Abstract
The astatine radionuclides 208,209,210,211At and the iodine radionuclides 120,121,123I were simultaneously produced by 7Li beam irradiation of a stack of lead and tin targets. The astatine and the iodine radionuclides each were separated from the irradiated target with a dry distillation method. No-carrier-added astatine and iodine solutions were prepared using ethanol or water as a solvent. Astatine in the aqueous solution was reacted with an oxidizing or a reducing agent. Separation of the astatine and the iodine ions in the solutions was conducted by thin layer chromatography on silica gel with an ethanol/water solution. Astatine was separated and identified as anions of At−, AtO3− and AtO4−, while iodine was I−, compared with the standard iodine species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astatine is a radioactive element. Short-lived astatine radionuclides 219At (half-life T1/2 = 56 s), 215At (T1/2 = 0.1 ms) and 218At (T1/2 = 1.6 s) are produced by the decay of naturally occurring radioactive substances such as 235,238U in nature. However, amounts of such short-lived astatine radionuclides are limited to study chemical properties [1]. Chemical properties of astatine have been generally studied by using the two longest-lived radionuclides 210At (T1/2 = 8.1 h) and 211At (T1/2 = 7.2 h) artificially produced using an accelerator [1,2,3]. The 210At and 211At nuclides have been generally produced through the 209Bi(4He,3n)210At and 209Bi(4He,2n)211At reactions, respectively [4,5,6]. Possible production routes of astatine radioisotopes have been recently studied in the 7Li induced reaction of natPb, natPb(7Li,xn)nat−xAt [7,8,9]. 7Li beams produce not only astatine radionuclides, but also its homologous element iodine radionuclides in the natSn(7Li,xn)nat−xI reaction [10]. Thus, the simultaneous production of homologous elements, astatine and iodine, is accomplished by irradiating a stack of lead and tin targets with the 7Li beams. This enables us to conduct control experiments. The control experiments of no-carrier-added astatine and iodine will be helpful and effective to study the chemical properties of astatine.
From the viewpoint of medical use of radionuclides, the 211At nuclide is a prospective candidate for utilization in targeted alpha therapy. A large number of studies in respect not only of production and separation of astatine from irradiated targets [3, 4, 11,12,13,14,15,16,17,18,19,20,21,22,23,24,25], but also of chemical and radiolabeling reactions for pharmaceuticals [4, 25, 26] have been carried out in the past. In the preparation of no-carrier-added astatine, chemical procedures based on dry- [3, 4, 11,12,13,14,15,16,17, 26] and wet-chemistry [20,21,22,23,24,25] have been conducted. In general, methods based on dry-chemistry, namely distillation of astatine from the melted bismuth, are simple but somewhat problematic in the repeatability of yields in the chemical and radiolabeling reactions [1, 2, 25]. In the production of iodine for medical use, however, dry-chemistry has been commonly used without such problem. The problem in dry-chemistry of astatine is still unsolved and could originate from its chemical properties. The understanding of basic chemical properties of astatine has been required to develop targeted alpha therapy agents for cancers [27].
Chemical species of iodine and astatine have been successfully separated and identified as iodide (I−), iodate (IO3−) and periodate (IO4−), and astatide (At−), astatate (AtO3−) and perastatate (AtO4−) by paper chromatography, respectively [28, 29]. These iodine chemical species were studied not only by paper chromatography [28] but also by thin layer chromatography (TLC) on silica gel plate [30]. In the case of astatine, TLC has been conducted to study the separation and identification only of its organic compounds [31] and mobility of its complexes extracted with organic solvents [32]. However, no experimental data are available on the separation and identification of astatine inorganic chemical species At−, AtO3− and AtO4− by TLC.
The aim of this work was to study astatine inorganic chemical species by TLC and the chemical properties of astatine prepared by dry distillation. The control experiment of TLC was carried out by using no-carrier-added astatine and iodine solutions prepared with a method based on dry-chemistry, after the irradiation using 7Li beam.
Experimental
Production of radionuclides
The thin targets of lead or tin of approximately 1 mg/cm2 in thickness were prepared by vacuum evaporation of lead or tin metal on a backing foil of 2.7 mg/cm2 aluminum. Each thin target of lead or tin was sandwiched between the backing foil and a catcher foil of 2.7 mg/cm2 aluminum. The first stack was composed of five lead targets and four tin targets. The second was composed of three lead and four tin targets.
The astatine and iodine radionuclides were simultaneously produced by irradiating the stacks of the thin targets of lead and tin with the 60 MeV 7Li3+ ion beams supplied from the Japan Atomic Energy Agency (JAEA) tandem accelerator. Irradiation was carried out using a water-cooled Faraday cup in the similar way as described by Nishinaka et al. [9]. In this work, however, the backing foils on which the target was evaporated were placed beam backward side and the catcher foils were placed beam forward side to collect products recoiled out from the target by the catcher foils. This enabled us to determine amounts of recoil products and chemical yields. The average beam current was 180 nA during 2 h for the irradiation of the first stack, and 160 nA during 12 h for the second stack.
After irradiation, the radioactivities of astatine or iodine radionuclides produced in the target on the backing and in catcher foil were each measured by a high-purity germanium detector in the same way as described by Nishinaka et al. [9].
Dry distillation and preparation of solutions
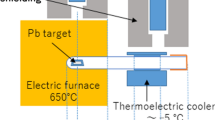
Dry distillation was carried out as described in Ref. [9]. An irradiated lead or tin target on the backing aluminum foil was put into the 180 × 16 mm (i.d.) glass test tube. The test tube was filled with nitrogen gas and was dually sealed with DuraSeal™. A third portion from the bottom of the test tube was inserted into a furnace at 650 °C and heated for 20 min. After the test tube was removed from the furnace and cooled to ambient temperature for 10 min in air, the lead or the tin target on the backing foil was taken out of the test tube.
The test tube was rinsed with 1.8 mL of ethanol or distilled water to elute astatine or iodine attached on the inner wall of the test tube. Ethanol or aqueous solution containing astatine or iodine was each stored in a glass vial by pipetting. Recovery of the solutions was determined by weighing the glass vial before and after pipetting. The four stock solutions of no-carrier-added astatine and iodine were prepared.
After dry-distillation, the activities of astatine or iodine in the glass vial, in the target on the backing foil, and on the DuraSeal™ were each measured by gamma-ray spectroscopy. These measured activities provided yields of dry distillation and elution.
In order to investigate oxidation–reduction degree of astatine ions, an oxidizing and two single reducing agents were used as follows; (1) A stock solution containing the oxidizing agent of potassium periodate (KIO4) was prepared by adding 0.05 mL of 3 × 10−2 M KIO4 into 0.2 mL of the astatine aqueous solution and heating at 90 °C for 20 min. (2) A stock solution containing the reducing agent of sodiumu sulfite (Na2SO3) was prepared by adding 0.05 mL of 5 × 10−4 M Na2SO3 into 0.2 mL of the astatine aqueous solution, and was kept at room temperature for 20 min. (3) The stock solution containing the reducing agent of hydrazine hydrate was prepared by adding 0.02 mL hydrazine hydrate into 0.2 mL of the aqueous solution of astatine and was kept at room temperature for 20 min.
TLC for radioactive astatine and iodine
TLC was used for the separation and identification of inorganic ions of astatine and iodine in the stock solutions. A silica gel TLC plate (Merck Silica gel 60 F254 aluminum sheet) was cut in strips 12 × 3 cm. A 2–5 μL portion of the stock solutions were spotted at 2 cm from the bottom of the silica gel TLC plate. The activities of radionuclides spotted on the plate were 15–18 Bq 123I and 25 Bq 121I for iodine, and 33–54 Bq 211At, 87–132 Bq 210At and 35–121 Bq 209At for astatine, respectively. After drying the spotted solutions, the plates were developed by ethanol/water solution (1:1, v/v) for approximately 90 min. The eluent front was allowed to advance 8 cm from the start line. After drying, the plates were exposed to imaging plates for approximately 12 h. The distribution of radioactivity on the plates was visualized by Bioimaging Analyzer System (BAS). The Rf value and its amount were obtained from the measured radioactivity of each spot obtained by the BAS.
It should be mentioned from a perspective of radiation safety that this TLC analysis for astatine solutions containing a reducing agent will release astatine activities in development. This aspect will discussed in more detail later.
TLC for non-radioactive iodide, iodate and periodate
The stable iodine chemical species in neutral solutions are iodide I−, iodate IO3−, and periodate IO4−. Thus, the Rf values of such standard iodine species were determined under the same condition of the TLC for radioactive astatine and iodine.
Reagents of 104 mg sodium iodide (NaI), 130 mg sodium iodate (NaIO3) and 21 mg sodium periodat (NaIO4) were each dissolved in 20 mL distilled water. A 10 μL of a portion of the aqueous solution of NaI, NaIO3 and NaIO4 was spotted at 2 cm from the bottom of a silica gel TLC plate. The development of the chromatograms was carried out with ethanol/water solution (1:1, v/v). After drying the TLC plate, the visualization of the spots was made in the same way as described by Näumann [33].
The detection of iodine was carried out by spraying successively with a starch solution and 0.1 M potassium nitrate (KNO2) solution, with a brown coloration indicating the presence of iodide. A solution of 2 M acetic acid and a solution of 5% pyrogallol in acetone were successively sprayed for the detection of iodate, showing a brown coloration. A mixture of 4,4′-methylenebis(2,6-diethylaniline) solution and a solution of 10% manganese (II) chloride tetrahydrate (MnCl2·4H2O) was sprayed for the detection of periodate, showing a blue coloration.
The starch solution was prepared by adding 0.2 g starch into 20 mL 2 M acetic acid, and then it was stirred and filtered. A 4,4′-methylenebis(2,6-diethylaniline) solution in 2 M acetic acid was prepared by adding 2 g 4,4′-methylenebis(2,6-diethylaniline) into 20 mL 2 M acetic acid, which was then stirred and filtered.
Results and discussion
Activities of produced astatine and iodine
The activities of astatine and iodine radionuclides determined by γ-ray spectroscopy are listed in Tables 1 and 2. Values were corrected for the counts of photopeaks for decays at measurements, corresponding to the activities at the end of bombardment (EOB). The denomination of 1Pb, 2Pb, 1Sn and 2Sn refers to each lead and tin targets. Two lead and two tin targets taken from the irradiated stacks were used in this work. The beam energy at the center of the target was calculated from the energy loss of 7Li beams in the targets and the aluminum foils [33, 34]. The activities of 211At were estimated from the excitation functions of 210,211At in the 7Li + natPb reaction [9]. Because intense γ-rays of the astatine isotopes 207−210At made impossible to measure the 687 keV photopeak (Iγ = 0.26%) of 211At by γ-ray spectroscopy. Activities for 2Pb were also estimated from the activities produced in the catcher foil by calculating an amount of nuclei recoiled out from the target and will be discussed in more detail later.
Dry distillation and preparation of solutions
The distributions of astatine and iodine activities in production and dry distillation, and yields in elution were determined from the measured activities before and after dry distillation and elution. The results converted to percentage are listed in Table 3.
The activities in the catcher foils represent amounts of nuclei recoiled out from the target and inplanted into the catcher foils. Such amounts can be calculated from the kinetics of recoils and the energy loss in the metal target [34, 35]. The calculated values in parentheses in the column for catcher foil of Table 3 are in reasonable agreement with the measurements for 1Pb, 1Sn and 2Sn. In the case of 2Pb, the calculated value of 17% had an error of 3%, which was taken from the difference between the experimental and calculated values of 1Pb. These calculated values and the experimental activity in the catcher foil of 2Pb provide an account of not only the activity produced in the corresponding target, but also the values calculated from it for 2Pb in Tables 1 and 3. The reason of this calculation is the accidential loss of the data file of a measurement for the activities produced in the target for 2Pb. However, the loss was reasonably overcome by the calculation. The calculated values involve relatively large ambguity but provide valuable data for considerations of dry distillation and elution. It is emphasized that this has no connection to the results of the TLC experiments.
In general, products recoiled out from the target became considerable amounts in the production of radionuclides by irradiating a thin target with energetic heavy ions. In this experiment using the thin targets of lead or tin of approximately 1 mg/cm2 in thickness, amounts of recoiled products for the Sn targets (~ 31%) are larger than those for Pb (~ 17%). This originates from the long range (~ 0.4 mg cm−2) of high energetic (~ 2.6 MeV) iodine products with small atomic and mass numbers compared with those of astatine products (∼ 0.2 mg cm−2, ∼ 1.7 MeV). The astatine and iodine nuclides inplanted into the catcher foil are unable to be released from aluminum metal by heating. Thus, the catcher foils were not used as samples for dry distillation.
The distributions of radionuclides in dry distillation were very different between astatine and iodine. It is well known that the volatility has advantage of astatine over iodine [1,2,3,4]. The chemical form At(0) is characterized by high volatility [1, 2]. This results in the high yields in the test tube and on the DuraSeal™, and the low yields in the target on the backing foil for astatine compared with those for iodine. An amount of activity on the inner wall of the test tube was not directly measured but it was able to be obtained from the measurements of target before and after dry distillation, and Dura seal. The yield of dry distillation corresponds to the sum of yields on Dura seal and in test tube. The yields of dry distillation for astatine (96%) were much larger than those for iodine (6–8%). A reaction between tin and iodine forms a specie of stannous iodide with high boiling point. This possibly affects low dry distillation yield of iodine compared with astatine.
The elution yields were obtained from the activities in the ethanol or water solutions of astatine or iodine. The activities in the target and on the DuraSeal™ after dry distillation were not used in elution of distilled astatine or iodine. Thus, the elution yield was obtained as the proportion of the measured activity in solution to that in the test tube. The elution yields include the recovery yields of solution by pipetting. The recovery yields of ethanol (∼ 92%) were slightly lower than those of water (∼ 99%) due to viscosity of the solutions. The elution yields show high values of more than 52%. However, astatine seems to show slightly lower elution yields than iodine. This could be related to the chemical properties of astatine and iodine prepared by dry-distillation. In order to understand the dependence of the elution yield on eluents, systematic studies of elution in this method are required. Finally, it is emphasized that both ethanol and water easily remove a large portion of astatine and iodine attached on the inner wall of the glass test tube although the eluent yields for astatine probably depend on eluents due to its chemical properties.
The chemical yields were obtained from the ratio of the activity in the solution to that produced in the target, approximately 60% for astatine and 6% for iodine. The chemical yield was composed of the yields of dry distillation, recovery of solution and elution. Among them, the yields of dry distillation play a crucial role in the determination of the chemical yields for astatine and iodine in this method.
Typical γ-ray spectra of astatine and iodine in the ethanol solutions are shown in Fig. 1. Some photopeaks are labeled by the nuclide with its photopeak energy and branch. The yields were obtained from 245.3 keV γ-ray activities of 210At for astatine and of 212 keV γ-ray activities of 121I for iodine, respectively. Photopeaks only of astatine or iodine were observed, indicating that the separations based on dry-distillation were successfully accomplished with high purities.
TLC for iodine and astatine
The results of TLC for non-radioactive iodide I−, iodate IO3− and periodate IO4− are shown in Fig. 2. The Rf values of the iodine anions under the condition of TLC in this work were determined to be Rf = 0.87 for I−, 0.78 for IO3− and 0.00 for IO4− and are listed in Table 4. The errors of Rf were estimated to be less than 0.01. The order of Rf values (IO4− < IO3− < I−) was consistent with the reported results of silica gel TLC system [29].
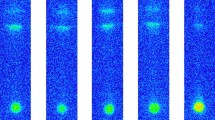
The results of TLC for radioactive astatine and iodine are shown in Fig. 3. The images of Fig. 3 were each individually visualized by BAS. TLC for iodine shows one spot with Rf = 0.84–0.86 (Fig. 3a, b) while that for astatine shows three (or two) spots with Rf = 0.74–0.82, 0.66–0.69 and 0 (Fig. 3c–g). The Rf value and amount determined from the measured radioactivity of each spot in the images of Fig. 3 are listed in Table 4. The amounts of spots correspond to the relative values to the total activity in all area of a plate after subtracting a background. Therefore, the sum of amounts of three spots for the anions in a plate becomes smaller than 100% due to tailing of spots.
Results of TLC experiments visualized by bioimaging analyzer system (BAS): a iodine ethanol solution, b iodine aqueous solution, c astatine ethanol solution, d astatine aqueous solution, e astatine aqueous solution + KIO4, f astatine aqueous solution + Na2SO3, g astatine aqueous solution + hydrazine hydrate
The Rf values of radioactive iodine, namely Rf = 0.86 for ethanol solution (Fig. 3a) and Rf = 0.84 for water solution (Fig. 3b), are almost identical to Rf = 0.87 for non-radioactive iodide I− (Fig. 2). This shows that the radioactive iodine is characterized and identified as I−. The amounts of IO3− (Rf = 0.78) and IO4− (Rf = 0.00) are negligibly small.
In contrast, astatine is clearly separated into at least more than three species by TLC and their relative amounts are largely different among the stock solutions. The similarity of the Rf values of astatine to those of iodine suggests possible astatine chemical species. Comparing with the Rf values of iodine anions, astatine species could be assigned to At− (Rf = 0.78–0.82), AtO3− (0.66–0.69) and AtO4− (0.00), respectively. Astatine anions show slightly smaller Rf values than those of corresponding iodine anions. Such statement was obviously demonstrated by analysis of the chromatograph behavior of halogenated aromatic compound [36]. This is reasonably explained by the different atomic volume for halogens [1]. The three oxidation states of astatine assigned by TLC are At(−I) for At−, At(V) for AtO3− and At(VII) for AtO4−.
Astatine is expected to possess a more electropositive character than the other halogens from the general trend in the periodic system [1]. This could reasonably explain differences of relative amounts of the three anions between astatine and iodine in ethanol and water (Fig. 3a–d). Besides, dependence of relative amounts of astatine anions on the solutions (Fig. 3c–g) of ethanol, water, aqueous solution of KIO4, Na2SO3 and hydrazine hydrate, respectively, strongly supports that the three oxidation states of astatine are assigned to At− (−I), AtO3− (V) and AtO4− (VII). This aspects are discussed below.
In the ethanol solutions (Fig. 3a, c), the most probable oxidation state is (−I) both for iodine I− and astatine At−. However, astatine in ethanol (Fig. 3c) has considerable amounts of the higher oxidation states of (V) and (VII); the relative amounts are 41% for At− (−I), 24% for AtO3− (V) and 14% for AtO4− (VII). It is clear that this comes from the more electropositive character of astatine than iodine. Changing astatine solution from ethanol (Fig. 3c) to water (Fig. 3d) results in the most probable oxidation state (VII); the relative amounts in astatine aqueous solution are 18% for At− (−I), 25% for AtO3− (V) and 44% for AtO4− (VII). This also means that astatine is more effectively oxidized in water in comparison with iodine (Fig. 3a, b) owing to the more electropositive character of astatine.
As shown in Fig. 3e–g, the presence of an oxidizing or a reducing agent controls the relative amounts of astatine species, At−, AtO3− and AtO4−, in comparison with the absence of agents (Fig. 3d). As shown in Fig. 3e, the presence of an oxidizing agent KIO4 largely increases the amount of AtO4− (VII) up to 94% and decreases that of AtO3− (V) down to 3%. Besides, the spot of At− (−I) was unobservable. In contrast, as shown in Fig. 3f, the presence of the reducing agent Na2SO3 decreases the amount of AtO4− (VII) down to 35% and increases those of AtO3− (V) and At− (−I) up to 29% and 19%, respectively. The stronger reducing agent hydrazine hydrate clearly disappers the spot of AtO4− (VII) as shown in Fig. 3g. The behavior of the relative amounts of the astatine species with oxidizing and reducing agents reveals that the astatine species are certainly identified as At−, AtO3− and AtO4−.
Such identification is consistent with the results of Dreyer and co-workers [28, 29] but inconsistent with the identification of At−, At+, AtO3− of Appelman [2] and Rössler et al. [37]. Appelman [2] claimed that no evidence has been found for AtO4−. Further research studies on astatine speciation has been carried out and identified some chemical species [38,39,40,41,42,43,44]. Champion et al. [43] identified At−, At+ and AtO+. HPLC analyses have recently isolated At− and AtO3− [24]. In general, identification of chemical species will be affected by the preparation of samples and analytical methods. It should be pointed out that the TLC analysis of this work successfully separates and identifies three anions, At−, AtO3− and AtO4−, in no-carrier-added astatine solutions prepared by dry distillation.
Finally, it should be mentioned that the TLC analysis has some disadvantages for the astatine solutions containing a reducing agent. Such solutions shown in Fig. 3f, g seem to show small activities compared with the neutral solutions (Fig. 3c, d), and the solution containing an oxidizing agent (Fig. 3e). The images of Fig. 3a–g individually visualized by BAS are unable to compare each other quantitatively. However, these trends have been apparently observable in our unpublished studies. As already discussed, the difference between the neutral solutions of Fig. 3c, d shows that astatine is oxidized even in water on a silica gel thin layer. Thus, the presence of a reducing agent in astatine aqueous solution on the silica gel thin layer could increase the probability of oxidation–reduction reactions among At− (−I), AtO3− (V), and AtO4− (VII) in dynamic equilibria. Such oxidation–reduction reactions between At− (−I) and AtO3− (V) would enhance the probability to form At0 (0) characterized by high volatility and release it from a silica gel thin layer in the development. This reasonably explains not only small activities but also the images of the results in Fig. 3g; the stronger reducing agent, hydrazine hydrate, doesn’t completely reduce astatine to At− (−I) and produces remarkable tailing of spots for At− (−I) and AtO3− (V). Such tailing generally shows that the chemical species in the ethanol/water solution are oxidized or reduced with some probability in the development.
The TLC analysis on a silica gel thin layer should not be applied to the astatine solutions containing a reducing agent without adequate ventilation because the disadvantage for astatine causes a marked loss of astatine activities in the development. In the conventional preparation of no-carrier-added astatine solutions, basic solutions containing a reducing agent has been used in order to control astatine chemical form as At−. Such solutions would lead to unsuccessful results for TLC on a silica gel plate. In contrast, the method based on dry distillation in this work has prepared astatine solutions with neutral solvents. This enables us to separate and identify the astatine chemical species of At− (−I), AtO3− (V) and AtO4− (VII) by TLC on a silica gel plate with ethanol/water solution.
Chemical properties of astatine prepared by dry distillation
It should be mentioned that the chemical species identified by TLC are not necessarily identical to those after dry distillation in the test tube and after extraction with ethanol or water. The zero oxidation state At0 (0) is characterized by high volatility and probably plays a crucial role in dry distillation [1, 2]. However, spotting portions of sample solutions on the TLC plate could oxidize or reduce astatine to the other oxidation states due to interactions with silica gel. In addition, during drying the sample solution on the TLC plate, the chemical species on a silica gel thin layer will be oxidized or reduced to the more stable astatine chemical species, namely At− (−I), AtO3− (V) and AtO4− (VII). The other chemical species with intermediate oxidation states between (−I) and (VII) are probably present in solutions due to oxidation–reduction reactions between the stable chemical species in dynamic equilibria. However, the other chemical species might not be present without solvents. Thus, the amounts of the astatine species observed by TLC are not necessarily identical, but instead retain the memory in the dry distillation and extraction processes.
In the extraction process, as discussed in the subsection of dry distillation and preparation of solutions, astatine shows the slightly smaller elution yields than iodine. This could be also related with the different relative amounts of the three inorganic astatine anions between ethanol and water. Because such different composition of the three astatine anions means that astatine species produced by dry-distillation could differently interact with glass and eluents in the extraction process. Not only the elution yields but also the composition of the astatine anions determined in this method, therefore, involve information on chemical properties of astatine, which originate from a more electropositive character than the other halogens, prepared by dry distillation.
Conclusions
The simultaneous production of the astatine and iodine nuclides using lithium beams makes it possible to prepare no-carrier-added astatine and iodine solutions with high purities by the method based on dry distillation and to study chemical species of astatine in the control experiment.
The yields of dry distillation present advantages for astatine over iodine due to At(0) characterized by high volatility, resulting in the chemical yields of approximately 60% for astatine and 6% for iodine by heating in the glass test tube filled with nitrogen gas with a furnace at 650 °C for 20 min. Astatine and iodine separated from target by dry distillation are easily eluted both with ethanol and water although astatine shows slightly smaller elution yields than iodine.
This work revealed that astatine in the solutions prepared by the dry distillation based method can be successfully separated and identified as At−, AtO3− and AtO4−. These astatine anions are probably related with the slightly small elution yields of astatine compared with those of iodine in the extraction process.
Not only elution yield in the extraction process, but also relative amounts of the astatine anions At−, AtO3− and AtO4− involve information on the astatine species prepared by dry distillation. Thus, systematic studies of elution dependence on solutions and composition analyses of the astatine anions will be subjected to understand the chemical properties of astatine prepared by dry distillation.
References
Kugler HK, Keller C (1985) Gmelin handbook of inorganic chemistry: astatine, 8th edn. Springer, Berlin
Applemann EH (1960) The radiochemistry of astatine. US Atomic Energy Commission, Report NAS-NS 3012, Washington DC
Johnson GL, Leininger RF, Segrè E (1949) Chemical properties of astatine. I. J Chem Phys 17:1–10
Corson DR, MacKenzie KR, Segrè E (1940) Artificially radioactive element 85. Phys Rev 58:672–678
Kelly EL, Segrè E (1949) Some excitation functions of bismuth. Phys Rev 75:999–1005
Ramler WJ, Wing J, Henderson DJ, Huizenga JR (1959) Excitation functions of bismuth and lead. Phys Rev 144:154–162
Roy K, Lahiri S (2008) Production and separation of astatine radionuclides: some new addition to astatine chemistry. Appl Radiat Isot 66:571–576
Maiti M, Lahiri S (2011) Production cross section of At radionuclides from 7Li + natPb and 9Be + natTl reactions. Phys Rev C 84:067601-1–067601-4
Nishinaka I, Yokoyama A, Washiyama K, Maeda E, Watanabe S, Hashimoto K, Ishioka NS, Makii H, Toyoshima A, Yamada N, Amano R (2015) Production and separation of astatine isotopes in the 7Li + natPb reaction. J Radioanal Nucl Chem 304:1077–1083
Nishinaka I, Yokoyama A, Washiyama K, Makii H, Hashimoto K (2017) Production of iodine radioisotopes using 7Li ion beams. J Radioanal Nucl Chem 314:1947–1965
Beyer GJ, Dreyer R, Odrich H, Rösch F (1981) Production of 211At at the Rossendorf-cyclotron U-120. Radiochem Radioanal Lett 47:63–66
Doberenz V, Nhan DD, Dreyer R, Milanov M, Norseyev YV, Khalkin VA (1982) Preparation of astatine of high specific activity in solutions of given composition. Radiochem Radioanal Lett 52:119–128
Lambrecht RM, Mirzadeh S (1985) Cyclotron isotopes and radiopharmaceuticals—XXXV astatine-211. Int J Appl Radiat Isot 36:443–450
Larsen RH, Wieland BW, Zalutsky MR (1996) Evaluation of an internal cyclotron target for the production of 211At via the 209Bi(α,2n)211At reaction. Appl Radiat Isot 47:135–143
Lindegren S, Bäck T, Jensen HJ (2001) Dry-distillation of astatine-211 from irradiated bismuth targets: a time-saving procedure with high recovery yields. Appl Radiat Isot 55:157–160
Lebeda O, Jiran R, Ráliš J, Štursa J (2005) A new internal target system for production of 211At on the cyclotron U-120M. Appl Radiat Isot 63:49–53
Nagatsu K, Minegishi K, Fukuda M, Suzuki H, Hasegawa S, Zhang MR (2014) Production of 211At by a vertical beam irradiation method. Appl Radiat Isot 94:363–371
Henriksen G, Messelt S, Olsen E, Larsen RH (2001) Optimisation of cyclotron production parameters for the 209Bi(α,2n)211At reaction related to biomedical use of 211At. Appl Radiat Isot 54:839–844
Hermanne A, Tárkányi F, Takács S, Szücs Z, Shubin YN, Dityuk AI (2005) Experimental study of the cross-sections of α-particle induced reactions on 209Bi. Appl Radiat Isot 63:1–9
Neirinckx RD, Smit JA (1973) Separation of astatine-211 from bismuth metal. Anal Chim Acta 63:201–204
Roy K, Basu S, Ramaswami A, Nayak D, Lahiri S (2004) Incorporation of thiosemicarbazide in Amberlite IRC-50 for separation of astatine from α-irradiated bismuth oxide. Appl Radiat Isot 60:793–799
Groppi F, Bonardi ML, Birattari C, Menapace E, Abbas K, Holzwarth U, Alfarano A, Morzenti S, Zona C, Alfassi ZB (2005) Optimisation study of α-cyclotron production of At-211/Po-211 g for high-LET metabolic radiotherapy purposes. Appl Radiat Isot 63:621–631
Morzenti S, Bonardi ML, Groppi F, Zona C, Persocp E, Menapace E, Alfassi ZB (2008) Cyclotron production of 211At/211gPo by 209Bi(α,2n) reaction. J Radioanal Nucl Chem 276:843–847
Balkin EB, Hamlin DK, Gagnon K, Chyan MK, Pal S, Watanabe S, Wilber DS (2013) Evaluation of a wet chemistry method for isolation of cyclotron produced [211At]astatine. Appl Sci 3:636–655
Yordanov AT, Pozzi O, Carlin S, Akabani G, Wieland B, Zalutsky MR (2004) Wet harvesting of no-carrier-added 211At from an irradiated 209Bi target for radiopharmaceutical applications. J Radioanal Nucl Chem 262:593–599
Aaij C, Tschroots WRJM, Lindner L, Feltkamp TEW (1975) The preparation of astatine labbelled proteins. J Appl Radiat Isot 26:25–30
Wilbur DS (2013) Enigmatic astatine. Nat Chem 5:246
Dreyer I, Dreyer R, Norseev YV, Chalkin VA (1978) Untersuchungen zur elektrophorese und papierchromatographie radioaktiv markierter anorganischer halogenverbindungen im system dimethylformamid-ammoniak. Radiochem Radioanal Lett 33:281–290 (in German)
Dreyer I, Dreyer R, Norseev YV, Chalkin VA (1978) Synthese anorganischer astatverbindungen und untersuchung ihrer eigenschaften mittels papierelektrophorese und paierchromatographie. Radiochem Radioanal Lett 33:291–300 (in German)
Benes J (1979) Behavior of iodide, iodate and periodate in TLC with alchohol-aqueous ammonia eluent. Collect Czechoslov Chem Commun 44:1034–1039
Viesser GWM, Diemer EL, Kaspersen FM (1980) The preparation of aromatic astatine compounds through aromatic mercury-compounts. J Label Compd Radiopherm 17:657–665
Viesser GWM, Diemer EL (1983) Inorganic astatine chemistry: formation of complexes of astatine. Radiochim Acta 33:145–151
Näumann R (1965) Separation of iodine anions by glass fibre paper chromatography. J Chromatogr 10:385–389
Ziegler JF (1985) The stopping and range of ions in solids. Pergamon Press, New York
Ziegler JF, Biersack JP (2013) SRIM-2013 Code. http://www.srim.org. Accessed 8 June 2017
Meyer GJ, Walte A, Sriyapureddy SR, Grote M, Krull D, Korkmaz Z, Knapp WH (2010) Synthesis and analysis of 2-[211At]-l-phenylalanine and 4-[211At]-l-phenylalanine and their uptake in human glioma cell cultures in-vitro. App Radiat Isot 68:1060–1065
Rössler K, Tornau W, Stöcklin G (1974) Rapid separation of carrier-free inorganic and organic compounds of radioiodine and astatine by high-pressure liquid chromatograpy. J Radioanal Nucl Chem 21:199–209
Visser GWM, Diemer EL (1983) Inorganic astatine chemistry: formation of complexes of astatine. Radiochim Acta 33:145–151
Milanov M, Deberenz V, Khalkin VA, Marinov A (1984) Chemical properties of positive singly charged astatine ion in aqueous solution. J Radioanal Nucl Chem 83:291–299
Dreyer R, Dreyer I, Fischer S, Hartmann H, Rösch F (1985) Synthesis and characterization of cationic astatine compounds with sulpher-containing ligands stable in aqueous solutions. J Radioanal Nucl Chem 96:333–342
Ludwig R, Fischer S, Hussein H, Frind M, Dreyer R (1989) Stability constants of At(I)-complexes with thiourea, iodide and mixed ligands in ethanol and water. J Radioanal Nucl Chem 134:141–149
Champion J, Alliot C, Huclier S, Deniaud D, Asfari Z, Montavon G (2009) Determination of stability constants between complexing agents and At(I) and At(III) species present at ultra-trace concentrations. Inorg Chim Acta 362:2654–2661
Champion J, Alliot C, Renault E, Mokili BM, Chérel M, Galland N, Montavon G (2010) Astatine standard redox potentials and speciation in acidic medium. J Phys Chem A 114:576–582
Campion J, Sabatié-Gogova A, Bassal F, Ayed T, Alliot C, Gallland N, Montavon G (2013) Investigation of astatine(III) hydrolyzed species: experiments and relativistic calculations. J Phys Chem A 117:1983–1990
Acknowledgements
The authors thank the crew of the JAEA Tandem Accelerator for accelerator operation. We are thankful to Dr. M. Asai for his help for γ-ray and α-ray spectroscopies. This work was supported by JSPS KAKENHI Grant Numbers JP2360013 and JP15K04741.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nishinaka, I., Hashimoto, K. & Suzuki, H. Thin layer chromatography for astatine and iodine in solutions prepared by dry distillation. J Radioanal Nucl Chem 318, 897–905 (2018). https://doi.org/10.1007/s10967-018-6088-6
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6088-6