Abstract
Nanoparticle film of copper hexacyanoferrate (CuHCFIII) was developed and coated on the rolled sheet electrodes for column cesium separation from wastewater. Results indicated well balance of Cs adsorption and desorption can be achieved switching the potentials between anodes and cathodes. XPS and isotherm studies indicated the electrochemical oxidation–reduction of Fe(II/III) on the monolayer of the CuHCFIII film made contributions to reversible Cs separation. Minimization of secondary waste, simple regeneration and uniformly coating for any size, suggest a promising column technology for sequential removal of Cs from actual wastewater.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
As we all know, the earthquake and subsequent tsunami in north-east Japan on 11 March 2011 caused huge damage to the Fukushima Daiichi nuclear power stations, Japan, thousands of tons of water have been catastrophically contaminated with 137Cs. Because of its chemical similarity to K, Cs can be easily incorporated into terrestrial and aquatic organisms, and ingestion of its radioisotopes results in their deposition in the soft tissues all over the body creating an internal hazard, especially to the reproductive system [1]. Nowadays, removal of Cs from wastewater form Daiichi 1st plant is a challenging job for the environmental engineers.
One of the most practical methods of cesium removal is that of its incorporation into hexacyanoferrates (HCFs) of divalent transition or of heavy metal cations [2, 3]. Among them, copper(II) hexacyanoferrate(III) (CuHCFIII) is often selected as the agent in feasible analysis. Principally, this component exhibits an open zeolite type structure consisting of a cubic network of iron centers bound by bridging cyanide ligand [4], which facilitates the intercalation of alkali metal ions into the cubic network required for maintaining its charge neutrality.
Although the structural properties of CuHCFIII are useful for selective separation of cesium, small sized CuHCFIII particles can easily contaminate water, which restricts its direct use for column operations. To solve this problem, many previous studies introduced the concept of electrochemically switched ion exchange (ESIX) as a technology using MHCF for Cs separation [5]. MHCF films were electro- or chemical deposited on the electrode substance [6, 7], Cs adsorption and desorption can be easily controlled by regulating the redox states of the MHCF films to achieve ion separation and/or regeneration of the MHCF films [8]. However, such a technique is still difficult to apply in wider field due to small scale of film coating (both size and thickness).
Herein, we first synthesized water-dispersed nanoparticle CuHCFIII ink and then coated its nanoparticles (NPs) on electrodes to electrochemically remove Cs. The CuHCFIII NPs can be uniformly coated on electrodes by simple wet process like conventional printing methods, so the column sizes and patterns are feasible at low cost. This study evaluates the electrochemical Cs sorbability of CuHCFIII NPs film and estimates the potential as a promising sorption electrode in the columns for sequential removal of Cs from wastewater.
Experimental
As indicated in our previous paper [9], the synthesis of CuHCFIII NPs was shown as follow:
In order to get the soluble ink, the CuHCFIII NPs were surface-capped with [FeII(CN)6]4− after the successive reaction of their insoluble solid with Na4[Fe(CN)6]. Then, 5 % CuHCFIII ink was obtained for film coating on the stainless sheet (SUS316L) using a spray coater (Model HIM-01, MITSUI Electric Co., Japan). Finally the CuHCFIII film coated large electrode sheet was rolled into the column for further characterization as well as Cs separation.
As shown in Fig. 1, Cs separation was conducted in a specially designed column of 2 cm diameter and 7.5 cm length. In such column system, the packed electrode sized 375 cm2, and the ratio of volume/surface area was 0.062. A three-electrode set, in which a platinum stick as reference electrode, rolled SUS sheet with and without CuHCFIII film as the working and counter electrode, respectively, was tightly packed in the column. The polythene filter paper (R7030, SSK, Japan) was sandwiched between two SUS electrodes. Multi-potential step technique was conducted by stepping to 0.0 V to load the film, and to +0.4 V to unload the film. This process can be simply controlled by switching the potentials of CuHCFIII NPs film between 0.0 and +0.4 V. The chemical equation was assumed as follow:
The prepared influent Cs or Na solution was passed upward at a constant volumetric flow rate with a peristaltic Pump (AI800, Yamazen, Japan). Effluent solution samples were collected and analyzed for Cs concentration using an inductively coupled plasma mass spectrometer (ICP-MS, NexION 300D, PerkinElmer).
Results and discussion
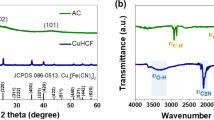
X-ray powder diffractometry (XRD, Ultima X, Rigaku, Japan) was carried out using a TF-XRD with CuKα beam in order to determine the crystalline structures. As shown in Fig. 2a, the peaks at 17.64°, 25.04°, and 35.71° are attributed to the Miller indexes of (2 0 0), (2 2 0), and (4 0 0) of the diffraction planes, respectively, indicating the cubic crystalline structure of Cu[Fe(CN) 6 ] 0.667 . According to Scherrer’s formula, D = K × λ/β × cos θ, the crystallite size of the CuHCFIII NPs can be evaluated from the full width at half maximum β of the diffraction peak with the Bragg angle of θ [10]. Since the particles are cubic, then K is equal to 0.943 and the average crystallite size of CuHCFIII NPs is 20 nm. The rough surface of CuHCFIII NPs film was also observed by atomic force microscopy (AFM) in Fig. 2b. Due to the estimation of the NPs agglomeration, the size ranged from 20 to 80 nm, which was a little larger than results from XRD calculation.
X-ray photoelectron spectroscopy (XPS) technique performed with a Shimadzu ESCA-3400 using an Al Kα X-ray source (1,486.6 eV) has been used for characterizing the structure of metal compounds and their interactions with CuHCFIII films. Figure 1c depicts the typical XPS spectra for CuHCFIII films before adsorption, and after 3 min, 90 min adsorption (coded as ad0, ad3 and ad90, respectively). Comparing with unused film, the appearance of peaks for Cs 3d at 724 and 738 eV indicated the Cs ions were adsorbed on film surface. Obviously, the peak of ad90 is much higher than that of ad3. The binding energy for Fe 2p are assigned at 707 eV to Fe(II), while Fe(III) are characterized by higher binding energies such as at 710 eV. Figure 1d indicated both Fe(II) and Fe(III) were observed in spectra of ad90, which demonstrated that the electrochemical oxidation–reduction of Fe(II/III) made contributions to Cs separation.
Influent solution of CsNO3/NaNO3 electrolyte was used for Cs uptake, and then pumped into 1 ppm NaNO3 electrolyte for Cs elution. Each process was performed at the flow rate 10 ml/min, and repeated for 5 cycles, the results were shown in Fig. 3. Well balance of Cs uptake and elution was achieved by electrochemical oxidation–reduction of CuHCFIII film, through switching the potentials between anodes and cathodes. In average, when the initial Cs concentration is 100 ppm about 723.6 μg was adsorbed on the CuHCFIII film, due to the electrochemical reduction from Cu(II)-CN-Fe(III) to Cu(II)-CN-Fe(II). Afterward, 709.4 μg was detected from the elution, indicting 96 ± 2.0 % of the loaded Cs can be desorbed from the used CuHCFIII film. The similar performance was also discovered when 80 ppm Cs solution passed through. This indicated that most of the sorption ability can be successfully regenerated by a reversible oxidation reaction from Cu(II)-CN-Fe(II) to Cu(II)-CN-Fe(III).
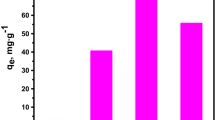
To investigate the characteristics of adsorption, the adsorption isotherms are of fundamental importance. The experiments were performed in an up-flow column at a constant volumetric flow rate of 10 ml/min, and the initial Cs concentration ranged from 6 to 100 ppm. Average data were reported and analyzed according to linearized forms of Langmuir and Freundlich isotherms [11], given as Eq. (3) and (4), respectively:
where C e (mg/l) is the pseudo-equilibrium concentration, q e (mg/g) is the amount adsorbed at pseudo-equilibrium, Q, n and b are isotherm constants. In Langmuir, Q and b, denoting monolayer capacity and energy of adsorption, which can be calculated from the slope and intercept of plot C e /q e verses C e, respectively. In Freundlich, b and n are the constants which are related to adsorption capacity and intensity, and can be calculated from the intercept and slope of the plot ln q e verses ln C e.
The results (Fig. 4) indicated that the experimental data better fitted Langmuir isotherm (R 2 = 0.993) than Freundlich isotherm (R 2 = 0.955), which was adopted to describe reversible chemical adsorption on the monolayer [11], rather than the monolayer sorption with a heterogeneous energetic distribution of active sites [12]. This is in agreement with the redox reaction, referring to Cs adsorption and desorption.
Summary
The synthesized CuHCFIII film, which has a crystalline structure, nanoparticles morphology, high selectivity for cesium, was proposed for Cs separation in an electrochemical column system. This system can be used for Cs separation without the costly ion-exchange membrane and extra chemical reagents. XPS and isotherm studies indicated the electrochemical oxidation–reduction of Fe(II/III) on the monolayer of the CuHCFIII film made contributions to reversible Cs separation. The well column performance, minimization of secondary waste, simple regeneration and uniformly coating for any size, suggest a promising column technology for sequential Cs removal.
References
Coughtrey PJ, Thorne MC (1983) Radionuclide distribution and transport in terrestrial and aquatic ecosystems, vol 2. A.A. Balkema, Rotterdam
Nilchi A, Malek B, Ghanadi MM, Khanchi A (2003) J Radioanal Nucl Chem 258:457–462
Pekarek V, Vesely V (1972) Talanta 19:1245–1283
Keggin JF, Miles FD (1936) Nature 137:577–578
Lilga MA, Orth RJ, Sukamto JPH, Rassat SD, Genders JD, Gopal R (2001) Sep Purif Technol 24(3):451–466
Sun B, Hao XG, Wang ZD, Guan GQ, Zhang ZL, Li YB, Liu SB (2012) J Hazard Mater 233–234:177–183
Hao XG, Li YG, Pritzker M (2008) Sep Purif Technol 63(2):407–414
Bocarsly AB, Sinha S (1982) J Electroanal Chem 140:167–172
Chen R, Tanaka H, Kawamoto T, Asai M, Fukushima C, Na H, Kurihara M, Watanabe M, Arisaka M, Nankawa T (2013) Electrochim Acta 87:119–125
Chan KY, Teo BS (2007) Microelectron J 38(1):60–62
Elizalde-Gonzalez MP, Mattusch J, Wennrich R, Morgenstern P (2001) Microporous Mesoporous Mater 46:277–286
LeVan MD, Vermeulen T (1981) J Phys Chem 85:3247–3250
Acknowledgements
A part of this study is the result of “Compact and reusable cesium recovery system by electrochemical adsorption/desorption” carried out under the Strategic Promotion Program for Basic Nuclear Research by the Ministry of Education, Culture, Sports, Science and Technology of Japan (MEXT).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, R., Asai, M., Fukushima, C. et al. Column study on electrochemical separation of cesium ions from wastewater using copper hexacyanoferrate film. J Radioanal Nucl Chem 303, 1491–1495 (2015). https://doi.org/10.1007/s10967-014-3588-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3588-x