Abstract
Patterning of sol gel based silica and silica–titania films has been developed at room temperature by soft lithographic technique. Corresponding metal alkoxides have been utilized for the preparation of precursor sols. Elastomeric stamps of polydimethylsiloxane (PDMS) are used to emboss patterns of a master grating on the as-prepared silica and silica–titania films obtained by sol gel process. Pressure-less capillary force lithography has been used to fabricate both 1-D and 2-D ordered structures of simple stripe patterns. A modified solvent assisted lithography and micro-molding in capillaries yielded stable and high fidelity 1-D structures for silica and silica–titania films over a large area.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Over the last few decades micro fabrication has gained a lot of prominence in the field of microelectronics and opto-electronics due to its wide applications in different areas such as information technology [1], fabrication of sensors [2], micro-reactors [3], combinatorial arrays [4], micro electro mechanical systems (MEMS) [5], micro analytical systems [6, 7] and micro-optical systems [8, 9]. Photolithography is the predominant one among the number of patterning techniques to form nano-sized structures. However, in photolithography optical diffraction limits the minimum resolution of the structures. In addition, creation of even small features demands high energy radiation which makes it very expensive and complicated.
Photolithography has been an important aspect for patterning which is required to fabricate electronic and optical devices. With ever increasing demand for smaller features in integrated circuits, lithography is fast approaching its fundamental limit and there is a need for new technologies for patterning of features on scales less than 100 nm [10–12] with the help of versatile lithographic techniques [13–16]. If economics is not a major concern, there are technologies already available for sub-100 nm features such as electron-beam lithography and lithographic techniques based on probe tips, including dip-pen lithography [17]. Large-area patterning involves patterning in one step several hundred millions of features of sub-micrometer size on an area not larger than a few square centimeters.
The patterning technique can be directly applicable to polymer films [18]. Soft lithography on the other hand, is low cost and convenient for transferring patterns (≥30 nm in dimension) and imprinting the patterns on the surface of other materials. While soft lithography based patterning typically finds its use in patterning of soft polymer thin films, patterning of inorganic thin films by this method is recently being investigated [13–15]. The precursor sol of inorganic system is a multiphase system where there is a large possibility of phase separation during patterning unlike a single phase melt for a polymeric thin film. Moreover, the presence of multiple solvents with different evaporation rates leads to difficulty in pattern replication and loss of structure fidelity. There is also a high possibility of structure damage during thermal annealing after patterning due to the removal of organics (a step not required for polymer films) which is not desirable for practical applications. Hence, it is a tricky thing to develop patterning in the oxide film from the sol–gel precursors. The critical aspect of the technique employed here is to ensure the capillary rise of the liquid precursor of the film under the cross linked PDMS based stamp in order to achieve the perfect negative replica of the stamp pattern. The precursor sol is usually hydrophilic in nature and the polymeric stamp to be used to emboss the grating patterns onto the film is of low surface energy and hydrophobic surface characteristics [19]. Due to the attractive interaction between the two contact media capillary action takes place by surface tension. When a liquid wets a capillary tube it would lead to the capillary rise of the liquid provided the wetting results in lowering the free energy. The conformal contact with the PDMS stamp for a couple of hours during drying resulted in development of the patterns [20] when solvents evaporated off leading to the increase in viscosity of the sol layer. This allows easy detachment of the sol–gel material from soft polymer structures without degrading the structural details. Capillarity is a useful concept for the patterning, particularly of polymeric materials. Hence, the lithography is based on capillary force which practically combines the essential feature of imprint lithography [21] with the prime element of micro contact soft lithography [22, 23], by using an elastomeric mold of PDMS. PDMS first attracts the sol and thereafter repels it. The precursor containing the metal alkoxide of relatively slow rate of hydrolysis such as silica system is suitable for this purpose.
Basically the capillary force lithography (CFL) technique has some versatility according to the adoption of specific steps in the CFL for patterning, as for example, solvent assisted CFL, micromolding in capillary (MIMIC) techniques. To increase the viscoelastic regime for the precursors having the metal alkoxides of high rate of hydrolysis, the solvent assisted CFL is important. Usually this is very much helpful for the precursors of titania, zirconia, silica–titania, silica–zirconia systems. In the case of low viscosity sol systems, MIMIC technique may be preferable because liquid pre-polymer placed at the open ends of the network of channels of the PDMS stamps spontaneously fills in the channels by capillary action between the surfaces which are in conformal contact and at least one of which possesses a recessed relief structure by a fluid. The fluid may be a liquid pre-polymer or suspension of materials. After filling the channels and curing, the pre-polymer transforms to a solid, cross-linked polymer. This technique is a convenient technique for generating three dimensional patterned micro/nano structures [24, 25] of polymers, ceramics.
The basic difference of the solvent assisted CFL and micromolding in capillary (MIMIC) techniques can be illustrated below:
-
1.
Solvent assisted CFL
-
(a)
PDMS based stamp is placed in conformal contact with the precursor coated film deposited on to a substrate
-
(b)
It seems capillary rise starts at a time for all microchannels as the film is conformal contact with the sol
-
(c)
Suitable for the precursors of relatively high viscosity and concentration
-
(d)
It is rather fast process and relatively large area can be covered; uniformity in patterning may be affected
-
(a)
-
2.
Micromolding in capillary (MIMIC)
-
(a)
The PDMS stamp is placed in conformal contact with the substrate and a drop of the sol is poured on the open sides of the stamp for capillary action to take place into the microchannels
-
(b)
Capillary rise starts from the edge of the microchannels as the added sol drop is allowed to pass through the microchannels
-
(c)
Suitable for the precursors of relatively low viscosity and concentration
-
(d)
It is rather slow process and relatively small area can be covered; hence better uniformity in patterning is expected.
-
(a)
In this report, 300–700 nm dip coated thick alkoxide (tetraethylorthosilicate) based silica films have been used for patterning on to quartz glass and silicon wafer substrates from the 16 wt% equivalent SiO2 precursors. In addition, 150–200 nm thick silica–titania films from the precursor sol of 10 wt% equivalent SiO2–TiO2 were also used for the same purpose. The key difference of patterning between inorganic and organic systems is the post processing after the pattern formation to generate oxide pattern from gel films by thermal curing. Controlled annealing of the patterned films up to 350 °C in oxygen atmosphere was an important target as considerable shrinkage of the films would possibly destroy the patterns. So thrust has been given to study the surface topography of the films by AFM in order to ascertain the stability of the imprinted structures.
2 Experiments
2.1 Materials
Tetraethylorthosilicate (>98% (GC) purum grade) and tetra-isopropyl-orthotitanate (TIOT, pract. grade) were procured from Fluka Chemika. Both 2-butanol (≥99%) and 1-propanol (≥99%) were of for synthesis grade of E. Merck India Ltd. (EMIL) while 2-propanol was of GR grade of EMIL which were used as solvents for the preparation of precursor sols. Concentrated HCl (GR grade, EMIL) after dilution was used as the acid catalyst for silica sol preparation. Acetylacetone (acac, for synthesis, ≥98%, SRL) was used as the complexing agent to prohibit the fast hydrolysis rate of TIOT (acac : TIOT = 1:2, molar ratio). The substrates were ordinary microscopic slides for initial experiments and the quartz silica substrates (Heraeus, Germany, Suprasil 2 Grade B, size: 75 × 25 × 1 mm3) were used finally. Sylgard Silicone Elastomer 184 and Sylgard 184 curing agent were bought from Dow Corning (Midland, MI). Double Distilled water was used as the hydrolyzing agent for the preparation of sols.
2.2 Silica sol
Tetraethoxyorthosilicate (TEOS) was used as the starting material for the preparation of silica precursor sols. The details of preparation have been described in our earlier work [26]. Silica sols of concentration range, 6.0–16.0 wt% equivalent silica were prepared for the film preparation.
2.3 Silica–titania sol
Tetra-isopropyl-orthotitanate (TIOT) was used as the source of titania and TEOS was used as the source of silica during the preparation of the silica–titania sol. The detailed preparation can be found in our earlier work [26]. Precursor concentration was 10.0 wt% equivalent mixed oxide system of silica–titania (SiO2:TiO2 = 50:50).
2.4 Patterning
Elastomeric stamps of PDMS containing the negative replica of the patterns found in commercially available CDs were used. For the preparation of the precursor of PDMS, requisite amounts of Sylgard Silicone Elastomer 184 and Sylgard 184 curing agent were thoroughly mixed and de-aerated in a vacuum desiccator by exposing it to a low vacuum. After the solution became completely free of air bubbles it was poured on to the exposed patterned face of the CDs and cured at 100 °C for 20 h. Cross linking takes place by which the inner surface of the PDMS film develops the grating patterns present on the patterned surface of the CD. After the completion of baking of PDMS mold in an air oven, small pieces were cut with the help of tweezers containing desired patterns for their use as stamps. Each stamp has parallel grating stripe lines of periodicity 1.5 μm and stripe height of ~120 nm and this can be used a multiple number of times.
All the substrates were cleaned following the steps outlined in our earlier work [27]. The 20 h aged silica sol was used for deposition of sol layer onto soda-lime silica glass substrate. We have used the dip–coating technique (Chemat 200 Dip Coater) with a withdrawal speed, 12 cm/min to yield the silica coatings. After 3 h of preparation of the silica–titania sol, coating was done on silica substrate by the dip-coating process, using the same coating machine at a lifting speed of 16 cm/min.
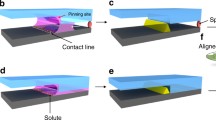
Patterning of the dip coated silica films has been achieved by pressure less Capillary Force Lithography (CFL). For our experimental purpose, we choose 16 wt% equivalent silica sol as this concentration is suitable for better pattern formation. During patterning the silica films we placed the PDMS stamps in conformal contact with the as-coated silica sol layer within 30 s of the completion of dip-coating as shown in the Scheme (Fig. 1a). During the CFL a negligible amount of pressure was applied with hand to adjust the stamps against the coated surface. Due to capillary action liquid rises (say 1 h) along the groove height. The stamps were removed after a time interval of the range, 3–16 h from the surface of the coated substrate and thus patterning in sol–gel silica film was formed.
During the process of patterning by MIMIC the stamps were pre-saturated in the silica sol (6.0 wt% equivalent silica) for about 5–6 h in an undisturbed condition. The presoaked stamp was placed in conformal contact with the slide and the open sides of the rectangular stamp were sealed with a drop of the silica sol. The substrate and the stamp in conformal contact were exposed to the solvent vapors of 1-prapanol and 2-butanol in a desiccator and left undisturbed for 12 h. The stamp was removed from the substrate after completion of 12 h of conformal contact to reveal the patterns.
In addition to creating a mere negative replica of the stamp, a novel combination of self organization and soft lithography shows that the elastic contact instability can generate a variety of complex ordered structures [28, 29]. Thin soft films confined by the long-range forces such as van der Waals interactions and electric fields which are inherently metastable or unstable, readily self-organize their shapes to reduce the total energy. Thus we have utilized a simple 1–D (stripe) patterned stamp to create ordered 2–D patterns. For this purpose, a stripe patterned polycarbonate (bare CD substrate without the protective foil) substrate has been used to coat the film, which was subsequently imprinted with another stripe patterned stamp, where the directions of the stripes on the stamp were perpendicular to those of the stripes on the substrate. The topographic contrast in the initial morphology of the film results in ordered 2–D structures under the confining stamp due to the substrate pattern combined with the capillarity driven self organization of the soft gel film.
During patterning the silica–titania gel films of regular nano scale patterns, we have adopted precursor sol assisted (Fig. 1b) capillary force lithography [30] as depicted in the scheme (Fig. 1). In this process, the PDMS is soaked in the precursor which subsequently leads to retention of solvent in it. For our experimental purpose, relatively low oxide concentration of precursor sol (8 wt% equivalent mixed oxides, SiO2:TiO2 = 50:50) produced incomplete replica of the patterns whereas the increase in concentration to 10 wt% equivalent silica–titania sol yielded encouraging results. Hence, we selected 10 wt% SiO2–TiO2 for our experimental purpose as homogenization and stability of the precursor became a major problem for the sols with even higher concentration. As for example, the stamps were initially presoaked in 10 wt% silica–titania sol for 1 min prior to patterning. Immediately after the completion of the dip-coating schedule the presoaked stamps were immediately pressed against the gel films of silica–titania without allowing much time lapse between the coating and patterning processes. Scheme (Fig 1b) shows the presoaked PDMS. All further steps are same as depicted the Fig. 1. The duration of conformal contact was increased up to an hour instead of 30 min as followed for silica films. Removal of the stamp was done in similar way as followed for silica system. The post processing of the patterned silica–titania samples was curing the samples at different temperatures (50–350 °C) starting from 50 °C in pure oxygen atmosphere. Silica–titania films were also patterned by the MIMIC processing following the same procedure as for silica films and heated following the same schedule.
All the patterned films are characterized by Atomic Force Microscope (Nanonics, Israel make NSOM AFM). The line scan images and their respective profiles are recorded. The thickness and refractive index of the samples were measured using an Ellipsometer equipped with He–Ne red laser of wavelength 632.8 nm (Gaertner Auto–Gain Ellipsometer, L-116 B). The FTIR spectra (corrected from silicon wafer substrate) were recorded using a FTIR instrument (Nicolet 5700, USA, wavenumber accuracy: 4 cm−1).
3 Results and discussion
3.1 Silica thin film
For the development of patterning in specific application, requisite stamp is required to emboss on to the gel film. Compact Disc (CD) having a grating structure of depth, ~120 nm and 1.5 μm periodicity was used as the master stamp for the generation of negative replica of the pattern of the CD in the PDMS film, illustrated in the Sect. 2. Figure 2 a, b shows the AFM image of a PDMS stamp and its cross-sectional line profile. Figure 2 c, d shows the AFM image and line scan of the pattern of a typical silica film cured at ~350 °C formed by Capillary Force Lithography on a silica film developed from 6.0 wt% equivalent silica precursor. The periodicity of the imprinted structures has been found to be identical to that of the patterns of the stamp, the height of the imprinted structure was 67 ± 2 nm (Fig. 2c, d) in contrast to the 120 nm heights of the stripes of the stamp. The incomplete replication occurs due to time lag between the coating and the application of the stamp. It may be probable that during this period, the viscosity of the sol increased significantly since no external pressure was applied. During the post processing by thermal curing up to 300 °C, there is a phase change of the imprinted structures (Fig. 3 a, c, e) which results in shrinkage of the films as evident from the line scans (Fig. 3b, d, f). It is evident from the cross-sectional profile in Fig. 3 that the height of the structures was reduced to ~84 ± 1.5 nm (cured at 100 °C, Fig. 3d) in contrast to 86 ± 2 nm (air drying) (Fig. 3b) developed after imprinting due to baking. The height further reduced to ~77.5 ± 2.5 nm (Fig. 3f) after curing at 300 °C. Thus for 16 wt% silica thin films, the observed shrinkage of the structures in the z direction is around 9.89% due to thermal curing from room temperature to 300 °C.
On the other hand, patterns developed by micro-molding in capillaries (MIMIC) showed encouraging results for silica films (Fig. 4) as the obtained depth of the patterns was nearly 70 nm. The cross sectional profile confirms around 42% replication of the structures present in the master. Understanding the rheological and fluid dynamic properties involved in MIMIC enabled us to develop highly reliable structures. These fabricated structures possess potential application in micro electronics for sensing biological and chemical environments. Several authors have highlighted the wide range of applications of MIMIC as a “soft” alternative route to optical lithography. The possibility of fabricating micron scale electronic devices by soft lithographic techniques in conjunction with CVD has been demonstrated by Erhardt and Nuzzo [31]. They implemented MIMIC to Schottky diodes.
Since CFL yielded encouraging results with 16 wt% silica sol we tried imprinting of patterned polycarbonate substrates to fabricate 2D structures with the same sol. But 16 wt% silica sol did not yield two dimensional structures as the patterns present on the substrate were immersed due to the occurrence of thick layer of sol. Thus a sol of low concentration (6 wt% equivalent silica) was finally chosen for our experimental purpose. By using the lower concentration of silica sol promising 2D structures was obtained for both perpendicular (Fig. 5a) and parallel (Fig. 5b) imprinting. The development of such structures can be attributed to capillary driven self organization of the sol gel film under the confining stamp. The obtained height of the structures was between 70 and 80 nm. The positive aspect of the patterning methods used here is that large areas with even more complex nano structures can be patterned in a very simple manner.
3.2 Silica–titania thin films
Both CFL and MIMIC yielded positive results for the silica system but in the case of silica–titania system CFL or MIMIC alone did not produce encouraging results. This was due to the rapid evolution of the solvents and an instantaneous viscosity gain during the intermediate period between the coating and patterning. Hence, we adopted another technique of patterning where we presoaked the stamps in a diluted solution of the silica–titania sol for 15 min prior to patterning. We selected a silica–titania sol of medium concentration (10.0 wt% equivalent mixed oxide; silica–titania, silica:titania = 50:50) for deposition on silica substrates. After the coating was complete the presoaked stamps were immediately pressed against the films without allowing much time to elapse between the coating and patterning processes. The period of conformal contact was increased up to an hour instead of 30 min as done for silica films. The post processing of the patterned silica–titania samples constituted of curing the samples at different temperatures (50–350 °C) starting from 50 °C in oxygen atmosphere.
Figure 6 shows the AFM images of the patterns imprinted on the silica–titania films by the above mentioned process. The line scan images clearly depict that the height of the structures obtained in the ambient films is around 60 nm. The height does not decrease considerably upon heating at 100 °C as the height remains around 50 nm. The major shrinkage takes place when the films are heated in between 200 and 350 °C. At 200 °C the height is around 20 nm which shrinks to ~12 nm at 350 °C. Thus, it is evident that considerable shrinkage (~80%) of the structures (heights) has taken place when baking temperature increased. This shrinkage may interfere with the performance of a planar waveguide. Micro molding in capillaries (MIMIC) was also followed for patterning in silica–titania film. The structures imprinted by MIMIC were heated at 70 and 100 °C. At the former temperature the height obtained was around ~38 nm which shrunk to 16 nm at 100 °C showing shrinkage of 58% (Fig. 7).
3D AFM image a and line scan b of the grating developed by patterning in silica–titania film; c 3D AFM image and line scan d of the grating developed by patterning in silica–titania film cured at 100 °C; e 3D AFM image and line scan f of the grating developed by patterning in silica–titania film cured at 200 °C; g 3D AFM image and line scan h of the grating developed by patterning in silica–titania film cured at 300 °C; i 3D AFM image and line scan j of the grating developed by patterning in silica titania film cured at 350 °C
Patterning of sol gel based films has been developed at room temperature by CFL and MIMIC followed by baking the films up to 350 and 100 °C, respectively. The AFM images of the patterns developed by different methods have shown different shapes. As the contact angle of the sol for the glass is low and that for the PDMS is relatively high, a pretreatment of PDMS surface was done by the addition of precursor sol which would change the critical angle of PDMS leading to different wetting behaviour [32] resulting in different shapes.
3.3 Physical thickness and refractive index
Physical thickness and refractive index (RI) of the silica and silica–titania films developed under similar conditions were measured by ellipsometry (Table 1). This shows that the film from 16.0 wt% silica–sol was considerably high which is in the range 900–170 nm for baking from 100 to 350 °C. As the maximum height of the structures of air dried samples is ~90 nm, it implies that the structures are on the bed of silica films of ~800 nm thickness and the refractive index of this film was 1.405 (± 0.003). In the case of gel film derived from 6 wt% silica sols the physical thickness and refractive index of the films were ~500 nm and 1.410 (not shown in Table 1), respectively. This precursor was used for the preparation of pattern by MIMIC method. In this case, the maximum peak height of the gel film was around 70 nm which apparently indicates that the structure is placed on the silica gel bed of ~430 nm thickness. This precursor was also used for the development of cross pattern following capillary driven self organization of the film under the confining stamp. For silica–titania system the thickness of the gel film (as deposited) was ~350 nm, out of which 60 nm has been used for patterning (Fig. 6). This also indicates that the pattern is on the bed of silica–titania of ~290 nm thickness. Refractive index of the film baked at 200 °C was near 1.698 (± 0.003). The refractive index of the film on further baking was close to 1.700. This is possibly due to the fact that major transformation of the gel to oxide network has occurred even at 200 °C (Table 1). Considerable shrinkage occurred due to the increase in baking temperature. Shrinkage of the silica gel films was relatively low while for silica–titania system, it was relatively high. However, the shrinkage did not damage the patterns of both systems as evident from the AFM images of the patterns.
3.4 FTIR spectra
In order to study the structural properties of the films, substrate corrected FTIR spectra (Fig. 8) of the silica and silica–titania films on silicon wafer heat treated at different temperatures were recorded. With increasing curing temperature, the silica gel film containing organics transformed to oxide (silica) system. This is confirmed from the FTIR spectral (Fig. 8a) broadening in the region of 1,300–1,000 cm−1 due to increasing Si–O–Si network [33–35] enhancement in the film. On the other hand, as-prepared silica–titania film showed several prominent absorption peaks (Fig. 8b) within 740–1,590 cm−1. The vibrations in the region of 1,600–1,300 cm−1 are the characteristic vibrations of actylacetonate complex of Ti(IV) [35] which gives the stability of Ti-alkoxide in the mixed sol system. The asymmetric stretching vibrations of Si–O–Si bridging sequences [33, 34] appear in the range of 1,300–1,000 cm−1 and the absorption bands appeared at 818 and 934 cm−1 are attributed to the presence of Ti–O–Ti and [υ(C–C) + υ(C–O)] of Ti-actylacetonate complex and isolated silanol groups of hydrolyzed Si-alkoxide, respectively [36]. With increasing the baking temperature, intensity of the bands responsible for the complex becomes weak. All the characteristic bands of the Ti-actylacetonate complex disappeared at the curing temperature of 350 °C and a new band appeared at 965 cm−1 attributed to the formation of Ti–O–Si [36]. It is clear from Fig. 8 that the 1,300–1,000 cm−1 wavenumber region is broadened due to enhancement Si–O–Si network in the film. The shrinkage of patterned films has taken place during thermal curing as evidenced from AFM studies. It is known that the low temperature sol–gel film is porous in nature and the porosity decreases with increasing baking temperature. In the present work, we did not measure the porosity of the films but through the study of FTIR, the broadening of 1,300–1,000 cm−1 wavenumber region absorption band due to the enhancement of Si–O–Si film network make a sense of decreasing porosity. In Fig. 8b, the peak appeared at 965 cm−1 before heating the gel film because the formation of Si–O–Ti bond took place in the sol stage through the hydrolysis and condensation of the alkoxides of silica and titania.
4 Conclusions
Patterning of the dip coated silica and silica–titania films have been achieved by pressure less Capillary Force Lithography (CFL). Three dimensional patterned micro/nano structures have also been developed by MIMIC technique. 2D patterned structures have been developed by imprinting on to the patterned substrate. AFM images of the patterns developed at different temperatures suggests the grating structure which shrinkages at relatively high temperature for gel to oxide transformation. The FTIR spectral study also suggests the development of oxide pattern.
References:
Barrett CR (1993) Mater Res Soc Bull XVIII(7):3
Bryzek J (1996) Sensors July:4
Clark RA, Hieptas PB, Ewing AG (1997) Anal Chem 69:259
Wu MC, Lin LY, Lee SS, King CR (1996) Laser Focus World February:64
Briceno G, Chang HY, Sun XD, Schultz PG, Xiang XD (1995) Science 270:273
Bryzek J, Peterson K, McCulley W (1994) IEEE Spectrum May:20
Manz A (1996) CHIMIA 59:140
Kovacs GTA, Petersen K, Albin M (1996) Anal Chem 68:407A–4012A
Szendro I (2001) Proc SPIE 4284:80
Suh KY, Kim YS, Lee HH (2001) Adv Mater 13:1386
Xia Y, Rogers JA, Paul KE, Whitesides GM (1999) Chem Rev 99:1823
Xia Y, Whitesides GM (1998) Angew Chem Int Ed 37:550
Chou SY, Krauss PR, Renstrom PJ (1996) Science 272:85
Xia Y, Whitesides GM (1995) J Am Chem Soc 117:3274
Rogers JA, Meier M, Dodabalapur A (1998) Appl Phys Lett 73:1766
Schaffer E, Thurn-Albrecht T, Russell TP, Steiner U (2000) Nature 403:874
Piner RD, Zhu J, Xu F, Hong S, Mirkin CA (1999) Science 283:661
Drury CJ, Mutsaers CMJ, Hart CM, Matters M, de Leeuw DM (1998) Appl Phys Lett 73:108
Wang HY, Deeman N, Gauzner G, US Patent No. 7033683
Mukherjee R, Sharma A, Gonuguntla M, Patil GK (2008) J Nanosci Nanotech 8:1
Suh KY, Lee HH (2002) Adv Mater 14:346
Mukherjee R, Sharma A, Patil G, Faruqui D, Pattader PSG (2008) Bull Mater Sci 31:249
Xia Y, Kim E, Whitesides GM (1996) Chem Mater 8:1558
Fardad A, Andrews M, Milova G, Malek-Tabrizi A, Najafi I (1998) Appl Opt 37:2429
Herzig HP (1997) Micro-optics: elements, systems and applications. Taylor & Francis, London
Biswas PK, Kundu D, Ganguli D (1989) J Mater Sci Lett 8:1436
Atta AK, Biswas PK, Ganguli D (1990) J Non-Cryst Solids 125:202
Kim E, Xia Y, Whitesides GM (1995) Nature 376:581
Gonuguntla M, Sharma A, Mukherjee R, Subramaniam SA (2006) Langmuir 22:7066
Yu X, Wang Z, Xing R, Luan S, Han Y (2005) Polymer 46:11099
Erhardt MK, Nuzzo RG (1999) Langmuir 15:2188
Doshi DA, Huesing NK, Lu M, Fan H, Lu Y, Simmons-Potter K, Potter BG Jr, Hurd AJ, Brinker CJ (2000) Science 290:107
Jana S, Lim MA, Baek IC, Kim CH, Seok SI (2008) Mater Chem Phys 112:1008
Akram D, Ahmad S, Sharmin E, Ahmad S (2010) Macromol Chem Phys 211:412
Schrijnemakers K, Vansant EF (2001) Porous Mater J 8:83
Cheng P, Zheng M, Jin Y, Huang Q, Gu M (2003) Lett Matter 57:2989
Acknowledgments
The authors would like to thank the Director, CGCRI, Kolkata for support and encouragement for the work. This work has been carried out under the sponsorship of DST, Govt. of India (Sanction No.: DST/TSG/PT/2006/74). Two authors (DS and RDR) thankfully acknowledge DST for providing their research fellowships.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Sil, D., Deb Roy, R., Jana, S. et al. Patterning of sol gel thin films by capillary force assisted soft lithographic technique. J Sol-Gel Sci Technol 59, 117–127 (2011). https://doi.org/10.1007/s10971-011-2469-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10971-011-2469-9