Abstract
The possibility of production a medical radionuclide 177Lu by irradiating natHfO2 by bremsstrahlung photons up to 55 MeV was investigated. The yields of the main nuclear reactions were measured. A procedure for one-step separation of carrier free 177Lu via extraction chromatography on the LN resin sorbent (Triskem) was developed. The radiochemical yield was 98%, separation factor was higher than 105. Isomeric ratio 177mLu/177Lu was estimated. Simulation of 177Lu production in photonuclear reactions on 178Hf, 179Hf and natHf nuclei was performed. The possibility of production of medical quantities was discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
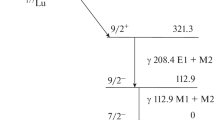
177Lu is one of the most promising radionuclides for endoradiotherapy. It has a half-life of 6.647 days optimal for therapeutic use. 177Lu is a medium-energy β-emitter (average energy of β−-radiation is 134.2 keV), which allows using it for the destruction of small objects—small tumors, micrometastases, etc. The average range of its radiation in soft tissues is 670 μm [1]. It also emits low-energy γ-radiation 112.9 keV (6.17%) and 208.4 keV (10.36%), which makes it possible to visualize the radionuclide distribution in the body by the SPECT. This opens the possibility of using 177Lu based drugs as theranostic agents for personalized medicine. A sufficiently long half-life allows organizing the delivery of the radionuclide from the production facility to clinic with relatively small losses due to decay.
A number of therapeutic radiopharmaceuticals have been developed based on 177Lu. The 177Lu-DOTA-octreotate binds to somatostatin receptors that are present on the surface of cells of many types of neuroendocrine tumors, and is used for the treatment of somatostatin receptor-positive gastroenteropancreatic neuroendocrine tumors. The use of 177Lu-PSMA-617 showed efficacy against prostate cancer [2].
177Lu is usually obtained in reactors by irradiating 176Lu by thermal neutrons, or indirectly from 176Yb by reaction 167Yb(n,γ)177Yb → 177Lu. 176Lu has a large thermal neutron capture cross section (σ = 2090 b, I0 = 1087 b), which makes it possible to obtain 177Lu directly with a high specific activity up to 20–30 Ci/mg, which is about a quarter of the theoretical (110 Ci/mg). Usually, this is sufficient for the production of labeled peptides and radioimmunoconjugates. The main disadvantage of the method is not a low specific activity, but the formation of a long-lived isomer 177mLu (T1/2 160.4 days). Its admixture is low (10−2% of 177Lu activity at EOB), and does not pose a risk in terms of additional exposure to the patient. However, the presence of a long-lived impurity brings a lot of inconvenience to clinics that have to deal with the disposal of radioactive waste.
The indirect method of obtaining 177Lu from 176Yb gives a product with a specific activity close to theoretical, but its yield is three orders of magnitude smaller because of the low reaction cross section (σ = 2.5 b). The main advantage of the method is the almost complete absence of a long-lived isomer (< 10−4% activity at EOB) [3]. But there is another difficulty—the separation of trace amounts of lutetium from hundreds of milligrams of ytterbium. In general, this problem is solved, although the proposed solutions are far from ideal [4,5,6,7]. The problems associated with obtaining 177Lu are considered in more detail in the reviews [1, 3].
The possibility of producing 177Lu by irradiating deuterons of ytterbium targets was investigated [8, 9]. The formation of 177Lu proceeds in two ways: 176Yb(d,p)177Yb → 177Lu and 176Yb(d,n)177Lu, the first is the main one. The (d,p) reaction cross section reaches a maximum value of 210 mb at 11.8 MeV [9]. According to the authors’ estimates [9], the method allows obtaining 60 GBq of 177Lu at 72 h irradiation of a thick 176Yb-target by a beam of 21 MeV deuterons with a current of 100 μA. The advantage of the method is the almost complete absence of a long-lived impurity 177mLu. Its detection by gamma spectrometry failed, the upper bound on EOB is 0.0045% [8]. The specific activity of the product obtained in this way is expected to be somewhat less than the theoretical due to the formation of stable isotopes of lutetium in reactions 176Yb(d,2n)176Lu and 176Yb(d,3n)175Lu.
Irradiation of ytterbium targets by α-particles also leads to the formation of 177Lu by the following ways 176Yb(α,p2n)177Lu and 174Yb(α,p)177Lu. The cross sections were investigated for ytterbium of a natural isotopic composition in the energy range of α-particles up to 45 MeV [10]. However, the conclusions were disappointing—in this way only about 10 kBq/μAh could be produced on a thick target, which is about four orders of magnitude lower than in the reaction 176Yb(d,p)177Yb → 177Lu.
When irradiating natural hafnium with protons, the formation of 177Lu is possible in several ways: 178Hf(p,2p)177Lu, 179Hf(p,3He)177Lu, 180Hf(p,α)177Lu. Also indirect formation through 177Yb is possible. Cross sections are investigated in the works [11, 12]. It is shown that the yield at 45 MeV is about 0.8 MBq/µAh [11]. The yield is small at relatively low proton energies (< 20 MeV) and the amount of long-lived impurities 172Lu and 173Lu increases with increasing energy.
At present, the possibility of photonuclear production of medical radionuclides using electron accelerators is being actively investigated [13,14,15,16]. It is shown that for some radionuclides (67Cu, 47Sc) photonuclear production can successfully compete with cyclotron and reactor methods. But there is only one publication in which the photonuclear production of 177Lu from the hafnium of a natural isotopic composition was investigated [17]. The authors studied the formation of 177Lu at bremsstrahlung photons energy up to 40 MeV and concluded that the method is ineffective.
In this work, we made a further study of the possibilities of obtaining 177Lu from hafnium targets by the photonuclear method. For this purpose, the yields of the reactions of formation of all nuclides were determined; a technique for separating tracer amounts of lutetium from macrophages of hafnium was developed, and the isomeric ratio 177mLu/177Lu was estimated.
Experimental
Determination of yields of nuclear reactions
Irradiation of the targets was carried out on a multipurpose pulse race-track microtron of Moscow State University with an electron beam energy of 55 MeV [18].
To determine the yields on a thin target, the natHfO2 powder (98% purity) with a mass of 1.200 ± 0.001 g was placed in a cylindrical plastic container with a diameter of 14.2 mm and a thickness of 3.6 mm. The bremsstrahlung target was a 0.2 mm thick tungsten plate. The photon beam was monitored by two targets of copper foil installed before and after the container. During the irradiation, continuous monitoring of the beam current was carried out. The duration of the irradiation was 1.1 h; the average current was 51 nA. To evaluate the yields after irradiation, a series of gamma-ray measurements with a total duration of 3 months was carried out.
The gamma spectra were registered on a gamma spectrometer of high-purity germanium detector Canberra Ind. GC3019. In order to make efficiency calibration of the detector, it was modeled using Geant4 software [19] and the 44Ti, 60Co, 94Nb, 133Ba, 137Cs, 152Eu and 241Am certified point sources were used.
Radiochemical separation of Lu(III) and Hf(IV)
For Lu(III) and Hf(IV) separation experiments, a thick target of natHfO2 with a mass of 3.44 g placed in the eppendorf was irradiated. The converter was a tungsten plate of 2.1 mm thickness. The eppendorf was placed at a distance of ~ 3 cm from the converter. The duration of irradiation was 1.4 h; the average current was 51 nA.
The irradiated target was dissolved by heating in HF conc.
The LN resin sorbent (Triskem Int; based on di(2-ethylhexyl)orthophosphoric acid, 100–150 mesh) was chosen for the extraction-chromatographic separation of Lu(III) and Hf(IV).
Distribution coefficients of Hf(IV) under stationary conditions in mixtures of fluoric and nitric acids were determined by the following procedure. 50 mg of the sorbent was added to an aliquot of the target solution. A solution of HF and HNO3 of the desired concentrations was added to a volume of 1 mL. The mixture was shaken for an hour and then centrifuged, 0.5 mL of the aqueous phase was taken, filtered, and the gamma spectrum was then registered. During the experiments, the temperature was maintained at 20–25 °C. The distribution coefficient was calculated by formula
where A0—label activity before sorption (Bq), As—the activity of the solution after sorption (Bq), V—volume of solution (mL), m—mass of sorbent (g), Kd—distribution coefficient (mL/g).
Extraction-chromatographic separation of Lu(III) and Hf(IV) was performed by the following scheme. The sorbent was preconditioned kept in 1 M HNO3 for at least 2 h, filled into a 3 mL column, washed with 10 mL solution of 0.1 M HF and 1 M HNO3 mixture. An aliquot of 3 mL of the target solution (~ 1.5 g of dissolved HfO2) were diluted by 1 M HNO3 to a volume of 45 mL. Then, all the resulting volume of solution was spilled through the column. The column was washed by a mixture of 1 M HNO3 and 0.1 M HF, then by 1 M HNO3. Finally, Lu(III) was eluted by 6 M HNO3. During this separation procedure, fractions of 5 mL were collected, their gamma spectrum was registered.
To determine the separation factor of Hf(IV) and Lu(III), the lutetium fractions were evaporated to dryness, diluted to 3 mL with water. The solution count rate was compared with the aliquot count rate of the solution prior to the experiment.
Measurements of the activity of hafnium and lutetium in experiments were carried out on gamma peaks of following isotopes 177Lu (208.4 keV), 179Lu (214.3 keV), 173Hf (123.7 keV) and 175Hf (343.4 keV).
Results and discussion
Estimation of yields of nuclear reactions on a thin target
EOB activities and radionuclide yields on a thin target are presented in Table 1.
The activity produced in the target at EOB depends on (a) the number of photons passing through the target, (b) their energy distribution, (c) the nuclear reaction cross section, and (d) the number of target nuclei:
Here A—activity (Bq), λ—decay constant (c−1), Ne—number of electrons on the converter, ns—number of nuclei per unit area (cm−2), σ(Eγ)—nuclear reaction cross section (cm2), N(Eγ)—the shape of the bremsstrahlung spectrum, expressed in terms of the number of photons per electron per unit energy of the spectrum (MeV−1).
The formula (2) is valid if (a) the target overlaps the entire beam, (b) the target is uniform in thickness and thin, that is, the multiple interaction of γ-quanta with the target can be neglected, (c) radioactive decay during irradiation can be neglected.
The integral on the right-hand side of formula (2) is the yield of the reaction under the conditions of a particular experiment, and depends on the thickness of the converter, which determines the shape of the photon spectrum N(Eγ).
There are no experimental data on the cross section of photoproton reactions on hafnium isotopes in the literature, so we used theoretical calculations. The cross section for photoproton reactions is significantly influenced by the isospin splitting of giant dipole resonance. In accordance with the model of the compound nucleus, the theoretical calculation of the cross section of photoproton reactions is divided into two stages. At the first stage, the gamma-ray absorption cross section is calculated taking into account the isospin splitting. Photon absorption cross-sections with excitation of states with ground state isospin T<=Tg.s and with T>=Tg.s. + 1 are calculated. Further, the decay of these excited states is calculated as a result of reactions with the emission of neutrons and protons. The component T< mainly decays as a result of reactions with neutron emission. The component T> is excited at a higher gamma-ray energy than T<, and is most likely to decay in reactions with proton emission.
Due to the isospin splitting effect of the giant dipole resonance, the cross section of photoproton reactions becomes much larger than calculated without taking it into account. However, in standard programs for modeling nuclear reaction cross-sections, for example, TALYS [21], isospin splitting is not implemented, therefore, the cross sections of photoproton reactions are significantly underestimated. For the calculations, we used a modified version of the program with the inclusion of isospin splitting—CMPR [22, 23]. To evaluate the applicability of these cross sections, we compared the absolute yields calculated from these cross sections and our experimental results. The experimental absolute yield (Y, 1/e−) can be calculated from the activity at the end of irradiation according to formula (3)
where t1 is the irradiation time and t is the activity measurement time. I(t) is the current of the accelerator during irradiation, determined with the aid of a monitor reaction 65Cu(γ,n)64Cu, for which the σ(Eγ) is reliably determined [24]. The radioactivity of 64Cu in monitors was measured in our experiment. Then, using the averaged activity of the monitors and σ(Eγ), the passed charge was calculated.
This yield is measured in the number of reactions per one electron of the accelerator and is applicable only to the conditions of a particular experiment, since it depends on the thickness of the converter and the geometry of the target. In the experiment, a natural mixture of isotopes was irradiated, so the formation of a final nucleus could occur in several ways. Thus, the isotope 177Lu is formed as a result of the reactions 180Hf(γ,p2n), 179Hf(γ,pn) and 178Hf(γ,p). Therefore, for comparison with the measured, the theoretical yield was calculated taking into account the contributions of all channels of formation of 177Lu:
Here \(n_{i}\)—the number of target nuclei per unit area (cm−2), taking into account the isotopic content, \(\sigma_{i} \left( {E_{\gamma } } \right)\)—the cross section of the reaction, which leads to the formation of the investigated isotope. The theoretical and experimental values of the yields obtained are presented in Table 2.
Table 2 compares the experimentally measured and theoretically calculated yields of isotopes 177Lu, 178Lu, 179Lu, 173Hf, 175Hf. In the case of photoneutron reactions, theoretical calculations coincide with the experimental data, in the case of photoproton reactions there is a discrepancy. The difference with theory was twofold for the 177Lu yield. The cross sections of the photoproton reactions leading to the formation of 177Lu, used to calculate the yields, are shown in Fig. 1. Isospin splitting was taken into account, which allowed us to approach the description of the experimental data. Without this effect, the theoretical yield is an order of magnitude lower.
In the literature there are no experimentally measured cross sections for total photon absorption for hafnium isotopes in the energy region of interest. Therefore, the calculation used the total cross section of photoabsorption, calculated from the systematics on neighboring nuclei.
According to calculations, photonuclear production of 177Lu from enriched 178Hf or 179Hf is of practical interest. Below we estimated the amount of activity that can be obtained from these isotopes.
Determination of the distribution coefficients of Hf(IV) on LN resin in mixtures of HF and HNO3
There are few ways to convert HfO2 into a solution: dissolving in HF with moderate heating, dissolving in H2SO4 with heating to 300 °C and sintering with alkalis at temperatures above 1000 °C. Obviously, the first method is the most suitable as it is easier to implement technologically for the production of 177Lu. In addition, it is known that in HF medium hafnium forms very strong anionic complexes like [HfOFx]n−, which could help in separation of the cation Lu3+ in this medium.
LN resin was chosen for the separation of Lu(III) and Hf(IV), since in the nitric acid medium Kd(Lu) takes a wide range of values [25]. At concentrations of nitric acid less than 3 M Lu(III) strongly binds to LN resin, while at concentration more than 6 M Lu(III) binds weakly. Hf(IV) binds with LN resin strongly at all concentrations of nitric acid. Small additions of HF to nitric acid solutions of Hf(IV) allow to easily elute it from the LN resin column [26].
We hypothesized that the addition of HF to the HNO3 solution will have small influence on Kd(Lu) in media where Lu(III) is strongly binds. Unlike Hf(IV), Lu(III) does not form anionic complexes in HF medium. It is worth remembering that the LuF3 is insoluble, however, we have dealt with the trace amounts of Lu3+, and the solubility product will not be achieved.
We planned to isolate the Lu(III) according to the scheme mentioned before in Experimental. For this experiment, it was necessary to determine Kd(Hf) in various mixtures of HF and HNO3 to make sure that small additions of HF allow elution of Hf(IV).
We varied the concentrations of both HF and HNO3 from 0.5 to 2 M in 0.5 M steps so that the total concentration of H+ does not exceed 3 M. Kd(Hf) was less than 1 for all selected combinations of concentrations.
These values of Kd(Hf) showed that the chosen technique could be realized.
Target dissolution and extraction-chromatographic separation of Lu(III)
A target of 3.44 g was dissolved in 14.6 g of 28 M HF, heating to boiling. An approximately 8 mL solution was obtained (~ 5 g evaporated on boiling); the process took no more than 30 min. 3 mL of the resulting solution was diluted 15 times with nitric acid of 1 M concentration so that the HF concentration did not exceed 2 M. 45 mL of the resulting solution was spilled through the column. Then, the Hf(IV) residues were eluted by a 16 mL mixture of 0.1 M HF and 1 M HNO3. The column was then washed with 5 mL of 1 M HNO3 to remove fluorides. Finally, Lu(III) was eluted by 30 mL of 6 M HNO3 solution. The elution profiles are shown in Fig. 2.
To determine the separation factor Lu/Hf, the evaporated lutetium fractions gamma spectra was measured for 18 h. The total activity of 175Hf before separation was ~ 3.5 kBq. Peaks of 175Hf were not detected in the gamma spectrum of the lutetium fractions. The detection limit of 175Hf for this measurement was 0.36 Bq. Thus, the estimated separation factor was not less than 105. The real value of the separation factor may be much higher, since the activity of 177Lu loaded on the column was very low (0.2 kBq). The gamma spectra of the irradiated thick target (4 h after EOB) and the fractions of the isolated Lu(III) are shown in Fig. 3.
During the elution of the first 17 column volumes, the total activity of Lu(III) in the fractions did not exceed 2% of all the activity of lutetium loaded onto the column. It is likely that the presence of Lu(III) in these fractions is due to the competing reaction of the formation of LuF3 or to the washout of the sorbent from the inert support. The radiochemical yield of Lu(III) was not less than 98%. The time from dissolving target to obtaining a carrier free Lu(III) in a nitric solution did not exceed 4 h.
An estimate of the isomeric ratio 177mLu/177Lu
One of the important parameters in the production of 177Lu is the isomeric ratio of 177mLu/177Lu at EOB. To evaluate it for photonuclear production of 177Lu, the purified lutetium fractions were evaporated to ~ 0.5 mL and the gamma spectrum of the solution was continuously measured for 2 months. The peaks of 177mLu were searched in the regions of 378.5 (29.7%), 413.7 (17.4%) and 418.5 (21.3%) keV. These peaks were not detected, so we estimated LU by the Currie method [27, 28]. The resulting upper limit was 0.0075 Bq at 95% confidence level. The 177Lu activity at the start of the measurement was 58 Bq, so the ratio of isomer activity was not more than 1.3 × 10−2%.
The possibility of photonuclear development of medical quantities of 177Lu
A lot of impurities are produced on natHfO2 targets: long-lived 173Lu, 174Lu; 176Lu (T1/2 = 7 days) and 179Lu, which activity at EOB is 2 orders of magnitude greater than the 177Lu activity. Obviously, photonuclear production of 177Lu requires enrichment of the target. The theoretical calculation of the cross sections showed (Fig. 1) that the cross section for the 177Lu formation reaction from 179Hf is higher than that from 178Hf. Experimental data on these two channels are not available in the literature and cannot be obtained with a natural mixture of hafnium isotopes. Therefore, we calculated the yields [MBq/μAh] on targets of 178HfO2 or 179HfO2, taking theoretical values of the cross sections (Fig. 1), and for the target from the natural mixture—our experimental ones. These yield values were used to estimate the possibility of 177Lu production by the photonuclear method on enriched targets.
To calculate thick target yields, experiment was simulated using Geant4. The following irradiation parameters were used in the calculation: the beam energy and width, the thickness of the target, the material and the geometry of the irradiated target. The result of the calculation was the distribution of the bremsstrahlung photons by energy in the volume of the target under study. By specifying the cross section of the reaction of interest, one could obtain its yield. Using the MCMC algorithm [29, 30], the selection of parameters was achieved to reach the greatest yield of the reaction.
The yields were calculated for sufficiently powerful 45 MeV linear accelerator with a magnetic mirror [31] for 1 cm3 (9.68 g) of hafnium oxide. The search for optimal parameters among the cylindrical targets of a fixed volume (varied parameters: the diameter of the cylinder and the thickness of the bremsstrahlung target) was carried out. The truncated cone target did not give an advantage in comparison with a cylindrical one. The following yield values were obtained in the optimal conditions for production: 0.08 MBq/µAh for 178HfO2, 0.19 MBq/µAh for 179HfO2 and 0.12 ± 0.01 MBq/µAh for natHfO2.
According to the calculations, when a target of mass 10 g is irradiated at a beam current of 0.1 mA for 10 days of irradiation, 1.2 GBq of 177Lu can be obtained on 178HfO2, 2.8 GBq—on 179HfO2 and 1.8 GBq—on natHfO2. Using an electron accelerator with a current an order of magnitude higher for irradiation, it is possible to obtain hundreds of mCi, which is equal to a therapeutic dose.
Conclusions
The thin and thick natHfO2 targets were irradiated by bremsstrahlung photons of energy up to 55 MeV. The total yields of nuclear reactions are determined on a thin target; the yields of each formation channel are calculated using a modified TALYS program. The distribution coefficients of Hf(IV) in HF-HNO3 mixtures were measured; it was found that at any concentrations 0.5 M < [HF] < 2 M Hf(IV) does not bind to the LN resin. A technique for one-step separation of carrier free 177Lu from an irradiated target by extraction chromatography was developed. The radiochemical yield was 98%; separation factor was higher than 105. It was shown that the ratio of the 177mLu/177Lu activities at EOB did not exceed 1.3 × 10−2%. According to the simulation results and experimental data, at a beam current of 1 mA, hundreds of mCi of 177Lu activity can be obtained on targets from enriched hafnium oxide.
References
Dash A, Pillai MRA, Knapp FF (2015) Production of 177Lu for targeted radionuclide therapy: available options. Nucl Med Mol Imaging 49:85–107. https://doi.org/10.1007/s13139-014-0315-z
Rahbar K, Ahmadzadehfar H, Kratochwil C et al (2017) German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 58:85–90. https://doi.org/10.2967/jnumed.116.183194
Mirzadeh S, Mausner LF, Garland MA (2011) Reactor-produced medical radionuclides. In: Vértes A, Nagy S, Klencsár Z, Lovas RG, Rösch F (eds) Handbook of nuclear chemistry. Springer, US, Boston, pp 1857–1902
Van So L, Morcos N, Zaw M et al (2008) Alternative chromatographic processes for no-carrier added 177Lu radioisotope separation Part I. Multi-column chromatographic process for clinically applicable. J Radioanal Nucl Chem 277:663–673. https://doi.org/10.1007/s10967-007-7130-2
Park UJ, Lee JS, Choi KH et al (2016) Lu-177 preparation for radiotherapy application. Appl Radiat Isot 115:8–12. https://doi.org/10.1016/j.apradiso.2016.05.028
Horwitz EP, McAlister DR, Bond AH et al (2005) A process for the separation of 177Lu from neutron irradiated 176Yb targets. Appl Radiat Isot 63:23–36. https://doi.org/10.1016/j.apradiso.2005.02.005
Boldyrev PP, Kurochkin AV, Proshin MA et al (2016) A modified electrochemical procedure for isolating 177Lu radionuclide. Radiochemistry 58:498–505. https://doi.org/10.1134/S106636221605009X
Manenti S, Groppi F, Gandini A et al (2011) Excitation function for deuteron induced nuclear reactions on natural ytterbium for production of high specific activity 177gLu in no-carrier-added form for metabolic radiotherapy. Appl Radiat Isot 69:37–45. https://doi.org/10.1016/j.apradiso.2010.08.008
Hermanne A, Takacs S, Goldberg MB et al (2006) Deuteron-induced reactions on Yb: measured cross sections and rationale for production pathways of carrier-free, medically relevant radionuclides. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 247:223–231. https://doi.org/10.1016/j.nimb.2006.03.008
Király B, Tárkányi F, Takács S et al (2008) Excitation functions of alpha-particle induced nuclear reactions on natural ytterbium. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 266:3919–3926. https://doi.org/10.1016/j.nimb.2008.07.002
Shahid M, Kim K, Naik H, Kim G (2014) Measurement of cross-sections for produced radionuclide in proton induced reactions on natHf up to 45 MeV. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 322:13–22. https://doi.org/10.1016/j.nimb.2013.12.029
Siiskonen T, Huikari J, Haavisto T et al (2009) Excitation functions for proton-induced reactions on natural hafnium: production of 177Lu for medical use. Nucl Instrum Methods Phys Res Sect B Beam Interact Mater Atoms 267:3500–3504. https://doi.org/10.1016/j.nimb.2009.08.016
Habs D, Köster U (2011) Production of medical radioisotopes with high specific activity in photonuclear reactions with γ-beams of high intensity and large brilliance. Appl Phys B Lasers Opt 103:501–519. https://doi.org/10.1007/s00340-010-4278-1
Szpunar B, Rangacharyulu C, Daté S, Ejiri H (2013) Estimate of production of medical isotopes by photo-neutron reaction at the Canadian light source. Nucl Instrum Methods Phys Res Sect A Accel Spectrom Detect Assoc Equip 729:41–50. https://doi.org/10.1016/j.nima.2013.06.106
Starovoitova VN, Tchelidze L, Wells DP (2014) Production of medical radioisotopes with linear accelerators. Appl Radiat Isot 85:39–44. https://doi.org/10.1016/j.apradiso.2013.11.122
Aliev RA, Aleshin GS, Belyshev SS et al (2017) Photonuclear production of carrier-free radionuclides: 69mZn. Russ Chem Bull 66:373–375. https://doi.org/10.1007/s11172-017-1743-6
Danagulyan AS, Hovhannisyan GH, Bakhshiyan TM et al (2015) Formation of medical radioisotopes 111In, 117mSn, 124Sb, and 177Lu in photonuclear reactions. Phys At Nucl 78:483–488. https://doi.org/10.1134/S1063778815030035
Ermakov AN, Ishkhanov BS, Kamanin AN et al (2018) A multipurpose pulse race-track microtron with an energy of 55 MeV. Instrum Exp Tech 61:173–191. https://doi.org/10.1134/S0020441218020136
Agostinelli S, Allison J, Amako K et al (2003) Geant4—a simulation toolkit. Nucl Instrum Methods Phys Res Sect A Accel Spectrom Detect Assoc Equip 506:250–303. https://doi.org/10.1016/S0168-9002(03)01368-8
(2018) Evaluated nuclear structure data file search and retrieval. http://www.nndc.bnl.gov/ensdf Accessed 22 June 2018
Koning AJ, Duijvestijn MC, Hilarie S (2007) Talys 1.0. In: International conference on nuclear data for science and technology. Nice, France, pp 211–214
Ishkhanov BS, Orlin VN (2015) Modified version of the combined model of photonucleon reactions. Phys At Nucl 78:557–573. https://doi.org/10.1134/S1063778815040067
Ishkhanov BS, Orlin VN (2007) Semimicroscopic description of the giant dipole resonance. Phys Part Nucl 38:232–254. https://doi.org/10.1134/S1063779607020049
Varlamov VV, Davydov AI, Makarov MA et al (2016) Reliability of the data on the cross sections of the partial photoneutron reaction for 63,65Cu and 80Se nuclei. Bull Russ Acad Sci Phys 80:317–324. https://doi.org/10.3103/S1062873816030333
Horwitz EP, Bloomquist CAA (1975) Chemical separations for super-heavy element searches in irradiated uranium targets. J Inorg Nucl Chem 37:425–434. https://doi.org/10.1016/0022-1902(75)80350-2
Bast R, Scherer EE, Sprung P et al (2015) A rapid and efficient ion-exchange chromatography for Lu–Hf, Sm–Nd, and Rb–Sr geochronology and the routine isotope analysis of sub-ng amounts of Hf by MC-ICP-MS. J Anal At Spectrom 30:2323–2333. https://doi.org/10.1039/C5JA00283D
Currie LA (1968) Limits for qualitative detection and quantitative determination. Application to radiochemistry. Anal Chem 40:586–593. https://doi.org/10.1021/ac60259a007
Gilmore G (2008) Practical gamma-ray spectrometry, 2nd edn. Wiley, Chichester
Hastings WK (1970) Monte Carlo sampling methods using Markov chains and their applications. Biometrika 57:97–109
Metropolis N, Rosenbluth AW, Rosenbluth MN et al (1953) Equation of state calculations by fast computing machines. J Chem Phys 21:1087–1092. https://doi.org/10.1063/1.1699114
Ermakov AN, Ishkhanov BS, Kamanin AN, et al Design of a linear accelerator with a magnetic mirror on the beam energy of 45 MeV. In: 24th Russian particle accelerator conference. Obninsk, Russia, pp 251–253
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazakov, A.G., Belyshev, S.S., Ekatova, T.Y. et al. Production of 177Lu by hafnium irradiation using 55-MeV bremsstrahlung photons. J Radioanal Nucl Chem 317, 1469–1476 (2018). https://doi.org/10.1007/s10967-018-6036-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-6036-5