Abstract
Direct and rapid determination of trace amounts of uranium in natural water remains a challenge. Herein, determination of uranium by differential pulse adsorptive stripping voltammetry (DPAdSV) in presence of cupferron and diphenylguanidine on glassy carbon electrode was investigated. Under optimized conditions, the DPAdSV peak current was proportional to the concentration of uranium in the range of 3–80 μg L−1 with the detection limit of 1.0 μg L−1 and a linear correlation coefficient of 0.999. The DPAdSV method based on GCE was successfully applied to direct determination of trace uranium in natural water samples.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As one of the primary clean energy sources, nuclear power can provide reliable and safe electricity power without emitting greenhouse gases. Uranium is called the new energy elite because it is one of the most important raw materials in nuclear energy industry [1]. However, its radioactive waste can endanger human health, like causing kidney damage and acute arterial disease. These potential hazards have aroused public concern, especially after the Fukushima Daiichi nuclear accident in 2011. The World Health Organization (WHO) has regulated the maximum concentration level of uranium in drinking water no more than 30 μg L−1 [2,3,4,5]. Thus, it is important to monitor the concentration of U(VI) in nuclear power plant, uranium mining area and environmental waters [6,7,8,9].
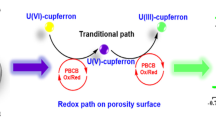
Many efforts have been made to detect uranium in environmental water samples using various of methods, such as inductively coupled plasma optical emission spectrometry (ICP-OES) and ICP-MS [10,11,12,13], fluorescence spectroscopy [5, 7, 8, 14,15,16], UV–vis spectrophotometry [17,18,19], neutron activation analysis [20, 21] and radiometric techniques [22, 23] (alpha- and gamma-spectrometry) combined with some separation techniques to further improve sensitivity. However, these methods are usually based on complex operation, high cost of apparatus and maintenance, which are not available for monitoring trace uranium in small laboratories and on-site environment [10]. Electrochemical analysis is relatively simple, convenient and low cost in operation [4, 6, 24]. Adsorptive stripping voltammetry (AdSV) based on complexing reagent of uranium [4, 25,26,27,28,29,30] on mercury electrodes provides low limits of detection and reliable analytical procedures [6, 31,32,33,34]. A parallel chemical re-oxidation of U(III) to U(V) was due to the complexation and oxidation of cupferron, and hence to an enhanced signal on mercury electrode [30]. The commonly used mercury electrode shows excellent electrochemical performances due to a fresh electrode surface and forming amalgams; however, its toxicity and the complex of operation device cannot be ignored [3, 27, 31].
The mercury-free electrodes hold promise to monitor uranium in environmental water samples [28, 35,36,37,38]. Zhang et al. [38] have made progress in the determination of uranium by tin–bismuth alloy electrode and proposed a more detailed mechanism of U(VI)-cupferron. Among these mercury-free electrodes, carbon material electrodes, like glassy carbon electrode (GCE), carbon paste electrode and graphite electrode, have received extensive attention in radioanalytical chemistry due to their non-toxicity, wide potential window and low cost [35, 39,40,41,42,43]. The GCE was employed as a common electrode material for trace uranium analysis combined with a complexing agent due to good electrical conductivity and electrocatalytic activity in diverse redox reactions. This suggests that GCE as a work electrode combined with cupferron is promising to determine trace uranium by DPAdSV [44, 45].
In this work, the GCE was used for direct determination of uranium by DPAdSV in presence of cupferron and exhibited decent sensitivity and reproducibility. Cupferron as a complexing agent and diphenylguanidine as a synergistic reagent were studied. Under the controllable conditions, the determination of uranium reached a lower detection limit of part-per-billion level. This method was applied for uranium detection in real water samples.
Experimental
Reagents and solutions
All chemicals, namely, cupferron, ethanol and HNO3 (Sinopharm Chemical Reagent Co., Ltd), diphenylguanidine (Shanghai Aladdin Biochemical Technology Co., Ltd) were used as received. Uranyl nitrate stock solution was prepared by dissolving U3O8 in diluted nitric acid. 0.1 M diphenylguanidine was prepared by diluting appropriate amount of reagent in ethanol. 0.1 M acetate buffer solution was obtained under different conditions by mixing the stock solution of acetic acid and sodium acetate. A fresh solution of 0.2 M cupferron was prepared in double-distilled water (DDW).
Apparatus
The cyclic voltammetry (CV) and DPAdSV were performed using PARSTAT 2273 electrochemical workstation (AMET Co., Ltd., Princeton, USA) and CHI 660D electrochemical workstation (Shanghai Chenhua Instrument Co., Ltd., China). A conventional three-electrode cell containing GCE (Φ 1, 3 mm) as the working electrode, a saturated calomel electrode (SCE) as the reference electrode and a platinum foil as the counter electrode was used. The pH measurements were performed with a PHS-3C pH meter (INESA Scientific Instrument Co., Ltd., China).
Electrode preparation
GCE was carefully polished to a mirror-like surface with 0.3 μm and then 0.05 μm gamma alumina slurry, rinsed with DDW in each polishing step, next ultrasonicated in DDW for 2 min to remove some adsorbed alumina particles or dirt from the electrode surface [46, 47].
Analytical procedure
The detection of UO22+ was performed in an electrochemical cell containing 0.1 M acetate buffer solution and a certain concentration of cupferron and diphenylguanidine. The deposition of UO22+ was carried out at − 0.95 V (vs SCE) under stirring with a deposition time of 120–180 s, and then the stripping voltammogram was recorded in quiescent solution from − 0.8 to − 1.3 V (vs SCE) by DPAdSV. Each measurement was repeated thrice. All electrochemical experiments were carried out at room temperature.
Results and discussion
Voltammetry determination in uranium–cupferron system
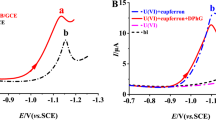
An excellent complexing agent cupferron was used to determine metal ions by voltammetry [6, 48]. The uranium–cupferron complex formed with diphenylguanidine synergistic effect was strongly adsorbed on the surface of GCE, leading to higher peak current of uranium [30, 38]. Figure 1a shows the cyclic voltammogram of 0.5 mg L−1 U(VI) in 0.1 M pH 4.5 acetate buffer solution. The cathodic peak current of uranium (solid curve) was obtained obviously while not observed at concentration of 10 μg L−1 U(VI). Figure 1b shows the DPAdSV voltammogram of 10 μg L−1 U(VI) in 0.1 M pH 4.5 acetate buffer solutions. Compared to the uranium stripping peak current, it can be clearly seen that a sharper increase of the uranium stripping current was exhibited with the addition of diphenylguanidine in the cupferron and uranyl solution. The diphenylguanidine radical cations may induce adsorption of uranium–cupferron complexes during preconcentration, resulting in higher response currents [38].
a Cycle voltammetry in 0.1 M acetate buffer of pH 4.5 for 0.5 mg·L−1 U(VI) with 160 μM cupferron and 40 μM diphenylguanidine (solid), 0.5 mg·L−1 U(VI) with 160 μM cupferron (short dash dot), 0.5 mg·L−1 U(VI) (dash dot) and reagent blank (dash). Scan rate, 100 mV s−1. b DPAdSV in 10 μg L−1 U(VI) in the same solution composition as a. Scan rate, 50 mV s−1; pulse height, 25 mV; step height, 5 mV; step time, 0.1 s, deposition time 120 s, deposition potential − 0.95 V
Effect of pH
The pH values play an important role on the complexation of uranyl ions in solution [49]. Herein, acetate buffer solution chosen as the supporting electrolytes is ascribed to the stable complexes of acetate and uranyl ion [50]. As shown in Fig. 2a, uranyl species with positive or neutral charge are present in the solution of the pH range of 3–4, whereas at pH > 4.5 neutral and anionic species prevail. In addition, the effect of pH suggests that the coordination interaction of the ligand cupferron with U(VI) competes with the acetate in solution. As shown in Fig. 2b, the uranium stripping peak current was strongly affected by pH values. The uranium stripping response decreased at pH < 4.5, which was a consequence of the reduction of cupferron to phenylhydrazine on the surface of electrode [6, 30]. The uranium peak decreased at pH > 4.5 because the hydrolysis of UO22+ ions resulted in great decrease of electroactive complex on the surface of electrode [38].
Effect of different pH on a U(VI) speciation [49], as predicted by MINEQL + for conditions of 10 μg L−1 U(VI) in 0.1 M acetate buffer, and b the peak current of 10 μg L−1 U(VI), with 160 μM cupferron and 40 μM diphenylguanidine in 0.1 M acetate buffer using deposition of 120 s and deposition potential of − 0.95 V
Effect of the cupferron and diphenylguanidine concentration
The impact of the concentration of cupferron and diphenylguanidine on the uranium stripping peak current was further investigated. As shown in Fig. 3a, it was clear that net current of cupferron and U(VI)-cupferron (∆Ip = Ip(U) − Ip(bl)) reached a maximum with the concentration of 160 μM cupferron and 40 μM diphenylguanidine. The ∆Ip increased at the concentration of cupferron lower than 160 μM, which was the effect of complex between cupferron and UO22+ in solution. The ∆Ip rapidly decreased at the concentration of cupferron higher than 160 μM, which was attributed to the increased ligand concentrations to reduce the stability of U(VI)-cupferron complex [38]. The effect of concentration of diphenylguanidine was exhibited in Fig. 3b. The ∆Ip increased rapidly with the diphenylguanidine concentration before the concentration of diphenylguanidine is up to 40 μM. In the weak acid buffering solution, the protonation of diphenylguanidine might induce uranium–cupferron adsorption on the surface of electrode, resulting in higher peak current of uranium [51]. However, the net current dropped sharply after the diphenylguanidine concentration was higher than 40 μM. It was probably because of diphenylguanidine affecting the coordination between cupferron and U(VI). Therefore, 160 μM cupferron and 40 μM diphenylguanidine were selected for the following experiments.
Effect of deposition potential and deposition time
The different deposition potential causes the change of interface potential, surface charge and potential distribution of the electrode/solution electric double layer of a solid electrode surface [52]. In order to further study the effect of the deposition potential on the peak current of uranium, it was investigated between − 0.75 and − 1.10 V during a constant deposition time of 120 s. The voltammetry scan was initiated after a pause of 10 s without stirring to allow the electrode to equilibrate. As shown in Fig. 4a, the uranium stripping peak current increased at potential range of − 0.75 to − 0.95 V because of high-efficiency deposition of U(VI)-cupferron complex on the surface of electrode [31]. The uranium peak decreased at potential range of − 0.95 to − 1.1 V as a result of the U(VI)-cupferron adsorption saturation of the electrode surface [29, 38]. The effect of deposition time on the uranium stripping peak current was investigated between 0 and 300 s at deposition potential of − 0.95 V. As shown in Fig. 4b, it was clear that the response current rapidly increased before deposition time up to 120 s and then almost leveled off, which indicated that electroactive substance adsorptive equilibrium was achieved at electrode surface [28, 29]. Therefore, deposition potential of − 0.95 V and deposition time of 120 s were chosen for the following investigation.
Real sample analysis
The main deficiency of stripping voltammetry methods based on the adsorption accumulation of U(VI) complexes is that they are susceptible to disturbances caused by organic compounds present in natural environmental samples, like surfactants [4, 29]. Some common surfactants are known to interfere with analysis quality of environmental water samples [32]. As shown in Fig. 5a, 2 mg L−1 Triton X-100 decreased the uranium signal for about 10%; however, caused peak sharp decrease at higher concentrations. Although the uranium signal decreased about 20% at the concentration of 4 mg L−1 SDS, uranium peak trended to level off with the concentration increasing. An interesting phenomenon observed was that the uranium peak decreased in the concentration range of 0 to 4 mg L−1 CTAB and then increased from 4 to 8 mg L−1 CTAB. It seems that the competitive adsorption of CTAB and uranium–cupferron was generated on the surface of electrode resulting in the change of the ratio of redox uranium species. Since environmental water samples usually contain 0.01–2 mg L−1 surfactant like Triton X-100 [53], there is almost no effect on the determination of uranium. The effect of foreign ions on the uranium stripping peak current was studied in Fig. 5b. It clearly shows that the uranium peak decreased more than 20% with the addition of Co2+, Cu2+ and Pb2+, but other ions have less effect on uranium response current. Considering the low concentration level of Co2+, Cu2+ and Pb2+ in the environment water, the uranium may be detected directly in real environmental water samples.
Figure 6 shows that the peak current rises linearly with the increase of U(VI) concentration based on DPAdSV with the following calibration equation: (a) y = 7.16 + 0.096 x (R2 = 0.999) and (b) y = 0.78 + 0.023 x (R2 = 0.997). The detection limit, calculated 3sbl/slope, was 1.0 μg L−1. The precise uranium stripping peak currents were obtained with a relative standard deviation (RSD) of 5.03% (n = 6). The stripping response of the GCE was examined by carrying out a series of repetitive experiments for a fixed concentration of U(VI) of 10 μg L−1. Repeatability and reproducibility of peak currents were obtained with RSD of 4.4% (n = 30) and 6.1% (n = 21), respectively.
The electrodes proposed in this work were used for direct determination of U(VI) in environmental water samples. The spiked method was used to detect low concentration of U(VI) in natural water to evaluate the performance of the proposed GCE in real sample analysis. The water used in this work was sampled from tap water and East China University of Technology Twin Lakes. The water samples were simply filtered with a long-neck funnel to remove insoluble substances, then the sample acidification before sealed for refrigeration. 5.00 mL water sample was fractioned in 25 ml volumetric flask containing 0.1 M acetate buffer pH 4.5, 160 μM cupferron, and 40 μM diphenylguanidine, and then detected using DPAdSV. Table 1 shows good recovery rates of spiked amount of uranyl ions in the tap water and lake water sample. The comparison test results obtained from the DPAdSV are in accordance well with those detected by ICP-MS, which confirmed the accuracy and precision of the proposed DPAdSV. In addition, Table 2 provides a comparison of the results of the present work versus that of other voltammetric procedures of U(VI) determination in natural waters. The GCE seems to be a promising chemical sensor for the determination of uranium in environmental water.
Conclusion
In summary, a convenient and sensitive differential pulse adsorptive stripping voltammetry method for the determination of trace uranium in presence of cupferrron on a GCE was developed. Effects of several parameters, like pH values, concentration of complexing agent, deposition potential and deposition time were investigated. The uranium was detected quickly and efficiently with a detection limit of 1.0 μg L−1, which was well below the concentration of 30 μg L−1 by WHO guideline in drinking water. In addition, the GCE based on DPAdSV was evaluated for its analytical performance using tap water, lake water and interfering substances, such as surfactants and some metal ions. Our results showed a very simple and economic approach for the determination of uranium in environmental water samples. Therefore, the GCE holds promise to be used in the field of electrochemical sensing in the environment water samples.
References
Liu C, Hsu PC, Xie J, Zhao J, Wu T, Wang H, Liu W, Zhang J, Chu S, Cui Y (2017) A half-wave rectified alternating current electrochemical method for uranium extraction from seawater. Nat Energy 2(4):17007–17014
Gupta R, Sundararajan M, Gamare JS (2017) Ruthenium nanoparticles mediated electrocatalytic reduction of UO2 2+ ions for its rapid and sensitive detection in natural waters. Anal Chem 89(15):8156–8161
Wu X, Huang Q, Mao Y, Wang X, Wang Y, Hu Q, Wang H, Wang X (2019) Sensors for determination of uranium: a review. TrAC Trends Anal Chem 118:89–111
Tyszczuk-Rotko K, Domańska K, Czech B, Rotko M (2017) Development simple and sensitive voltammetric procedure for ultra-trace determination of U(VI). Talanta 165:474–481
Wu P, Hwang K, Lan T, Lu Y (2013) A DNAzyme-gold nanoparticle probe for uranyl ion in living cells. J Am Chem Soc 135(14):5254–5257
Wang J, Setiadji R (1992) Selective determination of trace uranium by stripping voltammetry following adsorptive accumulation of the uranium—cupferron complex. Anal Chim Acta 264(2):205–211
Liu J, Brown AK, Meng X, Cropek DM, Istok JD, Watson DB, Lu Y (2007) A catalytic beacon sensor for uranium with parts-per-trillion sensitivity and millionfold selectivity. Proc Natl Acad Sci 104(7):2056
Chen X, Zhang K, Yu H, Yu L, Ge H, Yue J, Hou T, Asiri AM, Marwani HM, Wang S (2018) Sensitive and selective fluorescence detection of aqueous uranyl ions using water-soluble CdTe quantum dots. J Radioanal Nucl Chem 316(3):1011–1019
Farzin L, Shamsipur M, Sheibani S, Samandari L, Hatami Z (2019) A review on nanomaterial-based electrochemical, optical, photoacoustic and magnetoelastic methods for determination of uranyl cation. Microchim Acta 186(5):289–315
Santos JS, Teixeira LSG, dos Santos WNL, Lemos VA, Godoy JM, Ferreira SLC (2010) Uranium determination using atomic spectrometric techniques: an overview. Anal Chim Acta 674(2):143–156
Akl ZF (2018) Sensitive quantification of uranium using cloud point extraction coupled with inductively coupled plasma-optical emission spectrometry. J Radioanal Nucl Chem 315(1):21–28
Arnason JG, Pellegri CN, Parsons PJ (2015) Determination of total uranium and uranium isotope ratios in human urine by ICP-MS:results of an interlaboratory study. J Anal At Spectrom 30(1):126–138
Qiao J, Lagerkvist P, Rodushkin I, Salminen-Paatero S, Roos P, Lierhagen S, Jensen KA, Engstrom E, Lahaye Y, Skipperud L (2018) On the application of ICP-MS techniques for measuring uranium and plutonium: a Nordic inter-laboratory comparison exercise. J Radioanal Nucl Chem 315(3):565–580
Chen X, He L, Wang Y, Liu B, Tang Y (2014) Trace analysis of uranyl ion (UO2 2+) in aqueous solution by fluorescence turn-on detection via aggregation induced emission enhancement effect. Anal Chim Acta 847:55–60
Drobot B, Bauer A, Steudtner R, Tsushima S, Bok F, Patzschke M, Raff J, Brendler V (2016) Speciation studies of metals in trace concentrations: the mononuclear uranyl(VI) hydroxo complexes. Anal Chem 88(7):3548–3555
Jiang M, Xiao X, He B, Liu Y, Hu N, Su C, Li Z, Liao L (2019) A europium (III) complex-based surface fluorescence sensor for the determination of uranium (VI). J Radioanal Nucl Chem 321(1):161–167
Borák J, Slovák Z, Fischer J (1970) Verwendung mäßig dissoziierter komplexe bei spektralphotometrischen bestimmungen—II: Reaktionen von arsenazo iii mit uranyl und thorium(IV). Talanta 17(3):215–229
Shamsipur M, Ghiasvand AR, Yamini Y (1999) Solid-phase extraction of ultratrace uranium(VI) in natural waters using octadecyl silica membrane disks modified by tri-n-octylphosphine oxide and its spectrophotometric determination with dibenzoylmethane. Anal Chem 71(21):4892–4895
Jain VK, Handa A, Sait SS, Shrivastav P, Agrawal YK (2001) Pre-concentration, separation and trace determination of lanthanum(III), cerium(III), thorium(IV) and uranium(VI) on polymer supported o-vanillinsemicarbazone. Anal Chim Acta 429(2):237–246
Murthy RSS, Ryan DE (1983) Determination of arsenic, molybdenum, uranium, and vanadium in seawater by neutron activation analysis after preconcentration by colloid flotation. Anal Chem 55(4):682–684
Hosseini MA, Ahmadi M (2017) Miniature neutron source reactors in medical research: achievements and challenges. J Radioanal Nucl Chem 314(3):1497–1504
Hou X, Roos P (2008) Critical comparison of radiometric and mass spectrometric methods for the determination of radionuclides in environmental, biological and nuclear waste samples. Anal Chim Acta 608(2):105–139
Choi H-T, Kim T-R (2018) Necessity of management for minor earthquake to improve public acceptance of nuclear energy in South Korea. Nucl Eng Technol 50(3):494–503
Becker A, Tobias H, Mandler D (2009) Electrochemical determination of uranyl ions using a self-assembled monolayer. Anal Chem 81(20):8627–8631
Korolczuk M, Tyszczuk K, Grabarczyk M (2007) Determination of uranium by adsorptive stripping voltammetry at a lead film electrode. Talanta 72(3):957–961
Sander S (1999) Simultaneous adsorptive stripping voltammetric determination of molybdenum(VI), uranium(VI), vanadium(V), and antimony(III). Anal Chim Acta 394(1):81–89
Lin L, Thongngamdee S, Wang J, Lin Y, Sadik OA, Ly S-Y (2005) Adsorptive stripping voltammetric measurements of trace uranium at the bismuth film electrode. Anal Chim Acta 535(1):9–13
Kefala G, Economou A, Voulgaropoulos A (2006) Adsorptive stripping voltammetric determination of trace uranium with a bismuth-film electrode based on the U(VI) → U(V) reduction step of the uranium–cupferron complex. Electroanalysis 18(3):223–230
Tyszczuk-Rotko K, Jędruchniewicz K (2019) Ultrasensitive sensor for uranium monitoring in water ecosystems. J Electrochem Soc 166(10):B837–B844
Paneli M, Ouguenoune H, David F, Bolyos A (1995) Study of the reduction mechanism and the adsorption properties of uranium (VI)-cupferron complexes using various electrochemical techniques. Anal Chim Acta 304(2):177–186
Rashidi Nassab H, Bakhshi M, Amini MK (2014) Adsorptive cathodic stripping voltammetric determination of uranium(VI) in presence of N-phenylanthranilic acid. Electroanalysis 26(7):1598–1605
Grabarczyk M, Koper A (2011) Adsorptive stripping voltammetry of uranium: elimination of interferences from surface active substances and application to the determination in natural water samples. Anal Methods 3(5):1046–1050
Gholivand MB, Nassab HR, Fazeli H (2005) Cathodic adsorptive stripping voltammetric determination of uranium (VI) complexed with 2, 6-pyridinedicarboxylic acid. Talanta 65(1):62–66
Wang J, Wang J, Tian B, Jiang M (1997) Adsorptive stripping measurements of chromium and uranium at iridium-based mercury electrodes. Anal Chem 69(8):1657–1661
Dimovasilis PA, Prodromidis MI (2011) An electrochemical sensor for trace uranium determination based on 6-O-palmitoyl-l-ascorbic acid-modified graphite electrodes. Sens Actuators B Chem 156(2):689–694
Peled Y, Krent E, Tal N, Tobias H, Mandler D (2015) Electrochemical determination of low levels of uranyl by a vibrating gold microelectrode. Anal Chem 87(1):768–776
Piech R, Baś B, Kubiak WW (2007) The cyclic renewable mercury film silver based electrode for determination of uranium(VI) traces using adsorptive stripping voltammetry. Electroanalysis 19(22):2342–2350
Zhang L, Wang C-Z, Tang H-B, Wang L, Liu Y-S, Zhao Y-L, Chai Z-F, Shi W-Q (2015) Rapid determination of uranium in water samples by adsorptive cathodic stripping voltammetry using a tin-bismuth alloy electrode. Electrochim Acta 174:925–932
Rashidi Nassab H, Souri A, Javadian A, Amini MK (2015) A novel mercury-free stripping voltammetric sensor for uranium based on electropolymerized N-phenylanthranilic acid film electrode. Sens Actuators Chem 215:360–367
Gupta VK, Mangla R, Khurana U, Kumar P (1999) Determination of uranyl ions using poly(vinyl chloride) based 4-tert-butylcalix 6 arene membrane sensor. Electroanalysis 11(8):573–576
Guney S, Guney O (2016) A novel electrochemical sensor for selective determination of uranyl ion based on imprinted polymer sol-gel modified carbon paste electrode. Sens Actuators B Chem 231:45–53
Agarwal R, Sharma MK, Jayachandran K, Gamare JS, Noronha DM, Lohithakshan KV (2018) Poly(3,4-ethylenedioxythiophene)–poly(styrenesulfonate)-coated glassy-carbon electrode for simultaneous voltammetric determination of uranium and plutonium in fast-breeder-test-reactor fuel. Anal Chem 90(17):10187–10195
Ziółkowski R, Górski Ł, Malinowska E (2017) Carboxylated graphene as a sensing material for electrochemical uranyl ion detection. Sens Actuators B Chem 238:540–547
Pretty JR, Duckworth DC, Van Berkel GJ (1998) Electrochemical sample pretreatment coupled on-line with ICP-MS: analysis of uranium using an anodically conditioned glassy carbon working electrode. Anal Chem 70(6):1141–1148
Golikand AN, Asgari M, Maragheh MG, Lohrasbi E (2009) Carbon nanotube-modified glassy carbon electrode for anodic stripping voltammetric detection of uranyle. J Appl Electrochem 39(1):65–70
Ding M, Zhou Y, Liang X, Zou H, Wang Z, Wang M, Ma J (2016) An electrochemical sensor based on graphene/poly(brilliant cresyl blue) nanocomposite for determination of epinephrine. J Electroanal Chem 763:25–31
Xie F, Zhou Y, Liang X, Zhou Z, Luo J, Liu S, Ma J (2019) Permselectivity of electrodeposited polydopamine/graphene composite for voltammetric determination of dopamine. Electroanalysis 31(9):1744–1751
Jost CL, di Martos LM, Ferraz L, do Nascimento PC (2016) Sequential voltammetric determination of uranium, cadmium and lead by using the ex situ bismuth film electrode: application to phosphate fertilizers. Electroanalysis 28(2):287–295
Yantasee W, Lin Y, Fryxell GE, Wang Z (2004) Carbon paste electrode modified with carbamoylphosphonic acid functionalized mesoporous silica: a new mercury-free sensor for uranium detection. Electroanalysis 16(10):870–873
Sladkov V, Roques J (2016) Deactivation of lowest excited state of uranyl in the presence of acetate: a DFT exploration. J Photochem Photobiol A 322–323:10–15
Koll A, Rospenk M, Bureiko SF, Bocharov VN (1996) Molecular structure and association of diphenylguanidine in solution. J Phys Org Chem 9(7):487–497
Sun Y-C, Mierzwa J, Lan C-R (2000) Direct determination of molybdenum in seawater by adsorption cathodic stripping square-wave voltammetry. Talanta 52(3):417–424
Grabarczyk M, Koper A (2011) How to determine uranium faster and cheaper by adsorptive stripping voltammetry in water samples containing surface active compounds. Electroanalysis 23(6):1442–1446
Acknowledgements
This work is supported by National Natural Science-Foundation of China (No. 21667002, 21866001), Education Department of Jiangxi Province (No. GJJ150611, GJJ170435), Jiangxi Key Laboratory for Mass Spectrometry and Instrumentation (No. JXMS201507) and Jiangxi Province Key Laboratory of Polymer Micro/Nano Manufacturing and Devices.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
There are no conflicts to declare.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zhou, Z., Zhou, Y., Liang, X. et al. Sensitive detection of uranium in water samples using differential pulse adsorptive stripping voltammetry on glassy carbon electrode. J Radioanal Nucl Chem 322, 2049–2056 (2019). https://doi.org/10.1007/s10967-019-06892-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-019-06892-0