Abstract
A co-precipitation method followed by a liquid–liquid extraction and liquid scintillation counting is proposed to get low 226Ra detection limits when measuring complex samples. The sensitivity of the method is determined with an analysis of interfering agents on it. The ion-exchange followed by alpha spectrometry is also described and an analysis of the advantages and disadvantages of both methods is carried out. Then, a comparison between both methods, considering also time and cost analysis, is realised. Conclusions show that both methods provide similar values for uncertainties, critical values and chemical yields when similar sample size and measuring times are chosen.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
226Ra is one of the radionuclides to be measured when materials coming from NORM (naturally occurring radioactive material) and TE-NORM (technologically enhanced naturally occurring radioactive material) industries are analysed, and also when deciding if an industry should be considered as a NORM or a TE-NORM one.
There are different methods to determine 226Ra, depending on the pre-treatment of the sample and on the equipment used to carry out the radiometric measurement. Typical methods obtained from literature include gamma-spectrometry, direct or after a co-precipitation process, alpha-spectrometry after Ra radiochemical separation using ion-exchange and co-precipitation followed by proportional counter or liquid scintillation counter (LSC) [1, 2].
Among these methods, gamma-spectrometry measurements are the easiest ones. However, the 226Ra emission rate at 186.2 keV is quite small (3.6%) and its interference with 185.7 keV peak from 235U must be taken into account. Thus, once 226Ra/214Pb/214Bi reach radioactive equilibrium after ~ 25 days, gamma peaks from these last radionuclides are used to evaluate 226Ra activity [3]. Due to the low efficiency of these detection systems, the detection limits reached are quite high, as well as the sample size needed to get them and also the counting time (e.g. 35.3 Bq/kg for a sample mass of 0.2 kg and for 86,400 s of counting time).
Nevertheless, as the 226Ra regulation limits imposed for NORM materials are quite high (e.g. the value for exemption or clearance for NORM is 1 Bq g−1 [4]), the required detection limit for regulatory enforcement can usually be achieved by gamma-spectrometry measurements. However, in the cases when a NORM industry is studied from a radiological point of view to define radiation protection countermeasures for workers and general public, it is interesting to analyse the flow of different radionuclides in various products and by-products throughout the industrial process under study, to know their preferential pathways. In these cases, lower detection limits than those required by regulations are needed and, therefore, other methods should be applied.
The alpha measurement by a gas-flow proportional counter, after a Ra co-precipitation process, is not a radionuclide specific procedure, and thus it can have several interferences leading to non-reliable results. This method provides intermediate detection limits.
Hence, to achieve lower detection limits using a small sample size, two methods can be used: radiochemical radium separation by ion-exchange followed by alpha-spectrometry (hereinafter referred to as the “IE-A method”) and 226Ra determination via liquid scintillation counting after co-precipitation.
In this paper, a determination procedure for complex samples, based on the use of an HPA (High Pressure Asher), which allows to dissolve a great variety of samples, even flammable ones, followed by concentration of Ra by co-precipitation and the measurement of emanated Rn, using a liquid–liquid extraction step, by a LSC, is proposed. The method referred hereinafter as “C-LSC method” has the ability to isolate 226Ra from other interfering radionuclides and provides low level detection limits. The developed method is a coupling between a commonly used one to concentrate Ra [2] and a standard method for water samples [6]. The ability the proposed method shows for complex samples is analysed in this work.
There are different papers devoted to the evaluation of Ra in water samples by a LSC [1, 2, 5, 6], being the references 1 and 2 a good summary of most of them; however, the amount of papers devoted to the Ra determination in solid or complex ones is quite reduced [7,8,9,10,11,12]. Among these last ones, the paper from Kim et al. [9] presents a method similar to ours but with differences in the sample pre-treatment step and also in the tracer used; these choices determine not only some chemical steps of the method but also the analysis to be carried out in order to demonstrate its applicability.
In addition, in this paper, a comparison between this developed method (C-LSC) and the IE-A method is performed taking into account their advantages and disadvantages and also the detection limits and uncertainties they provide. Also, time and financial costs of both methods are analysed.
Various types of complex samples have been chosen for these purposes. Some of them are reference materials coming from Inter-Laboratory Comparison (ILC) exercises, while others were collected from production process associated to, mainly, gas and oil extraction industries.
These industries produce a spectrum of by-products, mainly water, scales, crude oil and sludge, which are very different from a radiological, physical and chemical point of view and should be treated as very complex matrices. These by-products have different compositions and densities and show different contents of organic matter and elements like barium and rare-earth, and some of them can also be flammable. Regarding their natural radioactive content, it will be quite different not only among by-products, but also among gas/oil wells or production processes. Despite these differences, the radiological characterization of these by-products can provide a prior knowledge of these samples and on how natural radioactivity is shared among them.
Thus, the test of the proposed method with all these samples can be a proof of its applicability for complex samples.
Materials and methods
Materials
Samples and sample pre-treatment
The samples used are the following.
Reference materials of calcium carbonate and soil, coming from different ILC exercises organised by International Atomic Energy Agency (IAEA) and by Consejo de Seguridad Nuclear (CSN) (Spain).
Crude oil, sludge and scales samples coming from oil industry, a sludge sample coming from gas drilling and a NORM industrial grease sample.
Samples coming from ILC exercises are supposed to be homogenized, while the pre-treatment process applied to the other samples includes a homogenization process using a sonicator system. After homogenization, an aliquot of solid and semi-solid samples is dissolved using a High Pressure Asher HPA-S (Anton Paar) following ASTM Standard C1234-11 [13], obtaining a sample solution.
Equipment
Alpha spectrometer
A Canberra alpha-particle spectrometer, equipped with 450 mm2 passivated implanted planar silicon detectors (PIPS), is used for measurements. These measurements are carried out at a 5 mm sample-detector distance, the efficiency being approximately 25% and the detector background around 5.8 × 10−5 cps.
Samples are prepared by micro-precipitation and the obtained filter is deposited on a stainless-steel planchet. After radiochemical separation, a week must be allowed before measuring for 217At to grow from 225Ra, which comes from 229Th tracer. Its 7.07 MeV peak is used to calculate the radiochemical yield while 226Ra is evaluated through its own 4.78 MeV peak. One week is used as counting time for both samples and background.
Liquid scintillation spectrometer
An ultra-low background liquid scintillation spectrometer 1220 QUANTULUS™, from Perkin Elmer, which provides excellent efficiency for alpha counting and background ranging from 0.05 to 0.1 cpm in plastic vials, is used.
The sample counting is performed in alpha/beta discrimination mode with an optimum PSA setting of 100, and a counting time of 1 day for blank sample and between 3 and 6 h for samples depending on their activity.
A water-immiscible scintillation cocktail Ultima Gold F (Perkin Elmer) is used to allow only 222Rn to get into the scintillation cocktail. PTFE coated 20 mL polyethylene vials are also used.
As 226Ra is determined through 222Rn, around 25 days should be allowed for them to reach secular equilibrium. Only alpha emissions of 222Rn (5.49 MeV), 218Po (6.00 MeV) and 214Po (7.69 MeV) are taken into account in spectra analysis. The total detection efficiency obtained for the three alpha emissions is around 250% and background in the alpha window is around 0.07 cpm.
Developed method (C-LSC)
Procedure
After digestion, the resulting sample solution is acidified to pH 3–4 with HNO3 65% followed by addition of 5 mL of 1 M C6H8O7·H2O and 12 mg of Ba carrier. The sample is heated at 90 °C for 25 min; phenolphthalein and NH4OH 25% to basic pH (> 10) until turning to get alkaline citrate. Methyl orange is added to see the turning to pink colour (< 3.2) when the solution is acidified with H2SO4 to co-precipitate radium with BaSO4. The solution containing the precipitate is centrifuged at 1300 rpm and decanted. The precipitate is washed with 65% HNO3 and twice with water. Then, it is dissolved using 20 mL of Na2-EDTA 0.25 M and radium is co-precipitated again with BaSO4 after pH adjustment to 4.5 with acetic acid. Radium and barium precipitates are dried in an oven at 50–60 °C and dissolved using EDTA 0.25 M and ammonia up to a 5 mL.
This is quite a common method used to separate Ra [2]. In this work, we have adapted it to be used as a preliminary step before using the ISO 13165-1 standard [6], devoted to the 226Ra measurement in water by using LSC.
According to this standard, a 10 mL water sample is added into the PTFE coated 20 mL polyethylene vial and then, a 10 mL water immiscible scintillation cocktail is added. Vials are then stored in the dark inside the scintillation chamber at a constant temperature, around 15 °C, to allow secular equilibrium to be achieved between 226Ra and 222Rn. The 222Rn emanation is retained in the scintillation cocktail and so, its alpha emissions together with those coming from 218Po and 214Po are recorded in the alpha spectrum. In our case, the cocktail Ultima Gold F (Perkin Elmer) has been chosen.
An aliquot of 0.5 mL from the 5 mL solution coming from the radium co-precipitation is taken to determine radiochemical yield through barium atomic absorption (AA) spectrometry. The rest of the solution is directly added to the vial, washing with DIW up to 10 mL, that becomes the test-portion. The blank sample is prepared by adding to the vial 10 mL of the scintillation cocktail, a solution with EDTA 0.25 M, ammonia and BaSO4, as these are the main components in the solution containing radium after co-precipitation. The calibration source is prepared in the same way as the blank sample, but adding a known amount of 226Ra from the certified standard solution. Both blank sample and calibration source are stored under the same conditions as the samples.
The elapsed time between Ra separation and measurement to allow equilibrium is chosen as 25 days. The elected measuring time is between 3 and 6 h and a test-portion size typically less than 1 g, depending on the desired detection limit, is needed.
Activity concentration, uncertainty and detection limits calculus
Following measurement, the sample’s 226Ra activity concentration is calculated following Eq. (1):
where ARa is 226Ra sample’s activity concentration, rg is the gross count rate of 222Rn + 218Po + 214Po and r0 that of the blank, m is test-portion mass, \(R\) is the chemical yield and ε is the total alpha detection efficiency. ε is calculated following Eq. (2):
\(r_{sg}\) is the count rate of 226Ra calibration source, \(A_{s}\) is the activity concentration of the 226Ra certified standard solution and \(m_{s}\) is the mass added of this 226Ra standard.
The chemical yield is calculated by adding a known amount of stable Ba to the sample before its digestion and measuring it by AA after co-precipitation. However, as barium could be naturally present in the sample to be measured, its amount should be previously determined and the chemical yield calculated using Eq. (3),
where Ba, m is the amount of Ba measured after separation; Ba, a is the amount of Ba added to the sample and Ba,e is the native Ba presented in the sample. The combined standard uncertainty of 226Ra activity concentration, u (ARa), is calculated using Eq. (4) [14]:
where ω is defined as:
and, u 2 rel (ω) is calculated using Eq. (6):
where terms, urel(x), represent the relative standard uncertainties for x and t, t0 and ts are sample, blank and 226Ra calibration source counting time, respectively.
The decision threshold (DT) and the detection limit (DL) are calculated, following ISO 11929 standard [15], using Eq. (7) and (8), respectively:
where k is the quantile of the standard normal distribution probability that takes a value of 1.65 for a confidence level of 95%; in this case, the probabilities 1-α and 1-β [15] are taken as equal.
Application of the developed C-LSC method to complex samples
To analyse the performance of the method two main points are considered. The first one is its sensitivity defined by the following parameters: its ability to remove most of the interfering substances in the sample and the chemical yield, uncertainties and detection limits obtained. The second one is the stability of the sample.
Method sensitivity
To establish the sensitivity of the method the following tests have been conducted.
Interferences can be radiological and chemical. Regarding radiological interferences, only the progeny from 224Ra in the sample should be taken into account, due to the overlap of the alpha energies of radionuclides from both 226Ra and 224Ra decay chains. However, considering that, the measurement is carried out after a 25-day span and the period of 224Ra (365 days), this interference will only appear in samples with almost 100 times more 224Ra than 226Ra. Anyway, the presence of 224Ra can be controlled in the sample spectrum through the appearance of the 212Po alpha peak at 8.8 MeV. If it appears, a new measurement some days later allows for the quantification of this 226Ra alone [16].
Moreover, in this case, chemical interferences could be more important than the radiological ones because of their potential ability to prevent the radon transferring from the final solution to the scintillation cocktail and, thus, to decrease the method efficiency.
Therefore, in this paper the ability of the co-precipitation process to remove conventional chemicals present in complex samples from oil and gas industries is analysed.
In a first step, we have prepared five synthetic solution samples simulating the worst possible composition of oil-production scales [17]. That means samples with high contents of Ca, Sr, Ba, Fe and Pb.
The proportion of each element in each prepared sample is shown in Table 1.
In these samples, after the application of the co-precipitation process, the residual amount of the considered chemical elements is measured. For Sr, Ba and Fe, AA procedure is used; for Ca, a test based on complexometric tritation (Titrimetric method, Merck) and for Pb, a gravimetric method. In the case of lead, we expect around 100% to be removed, that fact being one of the principles of the precipitation method chosen. Results will assess the ability of this co-precipitation process for removing specific sample chemical components.
The presence of Ba in those samples is used for evaluation of Ra chemical yield, Ba being the element used as a carrier, so the effects of other elements on Ba recovery would also be evaluated.
Secondly, real samples of scales and sludge from oil and gas industries are taken. Samples are dissolved and divided into two aliquots. In one of the aliquots, a conventional chemical analysis is performed while, in the other, the Ra co-precipitation process is applied. The liquid resulting of this process is divided once again into two aliquots, one for LSC measurement and the other for the same chemical analysis. This analysis is performed by inductively coupled plasma mass spectrometry (ICP-MS), due to the low amount of chemicals expected in the sample. Results will assess the ability of method procedure for removing specific sample chemical components and Ba recovery with real samples.
In addition, from the LSC measurements, uncertainty and detection limit values are obtained.
Sample stability
In this method, an organic scintillator cocktail is put together with a final solution with ammonia, BaSO4 and EDTA as main components. Hence, the stability of these counting samples has been checked in this work. For this, all samples are measured at different times over more than a month after radioactive equilibrium and activity concentrations obtained and compared.
In addition, the effect of mixing, by shaking, the final solution after preparation with the scintillator in the vial is analysed. This last point is not taken into account in the ISO 13165-1 standard [6]. For this, two 226Ra real samples with low activity concentration and two 226Ra synthetic samples with high activity concentration are prepared and one of each has been shaken just before storing them inside the scintillation chamber. Then, the four vials are measured at different moments to analyse the potential differences between them depending on whether they had been shaken or not and their stability through time.
IE-A method
In this section, the method used to obtain 226Ra by alpha-spectrometry is presented.
Procedure
After sample digestion, the obtained solution is passed through a column packed with 50WX8 cation resin to separate Th and Ca present in the sample, using nitric acid to elute radium isotopes from the resin and time is recorded (Th/Ra separation time). The eluant is evaporated, dissolved and then passed through a Sr Resin column to remove native Ba in the test-portions. Ca and Ba need to be removed for minimizing mass attenuation effect in high resolution and efficiency alpha spectrometric analysis. Before loading onto Ln Resin to separate radium from its progeny (recording Ra/Ac separation time) and from other residual interferences that could remain in the sample, the obtained solution is evaporated and re-dissolved with HCl. The obtained sample is micro-precipitated with barium sulphate on a filter and put on a planchet, later tested by alpha-spectrometry [18]. To obtain the radiochemical yield, a known amount of a certified standard solution of 229Th is added to the sample before its digestion and at least 12 h are allowed to reach isotopic equilibrium before starting it.
Given the short half-lives of the radionuclides belonging to the radioactive chain of 229Th (see Fig. 1) compared to its own, it can be considered that secular radioactive equilibrium among 229Th, 225Ra and the rest of its progeny have been already established in the standard reference solution of 229Th. The radiochemical yield can be calculated through the activity observed at 7.07 MeV peak of 217At. After radiochemical separation, a week must be allowed before measuring for 217At to grow from 225Ra. One week is used as counting time for both test-portions and background.
229Th radioactive chain [19]
Activity concentration, uncertainty and detection limits calculus
Following measurement, the sample’s 226Ra activity concentration is calculated following Eq. (9):
where ARa is 226Ra sample’s activity concentration, \(r_{g}\) is the gross count rate in 226Ra spectral ROI of the sample spectrum and r0 is that of the same ROI’s of the background spectrum, m is test-portion mass, ε is the detection efficiency and R is radiochemical yield, calculated as follows:
where ε is the detector efficiency, NAt,T is the calculated number of disintegrations of 217At during counting time t, rAt,g is the gross count rate observed in 217At spectral ROI of the sample spectrum and rAt,0 that of the same ROI’s of the background spectrum. NAt,T is calculated using Eq. (11), where t1 is the time elapsed between 229Th/225Ra and 225Ra/225Ac separation processes, t′ is the time elapsed between 225Ra/225Ac separation process and counting and t is the sample counting time, respectively:
λ1 is the disintegration constant of 225Ra, λ2 that of 225Ac and At is 229Th activity added to the sample.
u(ARa) is radium activity’s combined standard uncertainty and is calculated using Eq. (12).
where t0 is the background counting time and urel (analyst) is the standard relative uncertainty associated to the ROI selection and its value has been estimated to be about 2.5%. ω is defined as:
and its relative combined standard uncertainty calculated using Eq. (14):
The decision threshold (DT) and detection limit (DL) are calculated, following ISO 11929 standard [15], using Eq. (15) and (16), respectively:
The percentile k is considered as in Eqs. (7) and (8).
C-LSC and IE-A methods comparison
To carry out this comparison, firstly, both IE-A and C-LSC methods are applied to different complex samples also measured by gamma-spectrometry, uncertainties and detection limits are compared at this step; secondly, a comparative time and cost analysis is performed and finally, the advantages and disadvantages of both methods are analysed and compared.
Results and discussion
Application of the developed C-LSC method to complex samples
Method sensitivity
When the real samples taken for this proof are chemically analysed before and after the application of the co-precipitation process, the results show that the majority of elements are nearly completely removed; some anomalous data corresponds to those elements that appear at trace levels, Zn and Cu, and so results are close to the detection limit of the quantification method. However, there is one exception, removal percentage of Sr is not as good as for the other elements. Results are shown in Table 2.
The same results appear when synthetic samples are analysed. Sr is not fully removed from the sample with this method, and in some cases around 50% of it remains in the final solution after Ra separation. In addition, some Ca still remains, but in a lower amount; in the worst case, only 10% of it remains. Results appear in Table 3.
The recovery of Ba, which provides the Ra chemical yield, obtained in the presence of other elements, is found to be between 68 and 85% and independent of other elements in the sample.
The influence of the remaining of Sr and Ca in the sample is studied in two different aspects:
1. Their possible interference in the AA technique used to obtain the chemical yield in cases with a high concentration level of both elements, without having to dilute the samples to cope with these high levels. Eight synthetic samples are prepared, with typical Ba contents after co-precipitation process but with increasing amounts of Sr contents in four of them and with increasing amounts of Ca in the other four. For samples fortified with Sr, the measured Ba values by AA show deviations from the known one between − 2% and + 2.4% which are lower than the uncertainty of the technique. For samples fortified with Ca, only when Ca content is close to the saturation level of the AA instrument, the appearance of Ca affects the measured Ba values with deviations around 20%; in other situations, values obtained show deviations from the known value between − 3.5% and + 4.1%, which are lower than the uncertainty of the method.
2. The possible interference in the Rn liquid–liquid extraction step is analysed because the nature of the sample in the vial is not only an aqueous one, as in the ISO 13165-1 standard method [6], but an ammonia medium with presence of Sr, Ca, sulfates and EDTA. To carry out this analysis, four samples are prepared with a known amount of 226Ra, two of them with different amounts of stable Sr (0.6 mg and 8 mg) and the other two with different amounts of Ca (1 mg and 10 mg). Samples are measured and the results obtained for 226Ra differ from the known activity value by less than 3%.
Both results support the idea that Sr or Ca surviving the precipitation process does not interfere with the determination of Ba by AA in the chemical yield calculus neither in the measurement by LSC. If the chemical yield were obtained by gravimetry, the existence of Sr or Ca would provide a higher chemical yield and then, the value obtained for 226Ra would be lower than the real one.
Sample stability
Comparison of count rates when vials are and when they are not initially shaken, measured 25 days after Ra separation, shows that there are no noticeable differences, lower than 6%, between them. Therefore, we decided to apply the method without shaking before keeping the sample in the dark.
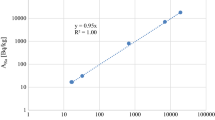
Regarding the stability of the samples, the results of a representative selection of measurements, carried out from the Ra separation until three months later, are shown in Fig. 2. After achieving 226Ra–222Rn equilibrium, these samples are highly stable, even with a high content of Sr or Ca, and therefore, the elapsed time between Ra separation and measurement, after equilibrium, is not a constraint of this method.
In addition, the possible appearance of a foamy interphase between the final solution and the liquid scintillator [16] is checked in all samples over time. This interphase has never appeared.
C-LSC and IE-A methods comparison
Results obtained by using C-LSC and IE-A methods in different complex samples
The results obtained when both methods are applied to two ILC exercise materials with a known 226Ra activity concentration and five real samples are shown in Table 4, together with the results obtained, for some of them, by direct gamma-spectrometry for comparative purposes. The uncertainties shown are the relative expanded uncertainties (coverage factor = 2).
As shown in Table 4, the values obtained by all methods match properly when there is a reference value. In the case of CaCO3, the relative differences between reference and experimental values are always lower than 2%. In the less contaminated soil, IE-A provides the worst results with a relative difference of 8%. These relative differences are lower in C-LSC and gamma-spectrometry techniques, 7% and 1.5%, respectively.
Regarding samples coming from industries, without reference values, the relative dispersion among the different methods is lower than 15%. For all samples, there is no significant statistical difference at 95% confidence level (coverage factor = 2).
As a conclusion, all the methods analysed provide comparable activity concentration values. Differences should be sought in their ability to provide the lowest detection limits using the lowest sample mass in the shortest time.
The results listed in Table 4 show that, as expected, in certain cases, detection limits obtained using gamma spectrometry are comparable to those obtained using the other methods, but the amount of mass needed is around thousand times higher. To properly compare detection limits and uncertainties of C-LSC and IE-A methods, a numerical simulation has been performed using the same conditions for both methods (24 h counting time and 0.5 g sample mass). The results are shown in Table 5.
As the results show in Table 5, detection limits are quite similar when both methods are applied in the same conditions. They are slightly lower in the C-LSC method than in the IE-A one, due to the higher total detection efficiency of LSC counter (around 250%) compared to the detection efficiency of alpha spectrometer (around 25%, at 5 mm sample to detector distance). In addition, combined standard uncertainties are slightly lower in the C-LSC method than in IE-A. The relative dispersion between highest and lowest activity concentration values is lower than or around 15% and for all samples, there is overlap among data considering their combined standard uncertainties at 95% confidence level. Crude oil activity concentration, in both methods, is below their detection limit.
Time and cost analysis
Regarding time analysis, after sample pre-treatment, both methods need less than 12 h to get the radiochemical separation. However, the time needed to get results is longer for the C-LSC method, which needs around 25 days, than for the IE-A method that needs around 2 weeks.
Counting times are higher in IE-A method, usually 1 week, compared to less than 1 day needed in C-LSC method. It is advisable to have longer counting times instead of using a greater amount of radiotracer 229Th to get the desirable number of counts necessary to evaluate the chemical yield. LSC counting exhibits higher detection efficiency, as it uses 4 π geometry and three alpha emissions detection, which allows shorter counting times to get similar detection limits than alpha-spectrometry, although this last technique takes advantage of lower background counting rates.
The cost analysis is carried out in comparative terms and separated in three items: equipment, personnel and chemicals, with the following considerations:
For equipment, amortization is taken into account for the specific large equipment, not for the common equipment (e.g. LSC and alpha spectrometer are considered large equipment), like stoves or heating systems. Very small equipment is considered as laboratory consumable.
For chemicals, all of them are type PA (Pro-analysis) and it has been considered that resins are not re-used.
For personal, times devoted to the measurements, data and chemical processes are similar for both LSC and alpha spectrometry systems, then this item is not taken into account.
A summary of the cost of both methods is shown in Table 6, where data has been normalized to the lowest value.
As shown in Table 6, the IE-A method is the most expensive method regarding conventional chemicals and equipment amortization, being a little bit less expensive when standard laboratory equipment is considered. The use of the resins is definitely the key point for this high cost of the IE-A method. However, it is worth pointing out that this analysis is based on not reusing the resin. Of course, if some of them are reused, the total price will decrease depending on the number of times they are reused. But, in any case, co-precipitation method followed by a LSC measurement is always the cheapest method.
Advantages and disadvantages of C-LSC and IE-A methods
C-LSC method
The disadvantages of this method, as previously described, are the followings.
From the analysed samples it is concluded that the influence of interfering substances in the method is not noticeable. However, when determining 226Ra in other type of complex samples, this issue should be previously considered to ensure that a loss of effectiveness of the co-precipitation process does not lead to a bad chemical yield by AA or an incomplete 222Rn emanation process. A comparison between gravimetric and AA yields is interesting to control the co-precipitation process. If gravimetric results are much higher than AA results, other chemical components remain in the final solution.
All samples measured by LSC systems have the problem that, the detector being an organic liquid, its stability when it is mixed with a sample solution should be checked especially in this case, where samples are measured 25 days after preparation. A poor performance of the co-precipitation process would make interferers appear in the final solution and perhaps stability problems. As in the previous point, a comparison between obtained AA yield and gravimetric yield will help to take care of this fact. Visual inspection of samples to take care of the appearance of a foamy interphase between the final solution and the liquid scintillator is recommended in all samples before taking any measurements.
Another disadvantage is that the possible presence of high activities of 224Ra in the samples should be considered as a potential interference. The presence of 224Ra should be controlled in the sample spectrum through the appearance of the 212Po alpha peak at 8.8 MeV. As established before, if this peak appears, a new measurement some days later should be taken to get rid of this interference.
Finally, the C-LSC method needs more equipment than the IE-A method. A liquid scintillation counter and an atomic absorption spectrophotometer (or similar systems) are needed because the simpler gravimetric method used to obtain chemical yields does not work properly with complex samples. Additionally, the time needed to obtain results is longer with the C-LSC method than with the IE-A method.
The most important advantages are that no radioactive tracer is needed in this method, the use of chemicals is lower and all the procedure is cheaper than the IE-A method. This method also minimizes the possible interferences from 210Pb, 228Ra and 224Ra with the introduction of the water-immiscible cocktail as it has been pointed out previously, resulting in a reliable and simple method for achieving low detection limits.
Moreover, C-LSC method offers a more reproducible and faster preparation as it has only one radiochemical separation while the IE-A method has two separation processes and a final microprecipitation procedure.
IE-A method
This method has no possible chemical and radiological interferences. However, there are some disadvantages in this method, some of them already described by EPA [18]:
229Th, in radioactive equilibrium with 225Ra, is used as a yield tracer and in the first step of the radiochemical process, 229Th is separated from 225Ra. If the efficiency of this separation process is lower than 100%, the alpha emissions of 229Th overlap with those from of 266Ra. In this sense, the spectra of the samples should be analysed looking for changes in the shape of the typical 226Ra peak due to the presence of 229Th. If 229Th is observed, the test-portion should be newly dissolved and then re-purified by passing it again through the cation exchange resin. Apart from this overlap, if thorium is not properly separated from radium, some 225Ra will be supported and some unsupported, which makes the yield evaluation from Eq. 10 impossible.
However, it is quite unlikely to find this 229Th interference and the separation efficiency is considered close to 100%.
In addition, it is necessary to ensure that all 217At counts are supported by 225Ra, which implies that all the 225Ac is removed from the sample during Ln resin separation step. This moment in time has to be registered as well and the efficiency of this step is assumed 100%.
However, it is possible to determine the accuracy of this last assumption by performing, as soon as possible after the sample preparation, an alpha measurement to register the counts at 225Ac energy. Considering the times involved in the determination process and the amount of 229Th tracer added, the removal efficiency is calculated by means of Bateman’s equations and therefore the fraction of the initial 225Ac activity that has been microprecipitated in the planchet. The number of counts that this 225Ac activity generates during counting time is evaluated and subtracted from the counts registered in the spectral region of 217At.
Finally, as usually done, time uncertainties are neglected in this method. However, during the radiochemical and measurement processes, tracer activity is varying and so, different times should be recorded during the whole determination process to ensure a proper activity concentration evaluation. Those times are the times of the first and second separation steps, the micro-precipitation time and starting and finishing counting times. Measurement times are properly recorded but separation and microprecipitation times cannot be well assessed due to the length of the processes involved.
Thus, to know the effect of this lack of proper time assessment has in activity determination, two analyses are conducted, one to evaluate its contribution to the global uncertainty of the method and another one to evaluate the sensitivity of the activity to those time assessments.
The first analysis is carried out by the variance partitioning analytical approach of Li and Wu [20] and so, the S(x) relative contribution of each variable x to the total uncertainty is calculated by the fraction of the terms associated with its variance (Eq. 17):
where ux is the standard uncertainty of a variable x and u(ARa) is the combined standard uncertainty of 226Ra activity. In this analysis, ux’s related to time have been assumed to be half the duration of each process.
Results show that the relative contribution of time uncertainties [S(t)] involved in activity calculation is minor, ranging from 0.03% to zero. This range does not change even for low and very high Ra activities.
The second analysis is carried out for the samples measured in this paper by determining the relative deviation between activities calculated using times values between t ± u(t). The results obtained show that these relative deviations are always lower than 1% in all cases, that is, lower than the uncertainties of the other variables considered.
So, the assessment of the time of each process has been that corresponding to half the length of the process.
Another point is the short half-life of 225Ra so that, some weeks after separation, tracer counts do not appear in the sample spectrum. Therefore, we must perform this measurement at the stipulated time and be very careful with the record of the times involved in this process.
Finally, it is the most expensive method, because of the use of resins. Of course, the method becomes cheaper if the resins are reused. However, in this case, a complete control on the re-generated resins after the first use should be performed.
Regarding its advantages, after waiting several months, it is also possible to get from it 228Ra activity concentration through the 224Ra alpha peak [1, 21, 22].
Other important advantage is the robustness and long life of the alpha-spectrometer and the very small maintenance it needs in comparison to the LSC. In addition, LSC needs efficiency calibration with 226Ra calibration sources and optimization of the counting conditions for alpha–beta discrimination before starting the measurements, while this is not the case for alpha-spectrometry, which is simpler as it does not require efficiency calibration. In addition, alpha spectrometry techniques allow internal control though the use of a tracer to assess the quality of each analysis.
Finally, the time needed to get results is shorter than that by C-LSC method. If the amount of 226Ra in samples is high or the detection limit required is not very low, results could be available in about 10 days.
Conclusions
In this paper, it is stated that the method developed to obtain 226Ra activity concentration contents in complex samples, through co-precipitation chemical procedure followed by a liquid–liquid extraction process by the use of an immiscible liquid scintillator to measure samples by means of a liquid scintillator counter (LSC), is a sensitive and robust method which allows to obtain 226Ra activity concentration with low detection limits (around 1 Bq g−1) and uncertainties (less than 10%) using a small sample (0.5 g) and taking short counting times (less than 1 day). In addition, the chemical separation yields obtained are quite high (from 68 to 85%).
Co-precipitation process is able to remove the most expected conflictive interfering substances, that is, those able to interfere in the achievement of adequate chemical yields and also those potentially able to interfere in radon emanation from the sample to the scintillation cocktail.
The samples remains stable for a long time and the spectra provided by the LSC system allow the control of radioactive interferences coming from the potential 224Ra contents in the sample.
A comparison between this method and the ion-exchange one, followed by an alpha-spectrometry measurement, shows that both methods provide comparable results on activity concentration, uncertainties and detection limits, when the same sample sizes and counting times are used. In addition, comparable results on chemical yields are obtained.
If detection limits and uncertainties are compared with those obtained by gamma-spectrometry, it is observed, as expected, that the second ones are always higher. The only possibility to obtain comparable values with gamma-spectrometry is using a large sample, approximately a thousand times larger.
An analysis of the advantages and disadvantages of both methods, C-LSC and IE-A methods, has been done. In summary, both methods have disadvantages that can be more or less easily solved. However, in the case of the IE-A method, we cannot solve its high cost, the lack of Ra tracer to be used and the short lifetime of 225Ra. In the case of C-LSC method, the disadvantages are related to the use of more equipment and the longest time needed to provide results.
The robustness of the alpha spectrometer and the rapidity to get results are key advantages for the IE-A method and the low price and short need of chemicals are the key advantages of the C-LSC method.
Both methods are applicable for any given purpose; the choice of a method for a laboratory will depend on its capabilities, available equipment and chemicals but also on the trained personnel. In any case, both methods allow achieving very low detection limits using small samples. They could also be used for water samples, getting even lower detection limits, as larger volumes of water could be pre-concentrated.
References
Jia G, Jia J (2012) Determination of radium isotopes in environmental samples by gamma spectrometry, liquid scintillation counting and alpha spectrometry: a review of analytical methodology. J Environ Radioact 106:98–119
IAEA (2010) Analytical methodology for the determination of radium isotopes in environmental samples. Analytical Quality in Nuclear Applications Series No. 19. International Atomic Energy Agency (IAEA), Vienna
Herranz M, Idoeta R, Abelairas A, Legarda F (2006) Radon fixation for determination of 224Ra, 226Ra and 228Ra via gamma-ray spectrometry. Radiat Meas 41:486–491
IAEA (2014) Radiation protection and safety of radiation sources: international basic safety standards. International Atomic Energy Agency, Vienna
IAEA (2014) A procedure for the rapid determination of 226Ra and 228Ra in drinking water by liquid scintillation counting. Analytical Quality in Nuclear Applications Series No. 39. International Atomic Energy Agency (IAEA), Vienna
ISO (2013) ISO 13165-1: water quality- 226Ra Part 1: test method using liquid scintillation counting. International Organization for Standardization (ISO), Geneva
Villa M, Moreno HP, Manjón G (2005) Determination of 226Ra and 224Ra in sediments samples by liquid scintillation counting. Radiat Meas 39:543–550
Moreno HP, Absi A, Vioque I, Manjón G, García-Tenorio R (2000) Application of a liquid scintillation technique to the measurement of 226Ra and 224Ra in samples affected by non-nuclear industry wastes. J Radioanal Nucl Chem 245:309–315
Kim H, Jung Y, Ji YY, Lim JM, Chung KH, Kang MJ (2017) Validation of a procedure for the analysis of 226Ra in naturally occurring radioactive materials using a liquid scintillation counter. J Environ Radioact 166:188–194
Jung Y, Kim H, Chung KH, Kang MJ (2016) Study of the determination of 226Ra in soil using liquid scintillation counter. Anal Sci Technol 29:65–72
Möbius-Ramamonjisoa TL, Andriambololona R (2009) Liquid scintillation methods for NORM in Madagascar. In: Eikenberg J, Jäggi M, Beer H, Baehrle H (eds) Advances in liquid scintillation spectrometry 2008. University of Arizona, Tucson, pp 287–292
Moebius S, Moebius R, Bartenbach M, Ramamonjisoa T (2008) Liquid scintillation for NORM in the oil and gas industry. In: 12th International congress of the International Radiation Protection Association (IRPA): strengthening radiation protection worldwide, Buenos Aires, Argentina, October 19–24, 2008
ASTM (2011) Standard practice for preparation of oils and oily waste samples by high-pressure, high-temperature digestion for trace element determinations. ASTM Standard C1234-11, ASTM International, West Conshohocken PA
GUM (2008) Evaluation of measurement data—guide to the expression of uncertainty in measurement. JCGM 100:2008
ISO (2010) ISO 11929: determination of the characteristic limits (decision threshold, detection limit and limits of the confidence interval) for measurements of ionizing radiation—fundamentals and application. International Organization for Standardization (ISO), Geneva
Forte M, Abbate G, Badalamenti P, Costantino S, Lunesu D, Rusconi R (2015) Validation of a method for measuring 226Ra in drinking waters by LSC. Appl Radiat Isot 103:143–150
Al Attar L, Safia B, Abdul Ghani B (2016) Chemical composition of scales generated from oil industry and correlation to radionuclide contents and gamma-ray measurements of 210Pb. Environ Pollut 210:246–252
EPA (2014) Rapid Radiochemical method for 226Ra in building materials for environmental remediation following radiological incidents. EPA 402-R14-002, U.S. Environmental Protection Agency (EPA), Office of Air and Radiation, Washington DC
Browne E, Firestone RB (1986) Table of radioactive isotopes. Wiley, Berkeley
Li H, Wu J (2006) In: Wu J, Jones KB, Li H, Loucks OL (eds) Scaling and uncertainty analysis in ecology: methods and applications. Springer, Dordrecht
Aguado JL, Bolivar JP, García-Tenorio R (2008) 226Ra and 228Ra determination in environmental samples by alpha-particle spectrometry. J Radioanal Nucl Chem 278(1):191–199
Jia G, Torri G, Innocenzi P, Ocone R, Di Lullo A (2006) Determination of radium isotopes in mineral and environmental water samples by alpha-spectrometry. J Radioanal Nucl Chem 267(3):505–514
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Idoeta, R., Rozas, S., Olondo, C. et al. 226Ra determination in complex samples using liquid scintillation counting. J Radioanal Nucl Chem 318, 1773–1784 (2018). https://doi.org/10.1007/s10967-018-6260-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6260-z