure formed by number of dendritic aggregates comprised SiO2 nanoparticles with mean diameter of 50 nm. With increasing treatment time with OTS ranging from 0 to 15 h, mean diameter of SiO2 nanoparticles remains the same but the dimensions of dendritic aggregates increase and porosity decline.
-
3)
As treatment time with OTS increase from 1 min to 3 h, the optical transmittance of glasses with SiO2 thin films in visible light region (400–800 nm) decrease with a maximum value of around 103.4% and a minimum value of 97.8%, but is still drastically higher than that of uncoated glass, ranging from 88.4 to 90.3%. The results indicate that SiO2 thin films exhibit excellent anti-reflection property.
-
4)
The SiO2 thin films present super-hydrophobic property with WCAs of 150.6° when treatment time is 3 h. Fortunately, anti-reflection OTS-treated SiO2 films with super-hydrophobic property present a promising application in various fields, especially in CSP for reducing specular reflectance efficiency.
References
Lien SY, Wu DS, Yeh WC, Liu JC (2006) Sol Energy Mater Sol Cells 90:2710–2719
Chabas A, Lombardo T, Cachier H, Pertuisot MH, Oikonomou K, Falcone R, Verita M, Geotti-Bianchini F (2008) Build Environ 43:2124–2131
Prado R, Beobide G, Marcaide A, Goikoetxea J, Aranzabe A (2010) Sol Energy Mater Sol Cells 94:1081–1088
Xu X, Vignarooban K, Xu B, Hsu K, Kannan AM (2016) Renew Sustain Energy Rev 53:1106–1131
Carnegie MR, Sherine A, Sivagami D, Sakthivel S (2016) J Sol–gel Sci Technol 78:176–186
Li X, Gao J, Xue L, Han Y (2010) Adv Funct Mater 20:259–265
Yang L, Jiang H, Feng X, Shen Y, Jia L (2016) J Sol–gel Sci Technol 79:520–524
Lei F, Li S, Li Y, Li H, Zhang L, Zhai J, Song Y, Liu B, Lei J, Zhu D (2002) Adv Mater 14:1857–1860
Huang Y, Liu W, Luo G (2008) Polym Mater Sci Eng 24:13–16
Duan H, Xiong Z, Wang H, Zhao H, Gao D (2006) Chem Ind Eng 23:81–87
Barthlott W, Neinhuis C (1997) Planta 202:1–8
Koch K, Bohn HF, Barthlott W (2009) Langmuir 24:14116–14120
Ennaceri H, Alami HE, Brik H, Mokssit O, Khaldoun A (2014) Compos Mater Renew Energy Appl 10:1–4
Ebert D, Bhushan B (2012) J Colloid Interface Sci 368:584–591
Nuria G, Esperanza B, Julio G, Pilar T (2007) J Am Chem Soc 129:5052–5060
Zhang X, Shi F, Yu X, Liu H, Fu Y, Wang Z, Jiang L, Li X (2004) J Am Chem Soc 126:3064–3065
Nicolas M, Guittard F, Geribaldi S (2006) Langmuir 22:3081–3088
Guo M, Diao P, Cai S, Liu Z (2004) Chem J Chin Univ 25:547–549
Li Z, Zhu Y (2003) Appl Surf Sci 211:315–320
Jeong A-Y, Koo S-M, Kim D-P (2000) J Sol–gel Sci Technol 19:483–487
Nishino T, Meguro M, Nakamae K, Matsushita M, Ueda Y (1999) Langmuir 15:4321–4323
Xiu Y, Xiao F, Hess DW, Wong CP (2009) Thin Solid Films 517:1610–1615
Guo Z, Zhou F, Liu W (2006) Acta Chim Sci 64:761–766
Mirji SA (2006) Surf Interface Anal 38:158–165
Rouchon D, Rochat N, Gustavo F, Chabli A, Renault O, Besson P (2002) Surf Interface Anal 34:445–450
Abdelghani A, Hleli S, Cherif K (2002) Mater Lett 56:1064–1068
Mirji SA (2006) Colloids Surf A Physicochem Eng Asp 289:133–140
Cai M, Ho M, Pemberton JE (2000) Langmuir 16:3446–3453
Qasim M, Singh BR, Naqvi AH, Paik P, Das D (2015) Nanotechnology 26:1–14
Zai-lan X, Wu Y, Hao G, Li-yan C, Ai-Ju Z, Jin-zhang G (2008) J Northwest Norm Univ Nat Sci 44:65–69
Angst DL (1991) Langmuir 7:2236–2242
Oh T, Kim JW (2008) International conference on condition monitoring and diagnosis. 2008:276–279. doi:10.1109/CMD.2008.4580280
Jian M, Bingqi L, Wenshen H, Jun Y, Xinxin L (2009) Opt Tech 35:513–516
Walheim S, Schaffer E, Mlynek J, Steiner U (1999) Science 283:520–522
Yang D, Xu Y, Xu W, Wu D, Sun Y, Zhu H (2008) J Mater Chem 18:5557–5562
Zhang X-T, Sato O, Taguchi M, Einaga Y, Murakami T, Fujishima A (2005) Chem Mater 17:696–700
Naganuma T, Kagawa Y (1999) Acta Mater 17:4321–4327
Wang B, Feng J, Gao C (2005) Colloids Surf A Physicochem Eng Asp 259:1–5
Lee JP, Kim HK, Park CR, Park G, Kwak HT, Koo SM, Sung MM (2003) J Phys Chem B 107:8997–9002
Lercel MJ, Craighead HG, Parikh AN, Seshadri K, Allara DL (1996) J Vac Sci Technol A Vac Surf Films 14:1844–1849
Carson G, Granick S (1989) J Appl Polym Sci 37:2767–2772
McGovern ME, Kallury KMR, Thompson M (1994) Langmuir 10:3607–3614
Acknowledgements
The authors would like to express sincere thanks for the financial support from the National Natural Science Foundation of China (51372179), the Science and Technology Planning Project of Hubei Province (2014BAA136), the Hubei Province Foreign Science and Technology Project (2016AHB027).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Li, H., Li, N., Zhang, Y. et al. Anti-reflection OTS-treated SiO2 thin films with super-hydrophobic property. J Sol-Gel Sci Technol 83, 518–526 (2017). https://doi.org/10.1007/s10971-017-4458-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-017-4458-0