Abstract
151Sm [half-life 94.7 (6) years] is a fission and activation product that requires accurate measurement as part of nuclear decommissioning. A procedure is outlined for the measurement of 151Sm in spiked graphite samples. Digestion is performed by automated lithium borate fusion, followed by extraction chromatography separation of 151Sm from interfering lanthanide isotopes, primarily europium. Measurement is carried out using tandem inductively coupled plasma mass spectrometry (ICP-MS/MS), using the integrated collision/reaction cell as a rapid support to radiochemical separation of 151Sm from isobaric 151Eu using oxygen as a reactive gas, achieving detection limits below the out-of-scope limit for 151Sm.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The first-generation nuclear power plants and reprocessing facilities are coming to the end of their operational lives. In the European Union alone, ninety-one power plants are currently being decommissioned, and the majority of the remaining 129 reactors and fuel cycle facilities are likely to begin decommissioning by 2030. The cost of decommissioning and waste management in the EU is estimated to be in excess of €150 billion [1, 2]. The key to safe and cost-effective disposal of the waste is accurate physical, chemical, and radiological characterisation of nuclear decommissioning waste, including accurate measurement of difficult to measure (DTM) radionuclides in a range of matrices including concrete, graphite, steel, plastics, and resins.
One DTM radionuclide is 151Sm, a fission product radionuclide (0.53% yield) also formed via neutron activation of stable Sm. Samarium-151 decays by low-energy β-particle emission (Eβ endpoint 76.6 keV (99.09%), Fig. 1) to 151Eu with a half-life of 94.7 (6) years [3], and is measurable by liquid scintillation counting (LSC).
151Sm decay scheme [3]
Accurate quantification of 151Sm by LSC can be affected by the presence of other β-emitting lanthanides, which can interfere with the measurement and make the deconvolution of the resulting spectrum rather challenging. Therefore, a chemical separation of 151Sm from interfering radionuclides is normally required prior to the LSC analysis to prevent spectral overlap.
The chemical separation of lanthanide elements represents a significant challenge due to similarities in their physical and chemical properties [4]. A summary of previously published procedures are shown in Table 1. The process of complex formation of lanthanide ions with organic ligands has been used to greatly enhance the selectivity of the separation methods [5]. The most effective complex formatting agent is 2-hydroxyisobutyricacid (α-HIBA), which has been used for the separation of rare earth elements by cation exchange chromatography [6, 7], capillary electrophoresis [5, 8] and high-performance liquid chromatography (HPLC) [9, 10].
Yoshida et al. reported a rapid separation method for the analysis of 151Sm and 147Pm in environmental samples based on HPLC in the presence of 0.2 M α-HIBA at pH 4.6. The two radionuclides were quantified by LSC, while ICP-MS was used for chemical yield determination [9]. HPLC has also been applied to the analysis of fissiogenic lanthanide elements Nd, Sm, Eu, and Gd in high burn up spent nuclear fuel [10]. The separation utilized a linear gradient elution of 0.04–0.26 M α-HIBA with a 0.30 ml/min flow rate, followed by ICP-MS detection [10].
Capillary electrophoresis (CE) has been successfully applied to the separation of fission products including lanthanide nuclides prior to measurement using quadrupole and sector field ICP-MS [8]. In this study, a CE buffer solution containing 0.8 mM picolinic acid, 10 mM HIBA and 25 mM formic acid at pH 4.7 have been employed for the determination of lanthanides. Separation has also been carried out using isotachophoresis, which, unlike other CE techniques, leads to a self-sharpening effect at the zone boundaries and pre-concentration capability all along the analysis. Lanthanide separation was carried out using 2-hydroxy-2-methylbutyric acid (HMBA) and acetic acid as complexing agents [5].
Extraction chromatography techniques have also been applied to lanthanide element separation. A method was developed for analysis of 151Sm and 147Pm in environmental and nuclear waste samples using LN resin [Lanthanide resin, composed of di(2-ethylhexyl)orthophosphoric acid (HDEHP)], followed by LSC measurement and yield determination by inductively coupled plasma optical emission spectrometry (ICP-OES) [7]. The samples were loaded onto the column in 0.15 M HNO3 before eluting 147Pm with 40 mL of 0.25 M HNO3 while less than 40% of 151Sm was removed from the resin at this stage.
Inductively coupled plasma mass spectrometry (ICP-MS) has been increasingly used over the last few decades for measurement of long-lived radionuclides as a rapid alternative to decay counting techniques [11, 12]. As the technique has advanced, the number of radionuclides measurable and sensitivities have improved, making the technique a valuable tool for nuclear decommissioning [13]. ICP-MS is now in a position where routine measurement of difficult-to-measure radionuclides, including 151Sm, is now a realistic possibility.
The presence of various interferences that overlap with the mass of the radioanalyte remains an issue for accurate ICP-MS measurement. In the case of 151Sm, the major challenge is removal of an isobaric interference from stable 151Eu (47.81% abundance) prior to detection. Additionally, polyatomic interference from 150Sm1H (7.38% Sm abundance), 150Nd1H (5.6% Nd abundance) must be considered, as well as others including 111Cd40Ar, 135Ba16O and 119Sn16O2. Finally, the half-life of 151Sm is relatively short with regards to mass spectrometry measurement, with an activity of 1 Bq/g equivalent to 1.08 pg/g, compared to 8.0 × 107 pg/g for the same activity of long-lived 238U.
There has been a limited number of past measurements of 151Sm by ICP-MS. The majority have focused on coupling high performance liquid chromatography (HPLC) online to ICP-MS [10, 14,15,16], whilst one study applied CE [8]. An alternative separation approach that has not been investigated is to use ICP-MS equipped with a reaction cell, which can potentially reduce the extent of offline chemical separation required prior to measurement, which is particularly beneficial when wet chemical separation is challenging.
Tandem ICP-MS/MS has been successfully applied to the measurement of multiple radionuclides, including 90Sr, 93Zr, 129I, 135Cs, 226Ra and 236U [17,18,19,20,21,22]. The instrument layout consists of a collision-reaction cell positioned between two quadrupole mass filters. The inclusion of the first quadrupole means that the ion beam is filtered prior to entering the cell, improving the understanding of the cell chemistry, and preventing the formation of secondary polyatomic interferences that can form in the cell (e.g., formation of oxides when using O2 reaction gas). Additionally, the first mass filter means the abundance sensitivity has been measured as < 10−10 [23], eliminating peak tailing that can otherwise prevent accurate measurement of radionuclides including 88Sr on 90Sr and 133Cs on 135Cs [17, 21].
This study presents the first known procedure for measurement of 151Sm by ICP-MS/MS. The procedure incorporates digestion and chemical separation of 151Sm, focusing on graphite. Graphite is a sample matrix of significant interest for nuclear decommissioning, with approximately 250,000 tons of radioactive graphite worldwide which must be characterized prior to disposal [24]. Although the main nuclides of interest in this matrix are currently volatile radionuclides (i.e., 3H, 14C and 36Cl), the radioactive inventory will vary depending on the reactor design and the use of graphite as fuel sleeves, making the presence of fission products including 151Sm possible. This study demonstrates that ICP-MS/MS removes the need for complete interference removal prior to measurement, and that high throughput measurement of 151Sm in graphite is achievable.
Experimental
Reagents and materials
Stable single element standards (100–1000 μg g−1) were used for initial testing of digestion and radiochemical separation procedures, and for tuning the ICP-MS/MS. 151Sm was standardised at NPL by the CIEMAT/NIST technique [25], and a range of standards prepared for active runs and as ICP-MS/MS calibration standards. Blank graphite was purchased from Fisher Scientific in pure powder form. Pre-packed 2 mL resins of TRU and Ln- extraction chromatography resins (Triskem International) were tested as part of the radiochemical procedure. A vacuum box with 12 positions was used to increase the separation speed by controlling the flowrate by the use of a vacuum pump (Eichrom Vacuum Box System). Hydrochloric acid, nitric acid and ammonia solution (Fisher Scientific, Analytical Reagent Grade) were used for radiochemical separations, and nitric acid (Fisher Scientific, Trace Analysis Grade) was diluted to 2% (v/v) in deionised water (ELGA, Veolia Water, Marlow, UK, 18 MΩ cm, < 5 ppb Total Organic Carbon) prior to ICP-MS/MS measurement.
Digestion and chemical separation
Samples containing 1–5 g of blank graphite were added to platinum crucibles and left in a furnace (Carbolite RHF 15/8) at 800 °C overnight to incinerate organic material and ash the samples. The resulting ash was then digested by automated lithium borate fusion (SPEX Katanax K2 Prime), and the fused material was then dissolved in 150 mL 5 M HNO3. The full procedure is described elsewhere [17, 26], and based on a procedure first used in the radioanalytical field by Croudace et al. [27]. Stable elements (including Sm) were measured in aliquots of the digested material using a semi-quantitative ICP-MS/MS screening mode to asses if the elemental concentration increased with the amount of graphite dissolved as an indication that the selected digestion technique was appropriate.
The radiochemical separation was developed using aqueous tracer solutions spiked with a known amount of a mix of stable elements tracers to study the behaviour of Sm(III), Eu(III) and other elements on extraction chromatography resins. For this, the uptake of the tracers by the resins was studied in nitric and hydrochloric media as a function of the solution acidity analyzing aliquots of the separated fractions by ICP-MS/MS.
In order to investigate the effect of sample matrix, samples containing 3 g of blank graphite were digested and analyzed using the procedure developed. Samarium recovery was traced by spiking graphite with a stable Sm standard, with aliquots following each digestion and separation stage measured by ICP-MS/MS.
ICP-MS/MS measurement
An Agilent 8800 was used for all measurements in this study. The instrument was fitted with the x-lens setup, with a quartz double-pass spray chamber, MicroMist nebuliser, quartz torch (2.5 mm internal diameter), nickel sample and skimmer cones. The instrument is equipped with four cell gas lines—H2, He, and corrosive and non-corrosive reaction gas lines. In this study, the corrosive line (10% NH3 balanced in 90% He) and the non-corrosive line (O2) were investigated (all gases were grade 6.0 (99.9999% purity), BOC).
Tuning was initially carried out using a mixed 1 ng g−1 standard solution in Single Quad mode (i.e., only the second quadrupole mass filter operating). For assessing recovery from chemical separation stages, no further tuning was carried out, with the Sm and Eu signals measured at m/z = 147 (14.99% abundance) and 151 (47.81% abundance), respectively. For investigating cell gases, a mixed 10 ng g−1 stable Sm and Eu standard was used. A product ion scan was used to assess elemental behaviour in NH3: the instrument was operated in MS/MS mode, with Q1 set to m/z = 147 and 151 for Sm and Eu, respectively, and then Q2 scanned the entire all masses up to a maximum of m/z = 260, returning the counts per second for each mass. For O2 reaction gas, Q1 and Q2 were set to m/z = 147 and 163 for Sm and SmO, respectively, and 151 and 167 for Eu and EuO, respectively. The formation of polyatomic 150Sm16O1H was also monitored in O2 mode.
Once the optimal instrument conditions were established, active 151Sm calibration standards were run to determine the instrument limit of detection. Active standards were also spiked with increasing concentrations of stable Eu and Sm to assess the cell-based decontamination factors achievable. Lastly, spiked graphite samples were run following digestion and chemical separation to assess the procedures capability for decommissioning samples.
Results and discussion
Digestion and chemical separation
Ashing and lithium borate fusion effectively digested graphite samples, with increasing concentrations measured for multiple elements (including Sm) measured as the graphite mass increased [26]. Volatile radionuclides e.g. 3H and 14C were lost during the digestion stage, and therefore a suitable extract system was required for the furnace and borate fusion instrument. The trapping of volatile radionuclides could be achieved by using a Pyrolyser prior to digestion, and the ashed samples prepared for borate fusion as described.
The final separation procedure for graphite samples is shown in Fig. 2. Extraction chromatography separation was preceded by an iron hydroxide co-precipitation stage to remove the sample matrix. An Fe carrier (5 mg) was added to the digested flux, and adjusted to pH 12–14 by addition of ammonium hydroxide. The precipitate formed was centrifuged at 4000 rpm for 2 min, and then dissolved in 5 mL 1 M HNO3 to enable direct loading onto TRU resin, pre-conditioned with 20 mL 1 M HNO3. The eluted load contained Fe and other matrix elements, whilst lanthanides were retained. Following Fe and matrix removal from the TRU resin column, Sm and Eu were then eluted with 5 mL 0.05 M HNO3. Some tests showed a yellow colouring to the eluted Sm fraction as a result of insufficient Fe and matrix removal, which could potentially affect the performance of Ln resin. To ensure sufficient Fe and matrix removal, a wash volume of 10 mL 1 M HNO3 was applied to the column prior to Sm and Eu elution. The Sm and Eu fraction was then directly loaded onto an Ln resin column conditioned with 20 mL 0.05 M HNO3, followed by a 3 mL 0.25 M HCl wash to elute other lanthanides. Samarium was then eluted in 3 mL 0.75 M HCl, whilst the majority of Eu was retained on the resin until higher elution volumes were applied.
Direct loading of the eluted Sm fraction from TRU onto Ln resin was critical in achieving the optimal Sm recovery (60–70%). In earlier tests using different elution conditions, if the Sm fraction was evaporated to dryness, the recoveries were as low as 20%, which was improved by evaporating to insipient dryness. The final procedure in Fig. 2 does not require any evaporation stages. Approximately 30% of Eu was recovered in the Sm elution fraction, demonstrating the challenge of Sm/Eu separation by extraction chromatography. The procedure from the resin manufacturer co-elutes Sm and Eu in 0.75 M HCl [28], with the separation in this study achieved by limiting the elution volume to 3 mL. The final sample is therefore contaminated with Eu, and reaction cell-based separation is required.
ICP-MS/MS
Optimisation using stable element standards
By operating in MS/MS mode, no ions other than those at m/z = 151 were able to enter the cell, enabling better understanding and control over the cell chemistry. The instrument sensitivity decreased by approximately 45% when operating in MS/MS mode compared to Single Quad mode. When operating with NH3 reaction gas, multiple cell products were formed for both Sm and Eu, however, the majority of the signal remained on mass for both ions (~ 80 and ~ 95% for Sm and Eu, respectively), in agreement with results from the instrument manufacturer [29]. The most significant cell product for Sm [Sm(NH2)+] only represented 5% of the total Sm signal, and therefore NH3 was not investigated further. By comparison, when operating with O2 reaction gas, the majority of Sm formed SmO+, whilst the Eu signal remained on mass. This is because the reaction to form SmO+ is slightly exothermic (ΔHr enthalpy of reaction = − 0.70 eV), whereas the reaction with Eu+ is not energetically favourable and does not proceed (ΔHr = 1.10 eV [30]).
Custom tuning of the instrument significantly improved SmO+ separation from EuO+, with the optimal operating conditions shown in Table 2. Other than the cell gas flow rate, the parameters that had the most significant impact were the octopole bias, and to a lesser extent the energy discrimination voltage. These parameters affect the ion energy, reactivity and ion rejection in the cell. A negative energy discrimination voltage speeds up the ions and increases their energy in the cell [30]. The reduction of residence time in the cell means that reactions have less time to proceed, suggesting that SmO+ is more readily formed than EuO+, which is supported by the thermodynamic data. As the energy discrimination is reduced further, the SmO+ formation decreases, suggesting that the residence time in the cell is too short. A negative octopole bias accelerates reactant ions so that they gain kinetic energy before reacting with O2. At more negative octopole bias voltages, the EuO+ formation rate increased, as the ion energy was high enough for endothermic reactions to proceed. At the optimal value of 2–4 V, Eu+ did not have sufficient energy to form EuO+ (Fig. 3).
Under the optimal instrument conditions, 89–91% of the total Sm signal was measured as an oxide, compared to 3–4% for Eu. At O2 flow rates > 0.25 mL min−1, the EuO+ signal increased, whilst also reducing the SmO+ sensitivity (Fig. 4). Q1 was set to m/z = 150 and Q2 set to m/z = 150, 151, 166 and 167 to determine the Sm+:SmH+:SmO+:SmOH+ ratio. The majority of Sm formed SmO+, with negligible formation of SmOH+. The measured Sm+:SmH+:SmO+:SmOH+ ratio was 0.11:5.14 × 10−6:0.89:5.36 × 10−4. For graphite samples, there was an increased background at m/z = 151 when operating in no gas mode, which was most likely the result of 135Ba16O+ formed during sample introduction as a result of Ba present in the sample. The low (pg g−1) levels of Sn, Nd and Cd meant that the increased background was unlikely to be due to the presence of 119Sn16O2+, 150Nd1H+, or 111Cd40Ar+, respectively. However, when operating in O2 mode, there was no increase in background at m/z = 167 for unspiked graphite samples, suggesting no formation of 135Ba16O2+, or indeed other polyatomics. Of the polyatomics described, Cd should be closely monitored for graphite samples, given its use as a neutron absorber.
Active 151Sm and spiked graphite measurement
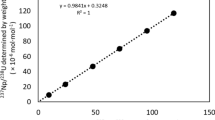
Calibration standards for 151Sm were run in the optimal instrument setup at activity concentrations from 0.25 to 250 Bq g−1, equivalent to 0.27–270 pg g−1 (Fig. 5). The standards were free from Eu contamination, which was monitored at m/z = 153 (52.19% abundance). The instrument detection limit was calculated as 1.0 Bq g−1 (1.08 pg g−1).
Multiple 100 Bq g−1 (108.09 pg g−1) 151Sm standards were prepared and then spiked with stable Sm at concentrations from 1 to 1000 ng g−1, and the signal monitored at m/z = 167. Even at a concentration of 1000 ng g−1, there was no increase in signal at m/z = 167 as a result of 150Sm16O1H+ formation (Fig. 6). This is in agreement with stable element results, with the majority of Sm forming SmO+, and the remainder staying on mass, with negligible hydride and hydroxide formation. Given that the stable Sm concentration in graphite following chemical separation ranged from the levels of 13.4–22.6 ng g−1, polyatomic 150Sm16O1H+ is not a significant concern for the samples studied. Additionally, given that the abundance sensitivity of ICP-MS/MS in MS/MS mode has been measured as < 10−10 [23], there is no tailing from 150Sm+ present as 150Sm16O+.
Signal measured for a 100 Bq g−1 151Sm in the presence of stable Sm under optimal instrument conditions (Table 2)
Repeat standards of 100 Bq g−1 (108.09 pg g−1) 151Sm were also spiked with 0.1–100 ng g−1 Eu and the signal monitored at m/z = 167 (Fig. 7). The majority of the Eu+ signal remained on mass, with only 3.4–3.7% of the total signal measured as EuO+, in good agreement with the results achieved during tuning. However, given the low activities of Sm being investigated, even a small concentration of Eu contamination can significantly contribute to the final Sm signal.
An Eu decontamination factor of one order of magnitude was achieved using ICP-MS/MS in O2 mode. The lowest Eu concentrations measured in final samples was around 1 ng g−1, enabling 151Sm quantification at the 100 Bq g−1 (108.09 pg g−1) concentration level. This is one order of magnitude lower than the 151Sm exemption limit of 1000 Bq g−1 [31], demonstrating that ICP-MS/MS is applicable to quantification of 151Sm in decommissioning matrices. Complete separation of Sm from Eu prior to measurement is not required, which is significant given the difficulty in separating lanthanide elements. Additionally, the procedural time is reduced, as reaction-cell separation is instant, and eliminates the need for additional and relatively time-consuming offline separation techniques, as well as reducing the number and volume of resins and reagents used. However, the relatively short half-life of 151Sm with regards to ICP-MS measurement means that even low-level Eu contamination has a significant impact on the detection limits achievable.
Alternative approaches to Eu interference removal include mathematical correction by monitoring contamination at m/z = 153 (52.19% abundance). In order to overcome instrumental mass bias, a reference solution with known 151Eu/153Eu ratio would need to be run prior to the samples in order to calculate the mass bias correction factor. This approach will increase the measurement uncertainty compared to the approach presented in this study. Alternative reaction gases may offer improved Sm/Eu separation compared to O2. For example, gases such as CH3F and N2O have been successfully applied to ICP-MS/MS separations of multiple elements [20, 32], but were not investigated as part of this study. An improved Eu separation may also be achieved by combining reaction cell separation with online separation, such as HPLC or CE, which have been proven to be efficient techniques for lanthanide separation.
Conclusions
The decommissioning of nuclear power plants represents a significant challenge with regards to the range of matrices and difficult-to-measure radionuclides that must be accurately measured. One such radionuclide is 151Sm, with interferences from other lanthanide elements (primarily Eu) significantly impacting accurate measurement. This study demonstrates the effectiveness of lithium borate digestion of graphite samples, and the difficulties related to the separation of Sm and Eu using offline extraction chromatography techniques. For the first time, ICP-MS/MS has been used for final quantification of 151Sm. By using O2 in the reaction cell, 151Sm+ is shifted to 151Sm16O+, whilst interfering 151Eu remains on mass. This approach offers a one order of magnitude cleanup, meaning that complete separation is not required prior to sample introduction. This improves the sample throughput through a reduction in the total procedural time. A detection limit of 1 Bq g−1 was achieved for interference-free 151Sm samples, increasing to 100 Bq g−1 in samples contaminated with 1 ng g−1 Eu. Further improvements in Eu decontamination may be achieved by combining ICP-MS/MS with online separation via HPLC or CE.
References
Nuclear Energy Agency (2014) R&D and innovation needs for decommissioning nuclear facilities. https://www.oecd-nea.org/rwm/pubs/2014/7191-rd-innovation-needs.pdf. Accessed 12 Feb 2018
SEC (2011) Seventh situation report on radioactive waste and spent fuel management in the European Union. Commission staff working paper. SEC 1007 final. https://ec.europa.eu/energy/sites/ener/files/documents/seventh_situation_report_corr_version_without_cover_page.pdf. Accessed 12 Feb 2018
LNE-LNHB Table de Radionuléides (2017) http://www.nucleide.org/DDEP_WG/Nuclides/Sm-151_tables.pdf. Accessed 29 Sept 2017
Schwantes JM, Sudowe R, Nitsche H, Hoffman DC (2008) Applications of solvent extraction in the high-yield multi-process reduction/separation of Eu from excess Sm. J Radioanal Nucl Chem 276(2):543–548
Vio L, Crètier G, Chartier F, Geertsen V, Gourgiotis A, Isnard H, Rocca J-L (2012) Separation and analysis of lanthanides by isotachophoresis coupled with inductively coupled plasma mass spectrometry. Talanta 99:586–593
Jerome S (1988) An improved method for the analysis of premethium-147. Sci Total Environ 70:275–298
Martin JP (1999) The determination of promethium-147 and samarium-151 using extraction chromatography. Spec Publ R Soc Chem 234:201–213
Pitois A, de Las Heras LA, Betti M (2008) Determination of fission products in nuclear samples by capillary electrophoresis-inductively coupled plasma mass spectrometry (CE-ICP-MS). Int J Mass Spectrom 270:118–126
Yoshida M, Sumiya S, Watanabe H, Tobita K (1995) A rapid separation method for determination of promethium-147 and samarium-151 in environmental samples with high performance liquid chromatography. J Radioanal Nucl Chem 197(2):219–227
Wolf SF, Bowers DL, Cunnane JC (2005) Analysis of high burnup spent nuclear fuel by ICP-MS. J Radioanal Nucl Chem 263(3):581–586
Hou X, Roos P (2008) Critical comparison of radiometric and mass spectrometric methods for the determination of radionuclides in environmental, biological and nuclear waste samples. Anal Chim Acta 2(11):105–139
Lariviere D, Taylor VF, Evans RD, Cornett RJ (2006) Radionuclide determination in environmental samples by inductively coupled plasma mass spectrometry. Spectrochim Acta B 61(8):877–904
Croudace IW, Russell BC, Warwick PE (2017) Plasma source mass spectrometry for radioactive waste characterisation in support of nuclear decommissioning: a review. J Anal Atom Spectrom 32:494–526
Alonso JG, Sena F, Arbore P, Betti M, Koch L (1995) Determination of fission products and actinides in spent nuclear fuels by isotope dilution ion chromatography inductively coupled plasma mass spectrometry. J Anal Atom Spectrom 10:381–393
Moreno JMB, Alonso JIG, Arbore P, Nicolaou G, Koch L (1996) Characterization of spent nuclear fuels by ion chromatography–inductively coupled plasma mass spectrometry. J Anal Atom Spectrom 11:929–935
Isnard H, Brennetot R, Caussignac C, Caussignac N, Chartier F (2005) Investigations for determination of Gd and Sm isotopic compositions in spent nuclear fuels samples by MC ICPMS. Int J Mass Spectrom 246(1–3):66–73
Russell B, Garcia Miranda M, Ivanov P (2017) Development of an optimised method for analysis of 90Sr in decommissioning wastes by triple quadrupole inductively coupled plasma mass spectrometry. Appl Radiat Isot 126:35–39
Amr AA, Helal A-FI, Al-Kinani AT, Balakrishnan P (2016) Ultra-trace determination of 90Sr, 137Cs, 238Pu, 239Pu, and 240Pu by triple quadruple collision/reaction cell-ICP-MS/MS: establishing a baseline for global fallout in Qatar soil and sediments. J Environ Radioact 153:73–87
Shikamori Y, Nakano K, Sugiyama N, Kakuta S (2012) The ultratrace determination of iodine 129 using the Agilent 8800 Triple Quadrupole ICP-MS in MS/MS mode. https://www.agilent.com/cs/library/applications/5991-0321EN_AppNote_8800_I.pdf. Accessed 29 Sept 2017
Zheng J, Tagami K, Bu W, Uchida S, Watanabe Y, Kubota Y, Fuma S, Ihara S (2014) 135Cs/137Cs isotopic ratio as a new tracer of radiocesium released from the Fukushima nuclear accident. Environ Sci Technol 48(10):5433–5438
Van Es EM, Russell BC, Ivanov P, Read D (2017) Development of a method for rapid analysis of Ra-226 in groundwater and discharge water samples by ICP-MS/MS. Appl Radiat Isot 126:31–34
Tanimizu M, Sugiyama N, Ponzevera E, Bayon G (2013) Determination of ultra-low 236U/238U isotope ratios by tandem quadrupole ICP-MS/MS. J Anal Atom Spectrom 28:1372–1376
Triple Quadrupole ICP-MS (2017) http://www.agilent.com/en/products/icp-ms/icp-ms-systems/8800-triple-quadrupole-icp-ms. Accessed 29 Sept 2017
Progress in Radioactive Graphite Waste Management, IAEA-TECDOC-1647 (2017) http://www-pub.iaea.org/MTCD/publications/PDF/te_1647_web.pdf. Accessed 29 Sept 2017
Pearce A (2001) CIEMAT/NIST—what is it? LS Users Forum 2001. http://www.npl.co.uk/upload/pdf/20010905_lsuf_pearce2_1.pdf. Accessed 29 Sept 2017
Suran J, Kovar P, Smoldasova J, Solc J, Van Ammel R, Garcia Miranda M, Russell B, Arnold D, Zapata Garcia D, Boden S, Rogiers B, Sand J, Peräjärvi K, Holm P, Hay B, Failleau G, Plumeri S, Beck YS, Grisa T (2017) Metrology for decommissioning nuclear facilities: partial outcomes of joint research project within the European Metrology Research Program. Appl Radiat Isot. https://doi.org/10.1016/j.apradiso.2017.08.032
Croudace IW, Warwick PE, Taylor R, Dee S (1998) Rapid procedure for plutonium and uranium determination in soils using a borate fusion followed by ion-exchange and extraction chromatography. Anal Chim Acta 371(2–3):217–225
Triskem Ln resin product guide (2018) http://www.triskem-international.com/iso_album/tki_1_binder_en.pdf. Accessed 14 Feb 2018
Agilent Technical Note (2017) Reaction data for 70 elements using O2, NH3 and H2 gases with the Agilent 8800 Triple Quadrupole ICP-MS. http://www.agilent.com/cs/library/technicaloverviews/public/5991-4585EN_TechNote8800_ICP-QQQ_reactiondata.pdf. Accessed 29 Sept 2017
Agilent O2 Technical Note (2017) Agilent 8800 Triple Quadrupole ICP-MS: understanding oxygen reaction mode in ICP-MS/MS. http://www.agilent.com/cs/library/technicaloverviews/public/5991-1708EN_TechOverview_ICP-MS_8800_ORS_mode.pdf. Accessed 29 Sept 2017
IAEA Safety Standard Series No. RS-G-1.7. https://www-pub.iaea.org/MTCD/publications/PDF/Pub1202_web.pdf. Accessed 12 Feb 2018
Bolea-Fernandez E, Balcaen L, Resano M, Vanhaecke F (2016) Tandem ICP-mass spectrometry for Sr isotopic analysis without prior Rb/Sr separation. J Anal Atom Spectrom 31:303–310
Acknowledgements
This study was supported by the European Metrology Research Program (EMRP) joint research project “Metrology for Decommissioning Nuclear Facilities” (MetroDecom). The European Metrology Research Programme (EMRP) is jointly funded by the EMRP participating countries within EURAMET and the European Union.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miranda, M.G., Russell, B. & Ivanov, P. Measurement of 151Sm in nuclear decommissioning samples by ICP-MS/MS. J Radioanal Nucl Chem 316, 831–838 (2018). https://doi.org/10.1007/s10967-018-5764-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-018-5764-x