Abstract
Strontium-90 emits beta-particles and decays into yttrium-90. At this time, the beta-particles released affect a human body, and liquid scintillation counting is used to detect such beta-particles. In general, cocktail method that mixes samples and liquid scintillators is used in conventional liquid scintillator counters. In the cocktail method, the degree of miscibility or solubility between samples and scintillators, precipitation phenomena, etc. reduce the reproducibility of the experiment. A new method for measuring beta-particles after separating samples and scintillators from each other is presented. In addition, several experiments are conducted to investigate the efficiency and tendency of the new method.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Radioactivity is the ability of elements, such as uranium, to release radiation as their atomic nuclei instability state. The metastable element emits radiation in the form of alpha-particles, beta-particles, gamma-rays or neutron particles. The radon (Rn), one of the radioactive materials, has been detected more than EPA standard level at 100 Bq m−3 [1] in products used in daily life or new apartments. Radon is a radiation nuclide that emits alpha-particles and was tested with strontium-90 (Sr-90), in order to verify the efficiency of the coefficient of liquid scintillation using the scintillation phenomenon during analysis of these nuclides.
Sr-90 has a half-life of 28.8 years, emitting 0.546 MeV of beta-particles and decaying to yttrium-90 (Y-90, halflife of 2.7 days). The Sr-90 is generated in large quantities by the fission of uranium-235, and its half-life is moderately long, making it useful in many applications. This chemical property of Y-90 is similar to calcium, so it is absorbed into the body, which has a significant adverse effect on human being such as bone marrow, leukemia, and genetic mutations [2].
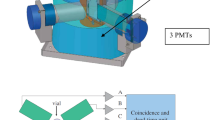
As can see in Fig. 1, liquid scintillator counter (LSC) produces instantaneous light in proportion to the energy lost in the fluorescent material when radiation is introduced into a substance consisting of several types of fluorescent material, which can be detected by amplifying the weak radiation emitted by Sr-90. However, existing LSC measures specimens by dissolving them into scintillators. Therefore, the effect of impurities on samples or solvents causes a quenching phenomenon in which the luminous efficiency of the scintillator decreases [3].
Experimental
As shown in Fig. 2, 1 mL of 90Sr/90Y standard solution provided by Korea Research Institute of Standards and Science (KRISS 09SR90V26, 1053.14 DPM) was passed through a column filled with 2.0 g of Sr-specific resin (Eichrom, Lisle, IL, USA; particle size: 100 to 150 μm) which was placed in 30 mL of 8 M HNO3 (Merck, Darmstadt, Germany) in advance. After then, about 20 mL of 8 M HNO3 solution was added to the column to make Sr-90 adsorb to the resin and Y-90 release [4], and deionized water was used to strip the adsorbed Sr-90 from the resin until the volume of receiving solution was up to 30 mL. The 30 mL of Sr-90 extract was marked to 50 mL with 0.1 M of HNO3.
Results and discussion
Unlike the typical cocktail method, the method for measuring beta-particles is to separate the Sr-90 containing sample and the scintillation cocktail (Ultima Gold and Ultima Gold-LLT, PerkinElmer, Waltham, MA) from each other, as shown in Fig. 3. The sample was placed in a 5 mL polyethylene vial, and then the vial was placed in a 20 mL low-potassium borosilicate vial containing the liquid scintillator. Finally, the 20 mL vial was shaken and counted by LSC (1220 Quantulus, Wallac, Perkin Elmer, Waltham, MA). The lower limit of detection (LLD) of Sr-90 was calculated to be 0.1 Bq kg-1 according to the reference method [5].
Several samples using the Sr-90 extract marked with 0.1 M of HNO3 were prepared to investigate the efficiency of the new method and are listed in Table 1. The amount of nitromethane (quenching agent) and 0.1 M HNO3 in the Sr-90 extract was varied in the J-LLT-R2 ~ R0 and J-UG-R2 ~ R0 samples, respectively. Meanwhile, the amount of nitromethane in the scintillation cocktail instead of the extract was varied too in J-LLT-R2a ~ R0a and J-UG-R2a ~ R0a samples, respectively. For comparison, the normal cocktail method was adopted as J-LLT-RS2 ~ RS0 and J-UG-RS2 ~ RS0, respectively. J-LLT-S2 ~ 0 and J-UG-S2 ~ 0 samples were prepared for another comparison with J-LLT-R2 ~ 0 and J-UG-R2 ~ 0 by utilizing the 90Sr/90Y standard solution provided by KRISS without separation of Y-90 using Sr-specific resin.
Since the amount of Sr-90 in the sample is the same, the value of DPM was 1053.14 as the following [6];
Each prepared sample was measured by LSC for 20 min as shown in Figs. 4 and 5, and the control test with the scintillation cocktail alone showed no specific peaks without Sr-90. However, in the presence of Sr-90 alone, a peak appeared in the channel range of 140–360, and the peak shapes and peak positions were the same as those obtained from the J-LLT-S, J-UG-S, J-LLT-R, J-UG-R, J-LLT-Ra, and J-UG-Ra series of spectra in Fig. 5. On the other hand, an additional peak in the channel ranges from 700 to 850 was detected in the series of J-LLT-S, J-UG-S, J-LLT-R, and J-UG-R, and shifted to 400 by increasing the volume of nitromethane in the series of J-LLT-Ra and J-UG-Ra. Nevertheless, existing conventional cocktail method has much broader peaks across the channel range from 200 to 850 in the series J-LLT-RS and J-UG-RS, as shown in Fig. 4. The long half-life (28.8 years) and high decay constant (6.60 × 10−5 d−1) of Sr-90 (the parent radionuclide), compared to the significantly shorter half-life (2.7 days) and lower decay constant (2.57 × 10−1 d−1) of Y-90 (the daughter radionuclide), put Sr-90 and Y-90 in secular equilibrium, which is the steady state. Therefore, the 14-day changes in radiative equilibrium with Y-90 after Sr-90 extraction using Sr-specific resin were confirmed, and the quenching by nitromethane (quenching agent) was confirmed. In the experimental method using the sample and scintillation cocktail separately, the peaks are sharper and better defined, but the efficiency is very low, about 10%. The distance between the two vials and the thickness of the vials may be the reasons for the low efficiency. The existing conventional cocktail method has a higher efficiency than the other series of the J-LLT-S, J-UG-S, J-LLT-R, J-UG-R, J-LLT-Ra, and J-UG-Ra. The energy of beta-particles is high, so the transmittance is not so good.
Conclusions
After separating the Sr-90 using resin, the radioactivity was measured using liquid scintillator counter. The separation method resulted in sharper peaks in the spectra than the conventional cocktail method, but it can be confirmed that the efficiency is inferior. When drawing the quenching curve, the linearity was poor, and it was a little difficult to grasp the measurement efficiency. However, when measured by the separation method, the peak did not appear sporadically but showed a certain trend. For the separation method, the peak in the channel range of 140 to 360 had the same peak shape and peak position as that obtained from a spectrum in the presence of Sr-90 alone. However, in the series of J-LLT-S, J-UG-S, J-LLT-R, and J-UG-R, additional peak was detected in the channel range of 700 to 850, and in the series of J-LLT-Ra and J-UG-Ra, this peak was shifted to 400 by increasing the amount of nitromethane. The intensity of the peak is not high, but if the peak is consistent, it can be used as an indicator of measurement. In addition, it is possible to measure the sample by preserving it intactly rather than using it as a one-time use. In situations where measurement is hindered by uneven mixing of the sample and scintillator, it is believed that a separate measurement method may be useful for several researchers to overcome the solubility issue in LSC method.
References
Shahrokhi A, Shokraee F, Reza A, Rahimi H (2015) Health risk assessment of household exposure to indoor radon in association with the dwelling’s age. J Radiat Prot Res 40(3):155–161
Goutelard F, Nazard R, Bocquet C, Coquenlorgea N, Letessier P, Calme D (2000) Improvement in 90Sr measurements at very low levels in environmental samples. Appl Radiat Isot 53:145–151
Vajda N, Kim CK (2010) Determination of radiostrontium isotopes: a review of analytical methodology. Appl Radiat Isot 68:2306–2326
Horwitz EP, Dietz ML, Fischer DE (1991) Separation and preconcentration of Sr from biological, environmental, and nuclear waste samples by extraction chromatography using a crownether. Anal Chem 63:522–525
Seymour R, Sergent F, Knight K, Kyker B (1992) Impact of sensitivity and throughput on optimum selection of a low-back-ground alpha/beta gross counting system. Radioact Radiochem 3:14–28
Douglas M, Bernacki BE, Erchinger JL, Finn EC, Fuller ES, Hoppe EW, Keillor ME, Morley SM, Mullen CA, Orrell JL, Panisko ME, Warren GA (2016) Liquid scintillation counting of environmental radionuclides: a review of the impact of background reduction. J Radioanal Nucl Chem 307(3):2495–2504
Acknowledgements
This work was supported by the research grant of Jeju National University in 2021. We thank Mr. H.-S. Kang of Jeju National University for partial assistance with the LSC measurements. It was presented at the International Conference on Nuclear Analytical Techniques in 2022 (NAT2022).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Jung, HS., Han, CH. & Im, HJ. Detection method of radioactive beta-particles for solving impurity and solubility problems. J Radioanal Nucl Chem 332, 5253–5258 (2023). https://doi.org/10.1007/s10967-023-09133-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-09133-7