Abstract
A dysprosium aluminum garnet (DAG) nanopowder was synthesized by aqueous sol–gel method using Al powder, HCl and Dy(CH3COO)3·4H2O as raw materials. The dried amorphous gel was heat treated in the range of 800–1,200 °C. The influence of heat treatment on crystallization and phase transformation of the dried gel was investigated using X-ray diffractometery, scanning electron microscopy, thermogravimetry and differential thermal analysis and Fourier transform infrared spectroscopy. It was shown that the gel calcined from 900 to 1,200 °C resulted in the formation of a crystalline DAG nanopowder with particle size distribution ranges from 26 to 98 nm.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Dysprosium aluminum garnet (DAG) is one of the traditionally antiferromagnetic materials which have been intensively investigated for years due to its interesting magnetic properties [1]. In DAG, anisotropy energy is extremely large, and the anisotropy axes are in three different directions at different sites in the cubic lattice. In addition to this, the narrow spectral line makes it a suitable material for observing spectral changes due to relatively small magnetic interaction [2].
In nuclear fields, boron carbide and some rare-earth elements are used extensively as neutron absorbing materials or protective materials because they are characterized by huge neutron absorption cross-sections (e.g. promethium, samarium, europium, gadolinium, and dysprosium) [3]. It is said that boron carbide is the first choice for absorbing material used in fast breeder reactors [4]. However, this application is limited by its poor sinterability. Rare-earth oxides and lanthanide aluminium garnets have been used as sintering aids to increase pressureless sintered density of boron carbide. Goldstein et al. [5] prepared B4C/metal boride composites with B4C and rare-earth oxides, such composites exhibit a sintering aptitude higher than that of monolithic B4C ceramic. In our previous work, we present a Si3N4 ceramic with the dry gel of YAG as sintering aid, and the addition of YAG may improve the α → β transformation of Si3N4 particles, leading to an enhancement in the mechanical properties of this material [6].
Sol–gel technology has been developed for the fabrication of high quality nanopowders. For complex powders, it achieves ultra-homogeneous of the several components in a molecular scale. Dubnikova et al. [7] recently have synthesized lanthanide aluminium garnets by complexing metal ions with ethane-1,2-diol in an aqueous media.
In the present work, a dysprosium aluminum garnet (DAG) nanopowder was synthesized through an aqueous sol–gel method using Al powder, HCl and Dy(CH3COO)3·4H2O as raw materials. The crystallization, microstructural evolution and effects of heat treatment processes on microstructure were investigated.
The purposes to synthesize DAG nanopowder are to prepare antiferromagnetic material with tailorable microstructure and properties, and to supply a desirable sintering aid for the densification of B4C ceramics and to improve the neutron absorption properties of B4C in nuclear applications.
2 Experimental
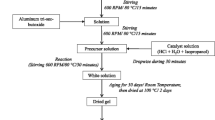
The precursor sol of DAG was prepared by using Al powder, HCl and Dy(CH3COO)3·4H2O as raw materials. The calculate amount of Al powder was firstly dissolved in HCl solution with molar concentration of 1–2 M. The precursor solution was refluxed at 90 °C for 5–8 h to dissolve the Al powder completely and then condensed at 80 °C for 2–3 h. The clear polyaluminium chloride sol was obtained. Based on the stoichiometric ratio of Dy3Al5O12, dysprosium acetate was added to the polyaluminium chloride sol. The solution was stirred at 60–65 °C for 2–4 h then aged at room temperature for 3–5 h, the homogenous DAG sol was prepared.
The resultant homogeneous sol was dried at 40 °C in oven for several days to obtain the gel finally. The gel was dried at 120 °C for 1 h to obtain a fine powder, and then heated at 10 °C/min to selected temperatures, with a holding time of 2 h for 400, 800, 900, 1,000, 1,100, 1,200 °C, respectively.
The thermal behaviors of the dry gel were investigated by thermogravimetry and differential thermal analysis (TG–DTA) (Shimadzu TG/DTA-40) analysis at a heating rate of 10 °C/min in air condition. The structures of gel were measured in a wave number range from 400 to 4,000 cm−1, using Fourier transform infrared spectroscopy (FTIR) spectroscopy (Bruker Vertex70). An X-ray diffraction (D/Max-γA, Japan) was used for identification of crystalline species of the heated gel. The morphology of samples was characterized by scanning electron microscopy (SEM) (EVO-18, CARL ZEISS SMT LTD). The average grain size of powders was calculated by the X-ray line broadening technique using the Scherrer formula [8]: D = 0.94λ/β cosθ, where D is the average size of the crystallites, λ is the X-ray wavelength (λ = 0.15405 nm), β is peak width at half maximum in radians and θ corresponds to the peak position of the maximum of diffraction.
3 Results and discussion
The X-ray diffractometery (XRD) patterns of the samples heated at different temperatures are shown in Fig. 1. The positions of the peaks were assigned to DAG (JSPDS42-0169). The XRD pattern of the sample heated at 800 °C indicated a large unidentified amorphous hump between 2θ = 17°–36°, reaching maximum height around 33° (Fig. 1a). However, by 900 °C the DAG was present in the XRD as the major phase (Fig. 1b), with a small amount of the amorphous phase present; According to the Scherrer formula, the average size of the crystalline particle was about 26 nm. When heated at 1,000 °C, the DAG was present as a single phase (Fig. 1c), with an average size of the particle of about 34 nm. Further heating of the sample up to 1,200 °C showed no change in phase composition except for an increase in the intensity of the peaks (Fig. 1d), indicating crystallite growth of the DAG powders as temperature increases; The average size of the crystalline particle was 98 nm. Therefore, it could be considered that DAG crystallizes directly from the amorphous precursor between 800 and 900 °C, which supported by the FTIR studies (Figs. 2, 3).
Figures 2 and 3 shows FTIR spectra of precursor and samples heated at different temperatures. The FTIR spectrum of gels clearly shows absorption around 3,500 cm−1, which is a characteristic stretching vibration of hydroxyl groups (O–H). Peaks located at 1,593 and 1,407 cm−1 are corresponded to asymmetrical and symmetrical stretching vibrations of carboxylate (O–C=O) respectively. When the sample is heated at 400 °C, the spectrum reveals that the carboxylate and hydroxyl groups of the precursor become weaker. Trace of DAG crystal character absorption peaks located from 800 to 400 cm−1 appears. In the spectrum of sample heated at 800 °C, the hydroxyl groups peaks (around 3,500 cm−1) disappear, new absorption peaks appear at 783, 720, 560, 506, 451 cm−1 that correspond to DAG crystal character absorption peaks[7]. However, carboxylate (O–C=O) absorption peaks still exist, indicates that the material is not fully crystallized, which is corroborated by the XRD (Fig. 1a). By 900 °C, the carboxylate absorption peaks disappear. The intensities of the DAG crystal character absorption peaks increase with the temperature increasing from 900 to 1,200 °C, which indicates continued crystallization and/or grain growth of DAG.
Figure 4 shows TG–DTA curve of the DAG precursor. There are three mass-loss steps during decomposition, according to the TG curve. The first step corresponds to the elimination of water and some organic matters from 50 to 330 °C (mass loss of 14.7 % by weight), which was associated with the endothermic peak of 104 °C in DTA curve. An exothermic peak at 227 °C was also observed, which might be the beginning of the formation of an amorphous garnet phase. The second mass loss, from 330 to 500 °C (mass loss of 37 % by weight), might be caused by decomposition of carboxylate, which was associated with the exothermic peak of 418 and 460 °C in DTA. The third mass loss, from 500 to 1,000 °C (mass loss of 3 % by weight), results from the oxidation of trace amounts of free carbonate. The DTA plot exhibits a distinct exothermal peak at 905 °C, and little weight loss in TG curve at this temperature, we consider it results from the crystallization of DAG.
The morphology obtained for the as-produced powders was wormlike agglomerates, as can be seen from SEM images in Fig. 5. Figure 5a show the SEM micrograph of powders heated at 900 °C. The powders had no discernible grain structure at this temperature, which was probably caused by incomplete crystallization. According to the Scherrer formula, the average size of the crystallites was about 26 nm. As with the calcining temperature increase, the grain boundary became more and more obvious, and the crystal grain size became larger, showing in Fig. 5b, c. Figure 5d show the SEM micrographs of DAG powders heated at 1,200 °C, the average size of the crystallites was about 100 nm.
The yield of dysprosium aluminum garnet nanopowder prepared from aqueous solution of Al powder, HCl and dysprosium acetate is about 80 wt%. After several times repeating experiments, we found that the synthesis was repeatable, the morphologies, properties, and yields of the DAG powder are similar.
4 Conclusions
Dysprosium aluminum garnet nanopowder was prepared from aqueous solution of Al powder, HCl and dysprosium acetate by sol–gel method. The temperature of forming crystallites is about 900 °C. Except for its use as an antiferromagnetic material, we believe it may be of high potentiality in nuclear applications, using as a desirable sintering aid for the densification of B4C ceramics and improving the neutron absorption properties of B4C neutron absorber.
References
Faulhaber R, Hufner S (1969) Z Physik 228:235–253
Aoyogi K, Tsushima K, Uesugi M (1969) J Phys Soc Jpn 27(1):49–56
Celli M, Grazzi F, Zoppi M (2006) Nucl Instrum Methods Phys Res Sect A 565:861–863
Wang LS, Fang YC, Wu F, Yin BY (2000) Mater Sci Eng Powder Metall 5(2):113–120 (in China)
Goldstein A, Yeshurun Y, Goldenberg A (2007) J Eur Ceram Soc 27:695–700
Zhao DL, Zhang YJ, Gong HY, Nie LF, Zhao L (2010) Mater Res Innov 14(4):338–341
Dubnikova N, Garskaite E, Pinkas J, Bezdicka P, Beganskiene A, Kareiva A (2010) J Sol-Gel Sci Technol 55:213–219
Jinshy T, Lin CH (1991) J Appl Polym Sci 42:3039–3044
Acknowledgments
This work is supported by the National Nature Science Foundation of China (No. 51072099).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, R.B., Zhang, Y.J., Li, X.N. et al. Synthesis of a dysprosium aluminum garnet nanopowder via sol–gel method. J Sol-Gel Sci Technol 65, 388–391 (2013). https://doi.org/10.1007/s10971-012-2950-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-012-2950-0