Abstract
Sorption of the 85Sr radionuclide from model seawater solutions on phosphate sorbents was studied. Sorbents were synthesized using natural dolomite by various methods. Effects of salinity and pH of solutions on the degree of sorption and the distribution coefficient of the 85Sr were investigated. Hydrolytic stability, phase transformation of sorbents in model seawater solutions and change of the pHpzc were established with the aim of identifying factors, which influence on the sorption efficiency towards the 85Sr radionuclide. The best sorption parameters in all range of salt concentration were obtained for sorbent containing tertiary calcium and magnesium phosphates and magnesium–ammonium phosphates.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High risk of radiation for human health makes continuous interest in finding effective methods for radionuclides immobilization. Depending on the level of radioactivity, sources, and volume of pollution different methods are used for immobilization of radionuclides, details of which are summarized in some review papers: precipitation, evaporation, solvent extraction, membrane technique, filtration, sorption and ion-exchange [1,2,3,4]. Each method has its advantages and limitations in use, so studies are underway in all these areas.
Currently, sorption technologies are developed very intensively for the decontamination of liquid radioactive waste (LRW) containing strontium radionuclides. The study of novel sorbents for strontium removal is the subject of research around the world. Due to the chemical and thermal stability and radiation resistance inorganic sorbents are the most promising materials for these purposes. One of the main required characteristics for sorbents is the high selectivity towards radionuclides due to the complex composition of LRW. Depending on chemical composition, LRW can be classified to low-salt (e.g. rinsing waters with salt concentration < 1 g L−1) and high-salt (e.g. decontamination solutions, ion-exchange regeneration solutions with salt concentration > 1 g L−1) [5].
At the present time there is a number of sorbents that demonstrate sorption-selective properties towards Sr2+-ions in saline solutions, e.g. natural and synthetic zeolites [4,5,6,7] and other raw materials [8,9,10], composite magnetic nanoparticles [11], manganese oxides [12], titanates and titanosilicates [5, 6, 13,14,15] etc.
Selective removal of Sr2+-ions from solutions with high salinity is a challenging task. One problem of strontium radionuclides removal from LRW is the presence of competing ions in solutions, especially calcium [5, 6, 8,9,10,11,12,13,14], magnesium [9,10,11, 14] and sodium [6, 8,9,10,11,12, 14, 15] that reduce the uptake of strontium. Another problem is the formation of strontium complexes with strong organic complexing ligands that lowers strontium sorption affinity [9]. These substances like oxalic acid and oxalates, citrate, Trilon B and others are often used as anti-fouling or decontamination reagents and at the technology end-point they become the component of LRW.
High characteristics for strontium sorption and ion exchange were shown by phosphate sorbents [16]. Most of the works are devoted to the sorption study of hydroxyapatite (HA) as the most common representative of calcium phosphates which is the main mineral component of bone tissue [7, 16,17,18,19,20,21,22,23]. Non-apatite calcium phosphates are much less studied [7, 24]. Despite the fact that the presence of competing ions negatively affects on the targeted ions sorption by phosphate sorbents, their use for the immobilization of radionuclides appear to be very promising because of the sorption capacity persistence in salt solutions, high affinity to strontium ion, its durable retention due to the formation of insoluble compounds, high performance in a wide pH range, especially in alkaline medium. The advantages of phosphate sorbents also include their safety for the environment and the possibility of using local natural resources to produce such sorbents in large quantities.
In particular, carbonate-containing minerals, such as calcite, can be used for obtaining of HA both of stoichiometric composition [25] and calcium deficient HA with incorporation of carbonate groups [26]. In some studies, the calcium oxide is obtained from calcium carbonate via its calcination, and then is neutralized with phosphoric acid [18, 28, 29]. In addition to the inorganic raw materials renewable organic resources also can be used, as for example in the case of sorbent Apatite II derived from fish bones [16, 17], and biogenic HA produced by bacterium Serratia sp. [19, 20].
The possibility of textural and morphological properties varying (degree of crystallinity, size of crystallites, porous structure) is a feature of HA and non-apatite calcium phosphates that determines their sorption capacity [21, 25, 27,28,29]. The sorption mechanism of Sr on HA is investigated in details. It has been showed that the sorption capacity of HA depends on its crystallinity [19, 22], morphology [21], solubility [22] and point of zero charge [18, 23]. According to the previous works, the dominant mechanism of Sr2+-ions sorption on HA is ion exchange, due to the similarity of the ionic radii of Ca2+ and Sr2+-ions [18, 21] and specific sorption mechanisms [18].
Calcium phosphates of non-apatite structure, as well as magnesium phosphates have been less studied as sorbents [7, 24, 30, 31]. Although the tertiary and hydrogen calcium and magnesium phosphates also show high sorption capacity, binding of ions occurs via the mechanism of dissolution–precipitation or ion exchange reactions. As the result of this interaction, the molar ratio of the extracted ions to ions released into solution can be increased to 1.3, 1.5 or 2 times depending on the concentration and pH of the initial solution [31]. Rokita et al. [24] found a fundamental difference in the mechanism of interaction of calcium phosphates with metal ions, for example Sr2+, depending on their structure. It was shown that during the removal of Sr2+ ions by phosphate with apatitic structure, strontium atoms are preferentially incorporated in fourfold calcium positions, while in the case of amorphous calcium phosphate and dicalcium phosphate dihydrate all calcium sites may be occupied by strontium atoms.
Using the ability of calcium phosphates to participate in exchange reactions with polyvalent metals ions in aqueous solutions it is possible to obtain substituted phosphates, in which the presence of impurity ions in the structure may increase their reactivity. In this way, sorbent Apatite II with Na+ ions has high reactivity towards numerous metal ions [16, 17]. Use of dolomite (calcium–magnesium carbonate) as a raw material makes it possible to obtain a magnesium containing calcium phosphates [32]. It is important to note that magnesium is a major impurity elements included in the structure of bone tissues and teeth, which ensures its higher reactivity [33].
Studies of the effect of magnesium addition to the composition of HA on removal efficiency of Cd2+ ions showed a significant increase in the sorption capacity with increasing of Mg2+ content and a higher value of enthalpy of ion exchange between Mg2+ and Cd2+ than that between Ca2+ and Cd2+ [34]. In previous works [35, 36] we found high efficiency of Ca-, Mg- and Ca–Mg phosphate sorbents of non-apatite structure in the processes of stable and radioactive Sr2+ ions removal both from aqueous solutions and model groundwater with hardness (in the presence of CaCl2). Sorption materials consisting Ca–Mg phosphates were synthesized from dolomite as a cheap and widespread natural raw materials representing a mixture of Ca and Mg carbonates. It was found that Mg phosphates retained a capacity for Sr2+ ions up to 100 mg g−1 in 0.05 M CaCl2 solution.
The aim of this work was to study the efficiency of Ca–Mg phosphate sorbents based on dolomite for radionuclide 85Sr removal from aqueous solutions modelling seawater depending on the salt content in the wide range of pH. It was important to determine the influence of competing ions in the solution on the stability of Ca–Mg phosphate sorbents and the effect of the surface changes of sorbents on their selectivity towards 85Sr removal from solutions with high salinity.
Experimental
Preparation of sorbents
The dolomite used was from the Ruba (Belarus, Vitebsk region) deposit with the following chemical composition (wt%): SiO2 1.1, Fe2O3 0.4, Al2O3 0.5, CaO 30.3, MgO 20.0, SO3 0.4, K2O 0.2, Na2O 0.1 and calcination loss 47.0. For the synthesis of sorbent with a high content of calcium and magnesium phosphates and high sorption properties it was previously suggested to activate the natural dolomite by its calcination at 800 °C. This allows to remove organic impurities contained in the natural dolomite, and to decompose dolomite to the magnesium oxide and calcium carbonate, that will significantly increase its activity in interaction with nitric and phosphoric acid.
The first sorbent (PD-1) was obtained after 24 h of activated dolomite stirring with a 20% phosphoric acid at dolomite:phosphoric acid weight ratio of 1:3. In order to prepare the second sorbent (PD-2), activated dolomite was dissolved in concentrated nitric acid, thereafter Ca and Mg phosphates were precipitated by ammonium phosphate at pH 10. The synthesis was realized by a slow controlled titration (5 mL s−1), and after total addition of the ammonium phosphate solution the suspension was stirred during 24 h. After the aging and washing with distilled water, the precipitate of Ca–Mg phosphate was rinsed with ethanol. Replacement of intermicellar liquid (water–ethanol) allows to obtain the sorbent with a more developed mesoporous structure, because ethanol has a lower value of the surface tension comparing to water. By this means, sorbent PD-1 represents a mixture of Ca–Mg hydrogen phosphates with the approximate composition (Mg,Ca)HPO4·xH2O and PD-2 represents a mixture of Ca–Mg tertiary phosphates with the total formula (Mg,Ca)3(PO4)2·yH2O. More details can be found elsewhere [32]. Sample PD-3 was obtained using soft non-acid method after 16 h of activated dolomite stirring with 0.2 M NaH2PO4 with dolomite:NaH2PO4 ratio of 1.4:33 g mL−1 [37]. Theoretical calculations suggest that this quantity is enough for full interaction between magnesium oxide and sodium phosphates.
Analytical methods
XRD patterns were collected on a DRON-3 powder diffractometer (CuKα radiation, 2θ = 10°–60°) (Burevestnik, Russia). Phase identification of the samples was carried out using XRD standard base JCPDS PDF2. The file number is provided in figure caption in the brackets.
The sorption and texture properties of the sorbents were assessed by isotherms of low temperature (−196 °C) physical adsorption–desorption of nitrogen, measured by the volumetric method on an ASAP 2020 MP surface area and porosity analyser (Micromeritics, USA). The surface area of pores per unit mass of the solid or the specific surface area were determined by the single point (A sp) according to Brunauer–Emmett–Teller (BET) theory (A BET). The single point method was used to calculate not only the specific surface area A sp, but also the adsorption and desorption volumes (V sp.ads and V sp.des) of pores and their average adsorption and desorption diameters D ads and D des. The relative error in determination of the pore volume was ± 1% for the surface area and ± 15% for the pore size.
For elemental composition analysis the sorbents were dissolved in 6 M nitric acid. Total calcium and magnesium concentrations were determined using complexometric EDTA-titration and magnesium concentration was determined using an atomic-absorption technique. The concentration of PO4 3− was analyzed spectrophotometrically as phosphovanadomolybdate complex at λ = 440 nm.
For studying of 85Sr uptake by phosphate sorbents, the 85Sr activity in the solution before (A init, kBq L−1) and after the sorption (A eq, kBq L−1) was measured by MKS AT1315 γ,β-spectrometer (Atomtex, Belarus). The degree of recovery (S, %) and the distribution coefficient (K d, cm3 g−1) of 85Sr were calculated by equations:
where V is the volume of solution (cm3), m is the mass of the sorbent (g). The initial activity of 85Sr(NO3)2 aqueous solutions was about 1 × 102 kBq L−1.The pH of the aqueous solutions was measured with a pH-meter OP-208/1 (Radelkis, Hungary).
Batch sorption studies
The batch experiments were conducted by mixing 0.10 g of sorbent with 50 mL of model seawater solutions with 85Sr radionuclide at controlled initial pH in plastic tubes from polyethylene with low sorption capacity (volume 100 mL) on open atmosphere. For pH adjustment HCl (0.1 M) and NaOH (0.1 M) solutions were used. The suspensions were agitated at the temperature 20 °C for 24 h with periodical rotation. After that sorbents were filtered by paper filter (3–5 µm pores) and 85Sr activity was measured by γ, β- spectrometer.
Seawater solutions preparation
The sorption experiments with radionuclide 85Sr were performed using the stock solution modelling seawater with total salt content 50.0 g L−1 and the diluted solutions with salt content 5.0, 10.0, 15.0, 20.0 and 35.0 g L−1. The stock solution was prepared by dissolving alkaline and alkaline-earth chlorides MCl (M = K, Na) or MCl2 (M = Mg, Ca) and MgSO4·6H2O in distillate water. The composition of the stock solution is presented in Table 1. At salt content 35.0 g L−1 the composition correspond to real seawater [38] and was used elsewhere [10, 20].
Effects of salt content and pH on the sorption of radionuclide 85Sr
Influence of salt content on the sorption process was studied in the solutions with various salt concentrations in the range from 0.0 to 35.0 g L−1 and radionuclide 85Sr. The solutions’ pH was adjusted to 8.1 ± 0.3.
The batch experiments for studying of pH effect were conducted by mixing of sorbent with model solutions (salt content 35.0 g L−1) at pH range from 2 to 8.5.
Effects of salt content and pH of solutions on the sorbents stability
These experiments were carried out at the same conditions as batch sorption experiments but in absence of radionuclide 85Sr. After suspension filtration pH of solutions were measured. This technique allows determine the point of zero charge (pHpzc) [18, 23]. The sorbents were washed with distilled water, dried at the temperature 70 °C and then phase composition was studied.
Results and discussion
Characteristics of the sorbents
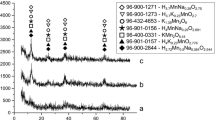
The sorbents for this study differ in chemical and phase composition. According to chemical analysis (Table 2) and XRD data (Fig. 1) the PD-1 sample is a mixture of calcium and magnesium hydrogen phosphates with gross composition Ca2Mg(HPO4)3·8.7H2O. Sorbent PD-2 is a mixture of calcium, magnesium and magnesium-ammonium secondary phosphates and its composition can be described by formula CaMg1.5(NH4)(PO4)2·12.5H2O. PD-3 is a mixture of calcium and magnesium tertiary and hydrogen phosphates, calcium carbonate and magnesium oxide with composition of Ca0.6Mg x (PO4) y (HPO4)1.6−y (CaCO3)4.7(MgO)5.1–x ·7·8H2O. The diffraction pattern of the PD-2 sorbent shows only the magnesium-ammonium phosphate because this compound is characterized by a high degree of crystallinity and is confirmed by corresponding intensive reflections, while the calcium and magnesium tertiary phosphates obtained by precipitation method are amorphous compounds and their reflexes are absent.
XRD patterns of PD-1 (1), PD-2 (2) and PD-3 (3). Phases: A—CaHPO4·2H2O [9-77], B—MgHPO4·3H2O [35-780], C—MgNH4PO4·6H2O [71-2089], D—CaCO3 [5-586], E—MgO [78-430]. The same phases are for Fig. 4
Table 3 shows the differences in the porous structure parameters of the obtained sorbents. The highest specific surface area was obtained for PD-2—A sp = 49 and A BET = 54 m2 g−1. For samples PD-1 and PD-3 the values were about 15–20 m2 g−1. The sample PD-2 had the highest pore volume 0.182–0.238 cm3 g−1, which was about 3–4 times higher than for other two sorbents. Distribution data for the average pore sizes indicated that the PD-3 samples had the smallest pore size about 11–12 nm.
Effects of salt content on the sorption of 85Sr
Figure 2a and b show the degree of 85Sr radionuclide sorption by phosphate sorbents from aqueous solutions depending on the salt concentration and the corresponding change of distribution coefficient of 85Sr. Thus, in aqueous solution without background electrolyte sorbent PD-2, which is a mixture of tertiary phosphates of Ca, Mg and ammonium, is the most active. The degree of 85Sr radionuclide sorption for this sorbent has reached 84% and the distribution coefficient 2.66 × 103 cm3 g−1. For sample PD-3, obtained under acid-free phosphating, the degree of extraction of 85Sr was 55%, and K d—0.63 × 103 cm3 g−1. The lowest activity towards 85Sr showed sorbent PD-1: S = 29%, K d = 0.21 × 103 cm3 g−1.
Introducing cations and anions modelling seawater to 85Sr radionuclide solution leads to the decrease of 85Sr sorption by all sorbents. Even the solutions with minimal salt concentration (5.0 g L−1) suffers a sharp decrease in sorption degree to 42% for PD-2, 16.7% for PD-3 and 11.5% for PD-1. The subsequent increase of salt concentration from 5.0 to 50.0 g L−1 does not cause sharp decrease in the degree of sorption, reaching at the end point values of 21.4, 6.8 and 7.0% for PD-2, PD-3 and PD-1, respectively. Similar changes occur with the value of the distribution coefficient.
Figure 2c shows the changes of the background pH of the solutions after contact with the sorbents. It demonstrates that all the sorbents increase the pH of salt solutions in comparison with the initial solution without sorbents. Changes in the pHf values relative to the initial pH of solutions are less for sorbents PD-1 and PD-3, than for the sorbent PD-2. Moreover, increase of the concentration of salt solution leads to the decrease of ∆pH that can be explained by the suppression of sorbent hydrolysis in the solution.
The comparison of obtained results with the data of [36] for the sorption of the 85Sr radionuclide on the background of CaCl2 suggests that Na+ ions have much less influence on the degree of 85Sr radionuclide sorption comparing to Ca2+ ions. Thus, in more mild conditions—with V/m ratio 250 mL g−1 and a saline background 0.5 g of Ca2+ ions per 1 g of sorbent, the degree of sorption for PD-1 and PD-2 decreased from 37 and 97% in water to 12 and 31% in the presence of 0.05 M CaCl2. The similar results of the influence of different alkali and alkaline earth metals cations on the extraction of the 85Sr radionuclide were obtained for sorbents with apatite structure [7, 18, 20], that is explained by the greater affinity of Ca2+ ions to the surface than others (Mg2+, Na+, K+) [18].
Effect of pH on sorption of 85Sr
Effect of initial solutions pH for the 85Sr sorption was studied in model solution with a salinity 35.0 g L−1, which corresponds to the salt content in seawater. The change in the degree of sorption and distribution coefficient depending on pHs solution is shown on Fig. 3a and b, which demonstrate different characters for the studied sorbents. So, for the most active phosphate sorbent PD-2, the maximum sorption efficiency in relation to the radionuclide is in the range of pH 3–9. The degree of 85Sr sorption is about 25%. At pH less than 3, the degree of soprption of the radionuclide decreases rapidly and is slightly more than 5%. The change in the distribution ratio is similar and the maximum value in the alkaline solution is 0.18 × 103 cm3 g−1. For samples PD-1 and PD-3 the dependence of degree of 85Sr sorption on the pH of the initial solution has an extreme character. The maximum absorption falls to the range of pH 5–6 and is 8 and 13% for PD-1 and PD-3 respectively. At pH below 3.0 and above 6.0 sorption effeciency is reduced 3 times for PD-1, and 2 times for PD-3. Thus, minimum sorption of 85Sr varies from 3% for PD-1 to 6.2% for PD-2 and PD-3 in the most acidic solutions for all sorbents. At pH 8.5 the sorption efficiency of the sorbents, with the exception of PD-2, is significantly reduced.
pHf of model solutions after contact with all three sorbents rised with increasing pHs of initial solutions, and graphics pHf versus pHs (Fig. 3c) are similar to S versus pHs (Fig. 3a) and K d versus pHs (Fig. 3b) for all three sorbents.
Effects of salt content and solutions pH on the sorbents stability
The results of the effect of initial solutions pH (for the model solution with salt content of 35.0 g L−1) and the salt concentration on the changes in pH after contact with the sorbents in the absence of radionuclides are present on Figs. 2c and 3c. Together with the results of the phase composition of the sorbents after contact with model solutions (Fig. 4) allow to assume stability characteristics of the sorbents in the considered conditions.
The pH of the aqueous extract of sorbents without the background electrolytes for all samples is increasing with the greatest increase (ΔpH about 2.7) observed for PD-2, while for PD-1 and PD-3, this difference is much smaller—1.4 and 1.6, respectively (Fig. 2c). The increase of salt content in solution is accompanied by pH decrease 0.68–0.78.
Such behavior of the sorbents can be explained by their different chemical composition, and complex multicomponent structure. The observed pH values of the solutions after contact with the sorbents are the cumulative results of many processes. The phosphate anion has a strong tendency to protonation of [39, 40]. Protonation of the phosphate and hydrophosphate anions included in the composition of the sorbents leads to alkalization of the solution.
At the same time, other components of sorbents undergo hydrolysis, in particular magnesium oxide and calcium carbonate.
The sorbent PD-1 is more homogeneous in the anionic composition, therefore in the aqueous medium mainly the protonation of hydrophosphate anion. As a result, at higher phosphorus content in the composition of this sorbent compared to the PD-2 (Table 2), the rise in pH is much smaller. Sorbents PD-2 and PD-3 are complex compounds with several anions and magnesium oxide. Phosphorus compounds are represented as the phosphate groups PO4 3− and hydrophosphate HPO4 2−. However, despite of the lowest phosphorus content of this sorbent, the pH change is strongly contributed by the hydrolysis of carbonate groups and magnesium oxide. As a result, solution has higher pH than after the contact with the sorbent PD-1.
Introduction of electrolytes into the solution causes a decrease in the degree of phosphate ions protonation due to the formation of associates with background cations [39], and it leads to a suppression in hydrolysis, which leads to the pH decrease in comparison with the system in a salt-free medium.
The character of the pH change depending on pH of initial solutions allows to define such an important property of the surface of the sorbent as the pH point of zero charge (pHpzc). Based on the pH curves of equilibrium from initial solutions for all sorbents are allocated two plots (Fig. 3c). At lower initial values pHs up to 4 increase in pH due to the processes of protonation and hydrolysis is observed, and in the interval pHs 4–8 the final pHf values for all sorbents remain constant, corresponding to the values pHpzc. The highest value of pHpzc is shown by a sorbent PD-2, for which pHpzc is 8.4. Sorbents PD-1 and PD-3 have values of 7.2 and 7.3, respectively.
In addition to the hydrolysis process solubility of the sorbents influences on their behavior in solutions with different pH. The analysis of X-ray diffraction data of the sorbents after interaction with model solutions with different salinity and pHs (Fig. 4) confirms the partial dissolution of the sorbents in the solution without background electrolyte, as evidenced by a observable decrease in the intensity of reflexes of all phases in the diffraction patterns (curve 1). For XRD data of the PD-3 sorbent (Fig. 4c) under all conditions of treatment the sorbent is characterized by the presence of reflexes of only calcium carbonate and magnesium oxide, while the reflexes of magnesium hydrogen phosphate are absent. The increase in the concentration of salt in solution is accompanied by the increase in the intensity of reflexes compared with diffraction patterns after treatment in salt-free solution, indicating a decrease in the level of dissolution in the presence of electrolytes. Lowering the pH to 2 causes the greatest changes of the phase composition of the sorbents PD-1 and PD-2 and the almost complete disappearance of the magnesium phosphate reflexes (curve 4).
The obtained data on the stability of the sorbents in model solutions allow to explain the regularities of 85Sr radionuclide sorption from solutions with different salinity and pH-value. It is known that Sr2+ ion has very small constant of hydrolysis (10−13.3 [23]), therefore, as a rule, it is not hydrolyzed and exists in solution as the divalent cation. As it was established for HA, the binding of these cations takes place most effectively on the surface with a negative charge at establishing pHf above pHpzc [18, 23]. Given the trace concentration of 85Sr in solution it is possible to compare values pHpzc obtained in the model solution at identical conditions as the experiment on the sorption of the radionuclide, and initial pH for the analysis of sorption processes. So, for the investigated sorbents the values obtained pHpzc increase in the row of PD-1 < PD-3 < PD-2, with the values of 7.2, 7.3, and 8.4, respectively. The sorbents are arranged in the same row by sorption parameters such as degree of sorption and distribution coefficient of 85Sr (Fig. 3b). However, only for sorbent PD-2 value pHpzc exceed pHs, resulting in the sorption of the radionuclide reaches high values and is practically independent on pH in the range of 3–8.
Higher sorption efficiency of the sorbent PD-3 compared to PD-1 despite the low content of phosphate phases, especially MgHPO4, most active towards 85Sr [35] is facilitated by the presence of CaCO3 and MgO, resulting in a higher value of pHpzc. Decreasing sorption of radionuclides by PD-1 and PD-3 sorbents with increasing pH in model solutions (Fig. 3) can be explained by the lower solubility of the sorbents, which prevents the occurrence of chemical reactions sites on their surfaces.
Comparison with other sorbents
Comparison of the obtained results for 85Sr sorption from solution using phosphate sorbents with literature data should be subject to the conditions of the sorption experiments. So, in this work, the 85Sr radionuclide sorption was studied in conditions close to the natural environment (high salt content and low ratio m sorbent:V solution) that is rare in the literature data. This leads to lower values of the degree of 85Sr sorption and K d for the studied sorbents in comparison with other inorganic sorbents [5]. Given in Table 4 comparative data on sorption of Sr2+ ions mostly in the form of a stable isotope by a variety of sorbents on the basis HA confirms that the most active among the calcium phosphates are compounds characterized by low crystallinity structure or an amorphous state. Such phosphate sorbents are superior to the natural zeolite–clinoptilolite for Sr2+ sorption from seawater [20]. Combining data of this study together with presented in [36], we can assume that under similar conditions the sorbent PD-2 according to its sorption characteristics may exceed that described in the literature, and that is why sorbent PD-2 may be of interest for practical applications.
Conclusions
Sorption of 85Sr radionuclide from model seawater solutions was studied on phosphate sorbents with complex chemical composition. It was found that for the studied phosphate sorbents the efficiency of 85Sr sorption from model solutions decreased with salinity increase, due to increase in ionic strength of the solutions and the competing effect of metal cations in the background electrolyte. For sorbent PD-2 containing tertiary calcium and magnesium phosphates and magnesium-ammonium phosphate the highest values for the distribution coefficient K d (from 2.66 × 103 to 0.14 × 103 cm3 g−1) and the degree of sorption S (from 84% in salt-free solution to 21% in solution with maximum salt concentration of 50.0 g L−1) were obtained. In solutions similar to seawater composition (35.0 g L−1), these values are 0.18 × 103 cm3 g−1 and 26% respectivly.
The influence of pH on the extraction efficiency of 85Sr is determined by the value of pHpzc, which depends on the composition of sorbents and characteristics of the processes of protonation-deprotonation on their surface. For the studied sorbents values pHpzc increased in the row of PD-1 < PD-3 < PD-2, with the values of 7.2, 7.3 and 8.4, respectively. Only for sorbent PD-2 value of pHpzc (8.4) exceeded pHs in solution with salinity 35.0 g L−1, which ensures the independence of the degree of sorption and distribution coefficient on solution pH in the pH range 3.0–8.5.
References
Abdel-Rahman RO, Ibrahium HA, Hung Y-T (2011) Liquid radioactive wastes treatment: a review. Water 3:551–565
Xu C, Wang J, Chen J (2012) Solvent extraction of strontium and cesium: a review of recent progress. Solvent Extr Ion Exch 30:623–650
Rana D, Matsuura T, Kassim MA, Ismail AF (2012) Radioactive decontamination of water by membrane processes—a review. Desalination 321:77–92
Misaelides P (2011) Application of natural zeolites in environmental remediation: a short review. Microporous Mesoporous Mater 144:15–18
Marinin DV, Brown GN (2000) Studies of sorbent/ion-exchange materials for the removal of radioactive strontium from liquid radioactive waste and high hardness groundwater. Waste Manage 20:545–553
Merceille A, Weinzaepfel E, Barre Y, Grandjean A (2012) The sorption behaviour of synthetic sodium nonatitanate and zeolite A for removing radioactive strontium from aqueous wastes. SepPurifTechnol 96:81–88
Nishiyama Y, Hanafusa T, Yamashita J, Yamamoto Y, Ono T (2016) Adsorption and removal of strontium in aqueous solution by synthetic hydroxyapatite. J Radioanal Nucl Chem 307:1279–1285
Milenkovic AS, Smiciklas ID, Sljivic-Ivanovic MZ, Zivkovic LS, Vukelic NS (2016) Effect of experimental variables onto Co2+ and Sr2+ sorption behavior in red mud-water suspensions. J EnvironSci Health 51(8):679–690
Khan SA, Riaz-ur- Rehman, Ali Khan M (1995) Sorption of strontium on bentonite. Waste Manage 15:641–650
Hong H-J, Ryu J, Park I-S, Ryu T, Chung K-S, Kim B-G (2016) Investigation of the strontium (Sr(II)) adsorption of an alginate microsphere as a low-cost adsorbent for removal and recovery from seawater. J Environ Manage 165:263–270
Tu Y-J, You C-F, Zhang Z, Duan Y, Fu J, Xu D (2016) Strontium removal in seawater by means of composite magnetic nanoparticles derived from industrial sludge. Water 8:357–368
Ivanets AI, Prozorovich VG, Kouznetsova TF, Radkevich AV, Zarubo AM (2016) Mesoporous manganese oxides prepared by sol–gel method: synthesis, characterization and sorption properties towards strontium ions. Environ Nanotechnol Monit Manage 6:261–269
Dhandole LK, Ryu J, Lim J-M, Oh B-T, Park JH, Kim B-G, Jang JS (2016) Hydrothermal synthesis of titanate nanotubes from TiO2 nanorods prepared via a molten salt flux method as an effective adsorbent for strontium ion recovery. RSC Adv. https://doi.org/10.1039/C6RA14769K
Decaillon JG, Andres Y, Mokili BM, Abbe JCh, Tournoux M, Patarin J (2002) Study of the ion exchange selectivity of layered titanosilicate Na3(Na, H)Ti2O2[Si2O6]2·2H2O, AM-4, for strontium. Solvent Extr Ion Exc 20:273–291
Oleksiienko O, Levchuk I, Sitarz M, Meleshevych S, Strelko V, Sillanpää M (2015) Removal of strontium (Sr2+) from aqueous solutions with titanosilicates obtained by the sol–gel method. J Coll Interface Sci 438:159–168
Nzihou A, Sharrock P (2010) Role of phosphate in the remediation and reuse of heavy metall polluted wastes and sites. Waste BiomassValor 1:163–174
Krejzler J, Narbutt J (2003) Adsorption of strontium, europium and americium(III) ions on a novel adsorbent Apatite II. Nukleonika 48:171–175
Smiciklas I, Onjia A, Raicevic S, Janackovic D, Mitric M (2008) Factors influencing the removal of divalent cations by hydroxyapatite. J Hazard Mater 152:876–884
Handley-Sidhu S, Renshaw JC, Moriyama S, Stolpe B, Mennan C, Bagheriasl S, Yong P, Stamboulis A, Paterson-Beedle M, Sasaki K, Pattrick RAD, Lead JR, Macaskie LE (2011) Uptake of Sr2+ and Co2+ into biogenic hydroxyapatite: implications for biomineral ion exchange synthesis. Environ Sci Technol 45:6985–6990
Handley-Sidhu S, Mullan TK, Grail Q, Albadarneh M, Ohnuki T, Macaskie L (2016) Influence of pH, competing ions, and salinity on the sorption of strontium and cobalt onto biogenic hydroxyapatite. Sci Rep. https://doi.org/10.1038/srep23361
Rosskopfova O, Galambos M, Rajec P (2011) Study of sorption processes of strontium on the synthetic hydroxyapatite. J Radioanal Nucl Chem 287:715–722
Heslop DD, Bi Y, Baig AA, Otsuka M, Higuchi WI (2005) A comparative study of the metastable equilibrium solubility behavior of high-crystallinity and low-crystallinity carbonated apatites using pH and solution strontium as independent variables. J Colloid Interface Sci 289:14–25
Lazarevic S, Jankovic-Castvan I, Tanaskovic D, Pavicevic V, Dj Janakovic, Petrovic R (2008) Sorption of Pb2+, Cd2+, and Sr2+ ions on calcium hydroxyapatite powder obtained by the hydrothermal method. J Environ Eng 134:683–688
Rokita E, Hermes C, Nolting H-F, Ryczek J (1993) Substitution of calcium by strontium within selected calcium phosphates. J Cryst Growth 130:543–552
Morgan H, Wilson RM, Elliott JC, Dowker SEP, Anderson P (2000) Preparation and characterisation of monoclinic hydroxyapatite and its precipitates carbonate apatite intermediate. Biomater 21:617–627
Ivanova TI, Frank-Kamenetskaya OV, Kol’tsov AB, Ugolkov VL (2001) Crystal structure of calcium-deficient carbonated hydroxyapatite. Thermal decomposition. J Solid State Chem 160:340–349
Riman RE, Suchanek WL, Byrappa K, Chen C-W, Shuk P, Oakes CS (2002) Solution synthesis of hydroxyapatite designer particulates. Solid State Ionics 151:393–402
Lazić S, Zec S, Miljevic N, Milonjic S (2001) The effect of temperature on the properties of hydroxyapatite precipitated from calcium hydroxide and phosphoric acid. Thermochim Acta 374:13–22
Smičiklas ID, Onjia A, Raicevic S (2005) Experimental design approach in the synthesis of hydroxyapatite by neutralization. Sep Purif Technol 44:97–102
Shul’ga NB, Samuskevich VV (2002) Lead(II) sorption by magnesium phosphates. Russ J Appl Chem 75(3):391–397
Shashkova IL, Rat’ko AI, Kitikova NV (1999) Removal of heavy metal ions from aqueous solutions by alkaline-earth metal phosphates. Colloid Surface A 160(3):207–215
Ivanets AI, Kitikova NV, Shashkova IL (2015) Sorption wastewater treatment from metal ions using modified phosphate dolomite. Environ Sci Eng. 4:284–312
Abbona F, Lundager Madsen HE, Boistelle R (1986) The initial phases of calcium and magnesium phosphates precipitated from solutions of high to medium concentrations. J Crystal Growth 74(3):581–590
Yasukawa A, Yokoyama T, Kandori K, Ishikawa T (2008) Ion-exchange of magnesium–calcium hydroxyapatite solid solution particles with Cd2+ ion. Colloid Surface A 317:123–128
Ivanets AI, Shashkova IL, Kitikova NV, Drozdova NV, Saprunova NA, Radkevich AV, Kul’bitskaya LV (2014) Sorption of strontium ions from solutions onto calcium and magnesium phosphates. Radiochemistry 56:32–37
Ivanets AI, Shashkova IL, Kitikova NV, Radkevich AV, Davydov YuP (2015) Recovery of strontium ions with calcium and magnesium phosphates from aqueous solutions against the background of CaCl2. Radiochemistry 57:610–615
Ivanets AI, Kitikova NV, Shashkova IL, Matrunchik YuV, Kul’bitskaya LV, Sillanpää M (2016) Non-acidic synthesis of phosphatized dolomite and its sorption behaviour towards Pb2+, Zn2+, Cu2+, Cd2+, Ni2+, Sr2+ and Co2+ ions in multicomponent aqueous solution. Environ Technol Innov 6:152–164
Campbell DM, Millero FJ, Roy R, Roy L, Lawson M, Vogel KM, Porter Moore C (1993) The standard potential for the hydrogen–silver, silver chloride electrode in synthetic seawater. Marine Chem 44:221–233
Ivanenko VI, Udalova IA, Lokshin EP, Kalinnikov VT (2002) The state of phosphate ions in aqueous solutions containing NaCl, KCl, NaNO3, and KNO3. Russ J Inorg Chem 47:923–929
Jeanjean J, Fedoroff M, Faverjon F, Vincent U, Corset J (1996) Influence of pH on the sorption of cadmium ions on calcium hydroxyapatite. J Mater Sci 31:6156–6160
Shashkova IL, Ivanets AI, Kitikova NV, Sillanpää M (2017) Effect of phase composition on sorption behavior of Ca-Mg phosphates towards Sr(II) ions in aqueous solution. J Taiwan Inst Chem Eng. https://doi.org/10.1016/j.jtice.2017.09.027
Acknowledgements
This work was partially financially supported by Belarusian Republican Foundation for Fundamental Research (Grant #X17MC-006).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kitikova, N.V., Ivanets, A.I., Shashkova, I.L. et al. Batch study of 85Sr adsorption from synthetic seawater solutions using phosphate sorbents. J Radioanal Nucl Chem 314, 2437–2447 (2017). https://doi.org/10.1007/s10967-017-5592-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5592-4