Abstract
For the first time we report the activity concentrations of 238U, 234U and 210Po and the 234U/238U ratio in drinking water from certain sources in South-Central Bulgaria. The results obtained for water samples varied from 79 to 826 mBq l−1 for 238U, 130 to 1623 mBq l−1 for 234U, < 0.5 to 25.5 mBq l−1 for 210Po and from 0.93 to 3.21 for the 234U/238U activity ratio. The annual effective dose from 238U, 234U and 210Po varied from 8.85 to 62.5 μSv y−1 with a mean of 30.1 μSv y−1 and is below the individual dose criterion of 100 μSv y−1. The highest contribution to the annual effective dose was found for 234U.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Humans are exposed to ionizing radiation from cosmic rays and natural radioactivity in air, food and water [1]. Water is the most significant vector for the transport of natural radionuclides (in solution or by erosion) in the environment and is the major source of radionuclides to the human body [1, 2]. Therefore, it is important to determine its radioactivity to prevent possible health risks. The permanent monitoring of water radioactivity allows the levels of radioactivity to be maintained below the normative limits for drinking water because the water is an important factor for increasing the total population exposure as a result of ingestion of natural radionuclides with it [3].
With respect to the effective dose for the public, the most significant radionuclides typically found in drinking water are the radionuclides of uranium, radium, polonium, lead, and short-lived 222Rn [4]. Human activity such as mining, fertilizing, water treatment, etc. can influence the composition of drinking water [2, 4, 5]. The determination of the radionuclides of uranium and polonium in water is of primary importance to human health due to their high toxicity and radiotoxicity [6].
Uranium has three alpha emitting radionuclides: 234U, 235U and 238U with a different atomic mass, which have different distribution and half-lives. More than 99% of natural uranium occurring in nature is 238U. Uranium is the heaviest naturally occurring radioactive element with high chemical toxicity, affecting the kidney function during the radionuclide excretion [7,8,9]. High concentrations of uranium are generally associated with the presence of granites, commonly found in fractured crystalline aquifers, and minerals such as pitchblende, silicates, vanadate, monazite and lignite sands and phosphates of uranium [7, 10]. The activity concentration of uranium in groundwater depends on many factors such as the presence of uranium in the aquifer matrix and the geochemical/hydrochemical conditions present [11]. In its hexavalent form, uranium is water-soluble, and in its tetravalent form it is relatively insoluble [10]. Usually, the uranium isotopes (238U and 234U) are the most important sources of natural radioactivity in water because of the great mobility and the long half-life, which makes these radionuclides long-term hazardous [2, 12]. Knowledge of the uranium concentration in ground and surface water is important in performing radiological impact assessment of various anthropogenic activities [13].
Regardless of radionuclide concentration levels in drinking water, radioactive disequilibrium between both 234U and 238U may be observed. The 234U/238U activity ratio in natural water is an important indicator of the origin of the uranium in the sample studied. Commonly observed disequilibrium between 234U and 238U in water is a result of nuclear recoil effects and extensive rock/water interactions [2, 11].
Polonium is also a naturally occurring radioactive element with an atomic number equal to 84. In nature several radioactive polonium isotopes are present. The most important radiologically polonium isotope is 210Po (T1/2 = 138.4 days), the last radioactive isotope in the 238U decay series [14,15,16]. It is one of the most radiotoxic substances to humans. This radionuclide is a relatively high energy alpha emitter (Eα = 5.305 MeV), being one of the main contributors to the committed effective dose via ingestion by the population [16]. It is well known that 10% of the 210Po is incorporated into the human body via inhalation and that 50% of the 210Po ingested goes into the circulatory system [16].
The reported measurements of 210Po in drinking water are rare. In the review paper of Seiler [17] 400 measurements in the US were identified, and world-wide only about 2200 have been identified and compared. The usual value is < 10 mBq l−1 with some high concentrations reported for US and Finland. It is because 210Po is strongly reactive under normal conditions and it binds to aquifer sediment [18].
The requirements for the radiological properties of drinking water are set by the World Health Organization (WHO) and were implemented into Bulgarian law by the drinking water ordinance [19]. WHO guidelines for drinking water quality set parametric values of 0.1 mSv y−1 for the annual effective dose, 0.1 Bq l−1 for 210Po, 1 Bq l−1 for 234U and 10 Bq l−1 for 238U [20].
The study of the natural radioactivity of drinking water in Bulgaria has become very important for the Bulgarian society after detecting of relatively high uranium concentrations in some sources of drinking water in the regions of Plovdiv and Haskovo in 2017. Due to a possible radiological hazard to the population in these regions, independent investigations of drinking water sources, complementary to the national regulatory control, have been performed at the Institute for Nuclear Research and Nuclear Energy-Bulgarian Academy of Sciences (INRNE-BAS).
The significance of drinking water for life and human health and increasing water scarcity has posed water quality in the focus of many investigations worldwide [4, 8, 21,22,23,24,25,26]. The available data on radioactive content of drinking water in Bulgaria are rather scarce. Until now only a few studies on the radioactivity of Bulgarian drinking water were published [27, 28]. However, activity concentration levels of uranium and polonium isotopes in drinking water from Plovdiv province and Haskovo province in South-Central Bulgaria and the radiological impacts of the ingestion of this water have not been reported previously.
The aim of this study is to determine the activity concentrations of naturally occurring radionuclides in drinking water samples collected from selected settlements located in South-Central Bulgaria and to assess the total annual effective doses received from these radionuclides for the Bulgarian population resulting from consumption of the water sources investigated. The survey is focused on three radionuclides, which are the most important ones from the point of view of public health in Bulgaria, because of their high toxicity and radiotoxicity in drinking water, namely 238U, 234U and 210Po.
Material and methods
Study area
The study area is located in the Upper Thracian Uranium Ore Region, in the central part of South Bulgaria, where uranium mining was carried out in the past. The ore region is characterized by exogenous uranium deposits formed in the Bartonian–Quaternary complex compound by sedimentary and less volcano-sedimentary rocks. Different granitoids and high grade metamorphic rocks are the sources of uranium. The ore bodies are localized within sandstone aquifer, less in aleurolite, tuff-sandstone, rare in clay, within reducing or neutral conditions [29].
Sampling
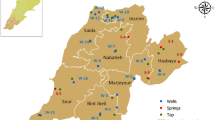
Thirty drinking water samples were collected from different settlements, located in South-Central Bulgaria in the boundaries of Plovdiv province and Haskovo province. Samples were selected randomly. Drinking water samples taken from the locations shown in Fig. 1 were collected in 10 l polyethylene bottles. The collected water samples were acidified with concentrated nitric acid to pH < 2, to prevent losses by sorption of the radionuclides studied onto the vessel walls. The samples were analyzed for 238U, 234U and 210Po.
Analytical procedures
Determination of uranium isotopes
Uranium isotopes were determined by alpha spectrometry after their isolation from the matrix. Fe3+ carrier (in the form of FeCl3 solution) for uranium co-precipitation and 232U tracer for determination of recovery were added to 2 l of each acidified water sample. The radiochemical procedure adopted for 238U and 234U determination is described in more detail by Slavchev et al. [28]. Radionuclides were concentrated from the water sample by co-precipitation at pH 9–10 using NH4OH, then separated from each other and from impurities through extraction chromatography. The supernatant was removed by decanting and the residue was centrifuged. The resulting precipitate was filtered through a 0.45 μm polypropylene filter, rinsed with water (to pH = 7) and dissolved in 3 M HNO3. The pure uranium fraction was obtained by use of Eichrom UTEVA resin which was preconditioned in 3 M HNO3. After interfering elements were removed by washing the column with 3 M HNO3, 9 M HCl and 0.5 M H2C2O4/5 M HCl, uranium radioisotopes were eluted with 0.01 M HCl. The source for alpha spectrometric measurement was prepared by microcoprecipitation with NdF3 and filtration on a polypropylene disk (0.1 µm) [8, 28, 30].
210Po determination
For the determination of 210Po in the water samples, the spontaneous deposition of Po from a solution onto a copper disc was used. The period of samples storage between sampling and analyses do not exceed 1–2 months thus ensuring no significant decay of the 210Po. A radiochemical procedure, based on extraction chromatography with a crown ether extractant, was applied to separate simultaneously the lead and polonium fractions. Pb carrier and 209Po tracer were added to 1 l water sample in order to correct for chemical recoveries and the sample was evaporated and then dissolved in 2 M HCl acid. Separation of polonium from lead was performed on Eichrom Sr spec resin preconditioned with 2 M HCl. 210Po was eluted from the column with 6 M HNO3 and the polonium fraction obtained was evaporated to dryness. A polonium source for alpha spectrometric measurement was prepared by self-deposition on a copper disk from 2 M HCl solution (pH = 1) with addition of 100 ml of distilled water. Spontaneous deposition of polonium was carried out at 50 °C for 4 h. The disk was rinsed with water and ethanol, and dried at room temperature [8, 28].
Instrument
The uranium and poloniun radionuclides were measured by means of high resolution ORTEC Octete Alpha Spectrometric system equipped with 8 chambers and ion implanted type ULTRA-AS detectors with 300 mm2 active surface (Fig. 2). The energy resolution (FWHM) for 241Am, 5.486 MeV line is 20 keV for 4 cm source to detector distance for all detectors. Energy calibration, as well as, efficiency calibration for source geometry (co-ppt. Source) is done by a mixed radionuclides standard containing 238U, 234U, 239Pu and 241Am with known activity. The efficiency calibration is performed with a 241Am Amersham standard [28]. The activity concentration of given radinuclide is calculated following Kanisch [31].
Selected alpha-spectra of uranium and polonium isotopes are shown in Fig. 3.
Results and discussion
Activity concentrations
The measurement of activity concentrations of 238U, 234U, and 210Po in drinking water samples collected from different water sources in South-Central Bulgaria, geographically distributed over 2 regions, was carried out for the first time. The results are given in Table 1.
The activity concentrations of 238U and 234U in drinking water varied in a wide range from 79 to 826 mBq l−1, with a mean value of 329 mBq l−1 and from 130 to 1623 mBq l−1 with a mean value of 508 mBq l−1, respectively. This high dispersion for 238U and 234U could be explained by the different geological characteristics of the aquifer rocks and the chemical composition of water [32]. In oxidizing conditions, uranium forms soluble stable complexes, e.g. carbonates, phosphates, etc. and can move a long distances, while in reducing conditions (absence of air) uranium precipitates, forming concentrated secondary deposits [4, 9]. The highest activity concentrations of uranium isotopes were registered in the sample DW18, located in Plovdiv region (Fig. 1). These high values are mainly due to uranium deposits in the geographical location of the aquifer and uranium mining activities in the 90s of the last century in this region. We have found that the total alpha activity from 238U and 234U in most of the samples exceeds the parametric value of 100 mBq l−1 for the total alpha activity, but the activity concentrations of each of the uranium isotopes do not exceed the guidance levels (3 Bq l−1 for 238U, 2.8 Bq l−1 for 234U) recommended by the Bulgarian regulation [19]. For comparison, 238U and 234U activity concentrations in drinking water from different countries in the world are shown in Table 2. Below listed values show the extremely wide activity concentration range of 238U and 234U in different parts of the world. The measured 238U activity concentrations in our investigation are higher than those observed in Poland, Hungary, Romania, Serbia, Croatia, Italy, Greece and Belgium and lower than those observed in Egypt and Germany. Our results for the 234U content in drinking water are generally higher than those given in the literature.
Significant (positive) correlation between 234U and 238U activity concentrations was established (Fig. 4). This correlation is consistent with other findings reported in the literature and is related to the high rate leaching of uranium isotopes to the underground water flowing through the faults and fissures between the grains of reservoir rock [15, 22, 34, 35].
It is observed that the activity concentrations of 234U in drinking water samples are higher than the activity concentrations of 238U, excluding one sample. A state of radioactive disequilibrium between 234U and 238U was observed in the water samples investigated. Usually the 234U/238U activity ratio in natural waters is in the range of 0.5–1.2, but it can reach 30 in extreme cases [2]. In this study 234U/238U activity ratios were found to vary between 0.93 and 3.21 (Table 1). From the activity ratio of 234U/238U, it is confirmed that in 90% of the cases the water is normal and comparable to worldwide values between 1 and 2. The activity ratio of 234U/238U in one water sample (DW28) was lower than 1 (0.93), indicating presence of younger water with a stronger contribution of U due to water–rock interaction and also a local recharge component to the groundwater [22]. The highest 234U/238U activity ratio values correspond to DW15 from Plovdiv province and DW28 from Haskovo province, indicating most probably old-type water [22, 35]. Higher 234U/238U activity ratios have been reported for other regions of Bulgaria. For example, Slavchev et al. [28] reported activity ratios in the range of 1.04–10.6 in drinking water samples from Southern Bulgaria. It is established that radionuclides produced by alpha decay are more readily driven out from the rock because the alpha decay causes the atom to recoil, which reduces the atom stability in the lattice, i.e. 234U activity concentration in water is higher than, or equal to, that of the parent 238U because the alpha decay-induced recoil can free 234U from the rock matrix [9]. In the bedrock material, 234U and 238U are usually in secular equilibrium, but because of the recoil energy in the disintegration of 238U, the 234U could be more loosely connected with the surrounding environment and in a different chemical state. In the hydrosphere and in ground water, the different chemical properties of the uranium isotopes, in particular the differences in their oxidation states can result in relative enrichment of 234U in water. Uranium is much less mobile under reducing conditions. The reduced U(IV) form may be stabilized as uraninite (UO2), although sorption of U(IV) to organic matter and clays has also recently been demonstrated [36].
The 210Po activity concentrations in water samples were found to be in the range between < 0.5 and 25.5 mBq l−1, with a mean value of 3.3 mBq l−1 and correlated with values obtained by Slavchev et al. in a previous study covering drinking water samples from different areas in the Southern part of Bulgaria [28].
As can be seen from Table 1 the activity concentrations of 210Po in drinking water samples are lower than the activity concentrations of 238U and 234U due to lower solubility in water of Po relative to U [15]. The results were compared with those obtained by other authors who studied the 210Po content in drinking water. The concentration values for drinking water in Belgium range from 0.2 to 3.1 mBq l−1 [26]. According to Jia et al. [25], the activity concentrations of 210Po in Italian drinking water varies in the interval 0.13–11.3 mBq l−1. The results for 210Po reported for drinking water in Romania, Galati and Vrancea counties range from 1.9 to 12.5 mBq l−1 [37]. In another survey of spring water in Eastern Carpathians, Romania 210Po have been detected at levels in the interval 18–64 mBq l−1 [38]. Activity concentrations of 210Po up to 114.2 mBq l−1 were measured in tap water samples in Western Australia [39]. Our maximum values obtained for all studied drinking water samples are comparable with data typical for drinking water and well below the guidance levels of 100 mBq l−1, recommended by WHO and the Bulgarian regulation.
Figure 5 shows a comparative overview of the mean activity concentration of 238U, 234U and 210Po in drinking water from the surveyed areas of South-Central Bulgaria. The mean activities of 238U, 234U and 210Po in the drinking water collected from Plovdiv region are 393 mBq l−1, 219 mBq l−1 and 4.3 mBq l−1, and from Haskovo region are 219 mBq l−1, 339 mBq l−1 and 1.5 mBq l−1, respectively.
It can be seen from Table 1 and Fig. 5 that the activity concentrations of 238U, 234U and 210Po in drinking water from Plovdiv region vary over a wider range than those in the Haskovo region, and the mean activity concentrations of the radionuclides studied in the Plovdiv region are higher than those in both the Haskovo region and the entire survey area.
Annual effective dose
On the basis of the mean concentration values of the analyzed radionuclides 238U, 234U and 210Po in drinking water samples the annual intake of these isotopes by an adult member of the population from Plovdiv province and Haskovo province was calculated. The results show that the consumption of drinking water by a statistical adult inhabitant of Plovdiv province leads to an intake about 287 Bq of 238U, 442 Bq of 234U and 3.12 Bq of 210Po per year. The annual intake of the already mentioned radionuclides by consumption of drinking water to the adult member of the population in Haskovo province is 160 Bq of 238U, 247 Bq of 234U and 1.10 Bq of 210Po.
The obtained activity concentrations of uranium and polonium isotopes in water samples were used for estimation of the committed effective dose for adults due to the annual intake of these isotopes by water consumption [5]. The dose reference level of 100 µSv y−1 has been used for comparison with our results. For the total annual effective dose calculation, dose coefficients of 0.045 μSv Bq−1 for 238U, 0.049 μSv Bq−1 for 234U, and 1.2 μSv Bq−1 for 210Po and annual water consumption of 730 l were used [8, 20, 40]. The annual effective dose was calculated separately from 238U, 234U and 210Po content.
The annual effective doses from the 238U, 234U and 210Po varied: from 2.60 to 27.1 µSv y−1 with a mean of 10.8 µSv y−1 for 238U; from 4.65 to 38.7 µSv y−1 with a mean of 16.4 µSv y−1 for 234U; and from 0.44 to 22.3 µSv y−1 for 210Po with a mean of 2.86 µSv y−1.
The estimated total annual effective doses for drinking water samples is presented in Fig. 6.
The total annual effective dose received by the population as a result of ingestion of drinking water was in the range 8.85–62.5 μSv y−1 with a mean of 30.1 μSv y−1. It is evident that the calculated dose varies over a wide range, but all values are below the recommended level of reference dose level of 100 µSv for 1 year’s consumption of drinking water. Consequently, the health hazards related to 238U, 234U and 210Po in drinking waters are expected to be negligible. The values of the total annual effective dose for adult received from the consumption of analyzed drinking water sources are in good agreement with the results obtained by us in previous studies [27, 28] for drinking water in the Southern regions of Bulgaria. Contribution of each radionuclide to the total annual dose is given in Fig. 7.
As seen from the results obtained, the highest contribution to the total effective dose in the water investigated comes from 234U (up to 54%). 238U dose contribution is around 36% for drinking waters. The lowest contribution was found for 210Po (up to 10%).
Based on the results obtained in this study, we can conclude that the main contribution to the formation of the total annual effective dose is due to the uranium radionuclides 234U and 238U.
Conclusions
Investigations of natural radionuclides in drinking water sources in South-Central Bulgaria are reported for the first time. The results show that the activity concentrations of 238U, 234U and 210Po varied from 79 to 826 mBq l−1, 130 to 1623 mBq l−1 and < 0.5 to 25.5 mBq l−1, respectively. This is the first detailed study of the uranium and polonium radionuclides in the drinking waters in South-Central Bulgaria.
A state of radioactive disequilibrium between 234U and 238U in water was detected. The 234U/238U activity ratio varied between 0.93 and 3.21.
The expected total annual effective doses for adults were calculated from the measured 238U, 234U and 210Po activity. The results show that the annual effective dose of ingestion of these drinking waters is lower than the recommended value of 100 µSv y−1.
According to the results of our study, the investigated drinking waters are suitable for human consumption without any radiological hazard. The new results obtained are used to assess the temporary radiological status of the water investigated, as well as, the related effective dose to the population. They will support timely and adequate measures to reduce the harmful impact of ionizing radiation on the population in cases of increased radioactivity.
References
de Oliveira J, Mazzilli BP, da Costa P, Tanigava PA (2001) Natural radioactivity in Brazilian bottled mineral waters and consequent doses. J Radioanal Nucl Chem 249:173–176. https://doi.org/10.1016/s0265-931x(00)00123-5
Nuhanović M, Mulić M, Mujezinović A, Grgić Ž, Bajić I (2015) Determination of gross alpha and beta activity and uranium isotope content in commercially available, bottled, natural spring waters. Bull Chem Technol Bosnia Herzeg 45:31–34
Marović G, Senćar J, Franić Z (1997) 226Ra in tap and mineral water and related health risk in the Republic of Croatia. Environ Monit Assess 46:233–239. https://doi.org/10.1023/A:1005794028679
Outola I, Nour S, Kurosaki H, Inn K, La Rosa J, Lucas L, Volkovitsky P, Koepenick K (2008) Investigation of radioactivity in selected drinking water samples from Maryland. J Radioanal Nucl Chem 277(1):155–159. https://doi.org/10.1007/s10967-008-0724-5
Jobbágy V, Kávási N, Somlai J, Dombovári P, Kardos R, Tibor Kovác T (2010) Radioanalytical investigations of uranium concentrations in natural spring, mineral, spa and drinking waters in Hungary. J Radioanal Nucl Chem 286:417–422. https://doi.org/10.1007/s10967-010-0711-5
Zehringer M (2019) Monitoring of natural radioactivity in drinking water and food with emphasis on alpha-emitting radionuclide. IntechOpen, London. https://doi.org/10.5772/intechopen.90166
Rojas LV, Júnior JAS, Alvarado JAC, Milan MO, Röllin S, Amaral RS, Fernández ZH, Santos JMN (2020) Natural uranium isotopes and 226Ra in surface and groundwater from a basin of a semiarid region in Brazil. J Radioanal Nucl Chem 326:1081–1089. https://doi.org/10.1007/s10967-020-07393-1
Rožmarić M, Rogić M, Benedik L, Štrok M (2012) Natural radionuclides in bottled drinking waters produced in Croatia and their contribution to radiation dose. Sci Total Environ 437:53–60. https://doi.org/10.1016/j.scitotenv.2012.07.018
Zapecza OS, Szabo Z (1986) Natural radioactivity in ground water—a review. In: Moody DW, Carr J, Chase EB, Paulson RW (eds) National water summary. Ground-water quality: selected events. USGS, Reston, pp 50–57
Abojassim AA, Mohammed HA-U (2017) Comparing of the uranium concentration in tap water samples at Al-Manathera and Al-Herra Regions of Al-Najaf, Iraq. Karbala Int J Mod Sci 3(3):111–118. https://doi.org/10.1016/j.kijoms.2017.07.001
El-Sharkawy AM (2018) 234U/238U activity ratios in groundwaters from two aquifers in Saudi Arabia, and correlation with water chemistry. J Rad Res Appl Sci 11(4):368–372. https://doi.org/10.1016/j.jrras.2018.07.005
Jia G, Torri G, Sansone U, Innocenzi P, Rosamilia S, Lullo AD, Gaudino S (2006) Concentrations and characteristics of uranium isotopes in drinking waters collected in Italy and the Balkan regions and their radiological impact on the public. Radioact Environ 8:223–234. https://doi.org/10.1016/S1569-4860(05)08016-2
Samaropoulos I, Efstathiou M, Pashalidis I, Ioannidou A (2012) Determination of uranium concentration in ground water samples of Northern Greece. EPJ Web Conf 24:03005. https://doi.org/10.1051/epjconf/20122403005
Ahmed MF, Alam L, Mohamed CAR, Mokhtar MB, Ta GC (2018) Health risk of polonium 210 ingestion via drinking water: an experience of Malaysia. Int J Environ Res Public Health 15(10):2056. https://doi.org/10.3390/ijerph15102056
Milena-Pérez A, Piñero-García F, Expósito-Suárez VM, Mantero J, Benavente J, Ferro-García MA (2018) Determination and dose contribution of uranium isotopes and 210Po activity concentrations of natural spring waters in the Province of Granada, Spain. Radiat Prot Dosim 181(4):350–359. https://doi.org/10.1093/rpd/ncy034
Francés ID, Mantero J, Manjón G, Díaz J, García-Tenorio R (2013) 210Po and 238U isotope concentrations in commercial bottled mineral water samples in Spain and their dose contribution. Radiat Prot Dosim 156(3):336–342. https://doi.org/10.1093/rpd/nct075
Seiler R (2016) 210Po in drinking water, its potential health effects, and inadequacy of the gross alpha activity MCL. Sci Total Environ 568:1010–1017. https://doi.org/10.1016/j.scitotenv.2016.05.044
Harada K, Burnett WC, LaRock PA, Cowart JB (1989) Polonium in Florida groundwater and its possible relationship to the sulfur cycle and bacteria. Geochim Cosmochim Acta 53:143–150. https://doi.org/10.1016/0016-7037(89)90281-0
Ordinance No 9 on the quality of water intended for drinking and domestic purposes, SG 30 of 2001, 2001 and Amendments, Ministry of Environment and Waters (in Bulgarian)
WHO (2011) Guidelines for drinking water quality, 4th edn. World Health Organization, Geneva
Radenković MB, Joksić JD, Kovačević J (2015) Natural radionuclides content and radioactive series disequilibrium in drinking waters from Balkans region. J Radioanal Nucl Chem 306:295–299. https://doi.org/10.1007/s10967-014-3858-7
El-Aassy IE, El-Feky MG, Issa FA, Ibrahim NM, Desouky OA, Khattab MR (2014) Uranium and 234U/238U isotopic ratios in some groundwater wells at Southwestern Sinai, Egypt. J Radioanal Nucl Chem 303(1):357–362. https://doi.org/10.1007/s10967-014-3461-y
Beyermann M, Bünger T, Schmidt K, Obrikat D (2010) Occurrence of natural radioactivity in public water supplies in Germany: 238U, 234U, 235U, 228Ra, 226Ra, 222Rn, 210Pb, 210Po and gross activity concentrations. Radiat Prot Dosim 141(1):72–81. https://doi.org/10.1093/rpd/ncq139
Walencik A, Kozłowska B, Dorda J, Szłapa P, Zipper W (2010) Natural radioactivity in underground waters. Pol J Environ Stud 19(2):461–465. https://doi.org/10.1016/j.apradiso.2009.09.056
Jia G, Torri G, Magro L (2009) Concentrations of 238U, 234U, 235U, 232Th, 230Th, 228Th, 226Ra, 228Ra, 224Ra, 210Po, 210Pb and 212Pb in drinking water in Italy: reconciling safety standards based on measurements of gross α and β. J Environ Radioact 100:941–949. https://doi.org/10.1016/j.jenvrad.2009.07.002
Vasile M, Loots H, Jacobs K, Verheyen L, Sneyers L, Verrezen F, Bruggeman M (2016) Determination of 210Pb, 210Po, 226Ra, 228Ra and uranium isotopes in drinking water in order to comply with the requirements of the EU ‘drinking water directive.’ Appl Radiat Isot 109:465–469. https://doi.org/10.1016/j.apradiso.2015.11.076
Slavchev B, Geleva E, Protohristov H, Dobrev L, Dimitrova D, Tonev D (2020) Investigation of natural radionuclides in drinking and mineral waters in Bulgaria and related dose assessment. C R Acad Bulg Sci 73(6):791–799
Slavchev B, Tonev D, Dobrev L, Geleva E, Veleva B, Protohristov H, Goutev N, Demerdjiev A, Dimitrova D (2022) Uranium and 210Po radionuclides in drinking water in southern Bulgaria and expected radiation doses. Radiat Prot Dosim 198(5):299–309. https://doi.org/10.1093/rpd/ncac039
Popov K, Velichkov D, Popov P (2016) The post-collisional Upper Thracian Rift system (Bulgaria) and the formed exogenous uranium deposits. Part 2—Metallogeny of the Upper Thracian uranium ore region. Rev Bulg Geol Soc 77(1):51–64
Dobrev L, Nonova T (2020) Determination of uranium content in ammonium uranyl carbonate (AUC) and triuranium octoxide (U3O8). J Radioanal Nucl Chem 326(3):1543–1550. https://doi.org/10.1007/s10967-020-07471-4
Kanisch G (2004) IAEA TECDOC-1401 alpha spectrometric analysis of environmental samples, Vienna
Ortega X, Vaks I, Serrano I (1996) Natural radioactivity in drinking water in Catalonia (Spain). Environ Int 22(1):S347–S354
Calin MR, Ion AC, Radulescu I (2014) Evaluation of quality parameters and natural radionuclides concentrations in natural mineral water in Romania. J Radioanal Nucl Chem 303:305–313. https://doi.org/10.1007/s10967-014-3401-x
Diab HM, Monged MHE, Khattab M (2013) Measurements of natural radioactivity in some granite samples using alpha spectrometric analysis. Open J Appl Sci 3:514–518. https://doi.org/10.4236/ojapps.2013.38060
Ioannidou A, Samaropoulos I, Efstathiou M, Pashalidis I (2011) Uranium in ground water samples of Northern Greece. J Radioanal Nucl Chem 289:551–555. https://doi.org/10.1007/s10967-011-1115-x
Cinelli G, De Cort M, Tollefsen T, Achatz M et al (2019) European atlas of natural radiation. Publications Office of the European Union, Luxembourg
Pintilie V, Georgescu L-V, Moraru L, Ene A, Iticescu C (2016) Natural radioactivity in drinking water from Galati and Vrancea areas, Romania. Rad Appl 1(3):165–170. https://doi.org/10.21175/RadJ.2016.03.031
Begy R-C, Savin C-F, Süle D-K, Nuhanovic M, Giagias E, Kovács T (2022) Radiological investigation of natural carbonated spring waters from Eastern Carpathians, Romania. J Radioanal Nucl Chem 331:1439–1450. https://doi.org/10.1007/s10967-022-08195-3
Walsh M, Wallner G, Jennings P (2014) Radioactivity in drinking water supplies in western Australia. J Environ Radioact 130:56–62. https://doi.org/10.1016/j.jenvrad.2013.12.016
ICRP (International Commission of Radiological Protection) (1996) Age-dependent doses to the members of the public from intake of radionuclides part 5, compilation of ingestion and inhalation dose coefficients. ICRP publication 72. Pergamon Press, Oxford
Acknowledgements
The research has been supported by Bulgarian Science Fund under Contract No. KP-06-N44/1, 27.11.2020 and the National Science Program “Environmental Protection and Reduction of Risks of Adverse Events and Natural Disasters”, approved by the Resolution of the Council of Ministers No 577/ 17.08.2018 and supported by the Ministry of Education and Science of Bulgaria (Agreement No D01-271/09.12.2022).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tonev, D., Geleva, E., Slavchev, B. et al. Investigation of natural radioactivity in drinking water sources in South-Central Bulgaria. J Radioanal Nucl Chem 332, 4641–4649 (2023). https://doi.org/10.1007/s10967-023-08983-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-023-08983-5