Abstract
A feasibility study was carried out to evaluate the potential of the thermal neutron capture prompt-gamma activation analysis (PGAA) for the measurement of low levels of boron in selected Canadian and Japanese foods using the PGA facility at the JRR-3 reactor of the Japan Atomic Energy Agency (JAEA) in Tokai. A method was optimized for this purpose. It is rapid and can be used without any chemical separation. The precision and accuracy of the method are good. The detection limit is around 0.5 mg kg−1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Boron is known to be an essential element for plants. Its deficiency may lead to reduced growth and lower yield of the plant. Excess boron may cause the death of the whole plant [1]. It has been postulated that boron perhaps is an essential element for both animals and humans but no characteristic biochemical function has yet been put forward [2]. Boron has been reported to be nutritionally important and to act as a co-factor for calcium metabolism. It is also toxicologically important as boron can accumulate in certain food items whose consumption may very well constitute a potential health hazard [3]. It is therefore necessary to have reliable methods available for the measurement of varying levels of boron in foods and diets.
The determination of light elements such as boron is rather difficult by almost all analytical techniques. In 1997 Sah and Brown [4] have extensively reviewed several sample preparation and analytical methods for measuring boron levels in a variety of matrices. Recently, colorimetry [5, 6], fluorimetry [7], solid-sampling graphite furnace atomic absorption spectrometry (AAS) [8], inductively-coupled plasma (ICP) atomic emission spectrometry (AES) [9], ICP-mass spectrometry (ICP-MS) [10], negative thermal ionization mass spectrometry (TIMS) [11, 12], and Laser-induced breakdown spectroscopy (LIBS) [13] have been used for analyzing boron.
Conventional instrumental neutron activation analysis (INAA) is not particularly suitable for boron determination. Boron consists of two stable isotopes, namely 10B (19.82 %) and 11B (80.18 %). 10B has a low thermal neutron capture cross section (300 mb) and produces the stable isotope 11B which has even a lower thermal neutron capture cross section of 6 mb producing 12B [14]. The thermal neutron absorption cross section of natural boron is around 760 b [15, 16]. 12B is a very short-lived nuclide with a half-life of 20.2 ms and emits Cerenkov radiation. A very fast pneumatic transfer system is required for its detection, as was available at Atominstitut Wien in Austria reported by Grass and coworkers [17] and the Risoe National Laboratory, Roskilde in Denmark reported by Heydorn and coworkers [18]. The transfer time of the Dalhousie University SLOWPOKE-2 Reactor (DUSR) pneumatic cyclic system was about 100 ms and therefore not suitable for the analysis of boron by conventional INAA. So other non-destructive techniques were investigated using the facilities available in our laboratory at that time. In one such method it was assumed that the magnitude of the neutron flux reduction in the reactor would be proportional to the amount of boron present in a sample. So experiments were carried out with varying amounts of boron standards and the depressions in the neutron flux were recorded. The results were quantitative but the reproducibility was no more 80 %. Then an indirect INAA method [19, 20], based on measuring the activity of an easily activated indicator element (such as vanadium) in a sample containing different levels of boron, was modified by Moir and Chatt [21–23] and applied to study sodium borosilicate glasses designed to be the hosts for immobilized high-level radioactive waste. The indirect INAA method was suitable for this purpose but the absolute detection limit of 100 μg was too high for the analysis of boron in food samples. Other elements including selenium, indium and titanium were investigated as possible indicators. It was hoped that selenium and indium would be good indicator elements due to their short-lived (77mSe, 17.4 s; 116mIn, 14.1 s) and very sensitive nuclides but again the detection limits for boron were not low enough for food analysis.
Three other nuclear analytical techniques are highly suitable for the direct determination of low levels of boron in solid samples, namely neutron activation-mass spectrometry (NA-MS) [24, 25], particle-induced gamma-ray emission (PIGE) [26–30], and neutron capture prompt-gamma activation analysis (PGAA) [29]. In PGAA, the 10B(n,α)7Li reaction with a very high neutron absorption cross section of 3837 b is used for the determination of boron down to ppb levels in a variety of matrices. PGAA has been extensively used for the determination of boron in foods, diets, diet supplements, and biological reference materials [31–45]. Both thermal and cold neutron PGAA methods have been used for this purpose [40]. Thermal neutron PGAA has been used in the present work to measure boron levels in a variety of Canadian and Japanese foods.
Experimental
Samples, comparator standards, and reference materials
Four groups of samples were analyzed for boron by PGAA. One group contained Canadian vegetables, namely beans, beets, broccoli, cabbage, carrots, cauliflower, celery, corn cucumbers, lettuce, mushroom (canned), onion, peas, and peppers. The second group was Canadian food samples, namely beef, fish, flour, french fries, milk, pancakes, poultry, puddings, and frozen dinner. These market basket foods were collected at the retail level in the Toronto area, Ontario, Canada. They were prepared and made to composites at the Kemptville Community College in Ontario, and sent to us by Health Canada samples in frozen condition. These samples were freeze dried, homogenized, and stored in Teflon containers in a freezer at −20 °C. The moisture content of the samples was calculated.
The third group contained several Japanese vegetables which were chosen for comparison purposes. These were; broccoli, brown mushroom, ginger, okra, pumpkin, red pepper, shiitake mushroom, and snow peas. These vegetables were bought from farmers’ market in Yamagata Prefecture, washed with tap water, freeze dried, homogenized, and moisture content calculated.
The fourth group contained different kinds of spice which were purchased in grocery stores in Sendai (S, Japan), Halifax (H, Canada) and Budapest (B, Hungary). These were: black pepper (S), cardamom (H), cinnamon (S), whole seed cloves (S), roasted garlic powder (H), ginger powder (H), paprika powder (B), and powdered tea (H). These samples were used on as is basis (dry weight) and moisture content was not available.
Boron levels were calculated from a calibration curve. A 1,000 ppm 99.999 % pure standard boron solution for atomic absorption spectrometry was purchased from the Wako Co. Ltd. and used as the stock solution. Comparator standards of boron were prepared by adding known amounts of the stock solution onto Whatman 42 filter papers and followed by drying under an IR lamp.
Two standard reference materials (SRM) purchased from the U.S. National Institute of Standards and Technology (NIST), namely SRM 1572 citrus leaves and SRM 1573 tomato leaves, were used in this work for the validation of the PGAA method.
Irradiation and counting
The PGA facility at the JRR-3 reactor of the Japan Atomic Energy Agency (JAEA) in Tokai was used in this work. The details of the PGAA system have already been published by Yonezawa and Wood [40]. Between 0.3 and 0.5 g of each sample was heat sealed in double FEP films of 15 × 15 mm. Then the sample bag was hung in PTFE frame by PTFE strings. Sample and sample holder were placed in PTFE sample box at an angle 45° to the neutron beam. The thermal neutron flux was 6.9 × 107 (5.7 × 108, when focused) cm−2 s−1 [46]. Air was purged from the sample box, and helium gas was allowed to flow through continuously during irradiation. Irradiation time varied between 1,000 and 3,000 s depending on the boron content of the sample.
The gamma-ray spectra were recorded using a Compton suppression system at JRR-3. This system has previously been described by Yonezawa [47]. The photopeaks were analyzed using the SEIKO EG and G gamma-ray spectrum analysis program.
Results and discussion
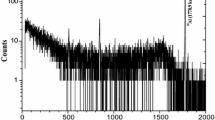
Boron levels were calculated from the calibration curve using the 478 keV photopeak from the l0B(n,α)7Li reaction. This peak is known to be broad due to the characteristic Doppler effect [16]. It is also well established that this peak could be interfered with by 472 keV peak of sodium which is known to be present in nutritional materials. The correction method proposed by Anderson et al. [32] was used in this work.
As noted by Anderson et al. [32], interferences from sodium, chlorine and nitrogen are significant around the 475 keV photopeak area. The interference from the 472 keV photopeak of sodium in fresh vegetables is not as severe as that in vertebrates, marine and cooked food samples. For correcting sodium interference, known amounts of sodium chloride were irradiated, counted, and the correction factors calculated from two different sodium peaks, namely 92 and 472 keV. Irradiation of 0.02445, 0.05648 and 0.11300 g of sodium chloride for 1,000 s gave a value of 1.057 ± 0.014 (mean + SD) on the average as the ratio of net counts in the 472/92 keV peak areas. Then known amounts of boron and sodium chloride were analyzed together. For example, as is shown in Fig. 1, net count for 10 μg of boron at the 478 keV peak was 5,040. The net counts of 10 μg of boron + 0.05600 g of sodium chloride were 3,222 and 8,165 for the 92 and 472 keV, respectively. The net count for boron was then calculated as: 8,165 − (3,222 × 1.057) = 4,759. It is evident that the net count after the correction (4,759) differs from the actual net count (5,040) by 5.6 %. The correction factor for boron could very well depend on the ratio of boron-to-sodium in a given sample. In future work, the correction factor will be checked for different boron-to-sodium ratio. The 92 keV peak of sodium was detected only in some Canadian vegetables.
The PGAA method was validated in this work using two control samples and the results are shown in Table 1. Our average value of 66.4 ± 5.0 mg kg−1 of three analyses for the NIST SRM 1572 citrus leaves agrees well with the literature values of 63.5 ± 0.2 mg kg−1 [32] and 65.8 ± 4.7 mg kg−1 [40]. No certified value for this SRM was provided by NIST. The average value of 34.7 ± 2.7 mg kg−1 of three measurements obtained in this work for the NIST SRM 1573 tomato leaves agrees with the literature value of 38.3 ± 0.7 mg kg−1 [32] and information value of 30 mg kg−1. The detection limit was 0.7 mg kg−1 for 1,000 s irradiation times for both of the SRMs. The limit for the vegetables with low sodium content was around 0.5 mg kg−1.
The reproducibility of the PGAA method was evaluated by analyzing two Canadian food samples, namely whole milk (A01) and baked beans (G01). Three portions of each sample were irradiated for two different times. The average values are presented in Table 2. It is evident that the values agree well within the experimental errors.
The PGAA method was applied to a small selection of Canadian and Japanese food samples. Boron levels in Canadian and Japanese vegetables are presented in Tables 3 and 4, respectively. These values are comparable to those published by Anderson et al. [36] for the U.S. vegetables. A few Canadian and Japanese spice samples were also analyzed for boron by the PGAA method; the results given in Table 5 show that the levels are low in general. A selection of items from various food groups are presented in Table 6. Sodium content of the foods analyzed is also given in Tables 3, 4, 5 and 6 to show the extent of correction needed to obtain boron values. It is evident that low levels of boron in these samples can be reliably measured by the PGAA method.
Conclusions
The PGAA method was found to be suitable for the determination of low levels of boron in presence of sodium in the Canadian and Japanese food samples analyzed. The method is applicable to solid samples without any pretreatment. The precision and accuracy of the method were good. The detection limit was around 0.5 mg kg−1. Since the feasibility project was completed, the disastrous earthquake and Tsunami on 3/11 in North East Japan has forced the reactor facility to shut down. No resumption of the facility has yet been announced.
References
Sah RN, Brown PH (1997) Plant Soil 193:15–33
Culver BD, Smith RG, Brotherton RJ, Strong PL, Gray TJ (1994) Boron. In: Clayton GD, Clayton FE (eds) Patty’s industrial hygiene and toxicology, pp 4411–4424. Wiley, New York
Yakimova VP, Markova OL (1992) J Anal Chem USSR 47:1477–1483
Sah RN, Brown PH (1997) Microchemical J 56:285–304
Zaijun L, Zhengwei C, Jian T (2006) Food Chem 94:310–314
Nakano E, Iwatsuki S, Inamo M, Takagi HD, Ishihara K (2008) Talanta 74:533–538
Economou A, Themelis DG, Bikou H, Tzanavaras PD, Rigas PG (2004) Anal Chim Acta 510:219–224
Resano M, Briceno J, Aramendia M, Belarra MA (2007) Anal Chim Acta 582:214–222
Dolan SP, Capar SG (2002) J Food Comp Anal 15:593–615
Madejczyk M, Baralkiewicz D (2008) Anal Chim Acta 617:11–17
Shen JJ-S, You C-F (2003) Anal Chem 75:1972–1977
Aggarwal SK, Wang B-S, You C-F, Chung C-H (2009) Anal Chem 81:7420–7427
Sarkar A, Aggarwal SK, Sasibhusan K, Alamelu D (2010) Microchim Acta 168:65–69
Vértes A, Nagy S, Klencsár, Z (eds) (2003) Handbook of nuclear chemistry, vol 3, App 2. Kluwer Academic
United Nations (1958) Proceedings of the 2nd United Nations International Conference on Peaceful Uses of the Atomic Energy, Geneva (1958), vol 16, p 11
Molnar GL (ed) (2004) Handbook of prompt gamma activation analysis with neutron beams. Kluwer Academic, Dordrecht
Hedrich E, Grass F (1981) J Radioanal Nucl Chem 61:295–305
Nielsen HK, Schmidt JO, Heydorn K (1987) J Radioanal Nucl Chem 114:237–241
Selecki A, Nowakowskia Z (1969) Radiochem Radioanal Lett 1:247
Sales HB, Dantas CC (1981) Radiochem Radioanal Lett 50:105
Moir DL, Chatt A (1987) J Radioanal Nucl Chem 116:389–400
Moir DL (1989) PhD Thesis. Dalhousie University, Halifax, NS, Canada
Moir DL, Chatt A (1995) Trans Am Nucl Soc 73:11–12
Clarke WB, Koekebakker M, Barr RD, Downing RG, Fleming RF (1987) Appl Rad Isotopes 38:735–743
Iyengar GV, Clarke WB, Downing RG (1990) Fresenius J Anal Chem 338:562–566
Chhillar S, Acharya R, Pujari PK (2012) DAE BRNS symposium on nuclear physics (SNP), Department of Physics and Astrophysics, University of Delhi, India, Board of Research on Nuclear Sciences (BRNS), Department of Atomic Energy (DAE), SNP-2012, vol 57, pp 920–921
Chhillar S, Acharya R, Pai RV, Sodaye S, Mukherjee SK, Pujari PK (2012) J Radioanal Nucl Chem 293:437–441
Chhillar S, Acharya R, Rao TVV, Bamankar YR, Mukherjee SK, Pujari PK, Aggarwal SK (2013) J Radioanal Nucl Chem 298:1597–1603
Kumar A, Chhillar S, Paranjape DB, Raut D, Acharya R, Jeyakumar S, Tomar BS (2014) Application of radiotracers and energetic beams in sciences (ARCEBS-2014). Saha Institute of Nuclear Physics, Kolkata, India, vol 4, pp 113–114
Chhillar S, Acharya R, Sodaye S, Pujari PK (2014) DAE BRNS 5th symposium on nuclear analytical chemistry (NAC-V), Mumbai, India, Book of Abstracts, pp 224–225
Failey MP, Anderson DL, Zoller WH, Gordon GE, Lindstrom RM (1979) Anal Chem 51:2209–2221
Anderson DL, Cunningham WC, Mackey EA (1990) Fresenius J Anal Chem 338:554–558
Anderson DL, Cunningham WC (1992) Trans Am Nucl Soc 65:141
Anderson DL, Cunningham WC, Alvarez GH (1993) J Radioanal Nucl Chem 167:139–144
Hight SC, Anderson DL, Cunningham WC, Capar SG, Lamont WH, Sinex SA (1993) J Food Comp Anal 6:121–139
Anderson DL, Cunningham WC, Lindstrom TR (1994) J Food Comp Anal 7:59–82
Anderson DL, Cunningham WC (1995) J Radioanal Nucl Chem 194:351–357
Anderson DL (2000) J Radioanal Nucl Chem 244:225–229
Anderson DL, Mackey EA (2005) J Radioanal Nucl Chem 263:683–689
Yonezawa C, Wood AKH (1995) Anal Chem 67:4466–4470
Yonezawa C, Matsue H (2000) J Radioanal Nucl Chem 244:373–378
Yonezawa C, Matsue H, McKay K, Povinec P (2001) J Radioanal Nucl Chem 248:719–725
Matsue H, Yonezawa C (2001) J Radioanal Nucl Chem 249:11–14
Magara M, Yonezawa C (1998) Nucl Instr Meth Phys Res A 411:130–136
Sakai Y, Kubo MK, Matsue H, Yonezawa C (2005) J Radioanal Nucl Chem 265:287–290
Yamada S, Shinohara T, Sasaoa H, Okua T, Suzuki J, Matsue H, Shimizu H (2006) Phys B 385–386:1243–1246
Yonezawa C, Magara M, Sawahata H, Hoshi M, Ito Y, Tachikawa E (1995) J Radioanal Nucl Chem 193:171–178
Acknowledgments
The authors would like to thankfully acknowledge the cooperation of the staff of the PGA facility at the JRR-3 reactor of the Japan Atomic Energy Agency (JAEA) in Tokai for irradiations, Ishinomaki Senshu University for research grants to MF, the Natural Sciences and Engineering Research Council (NSERC) of Canada for Research Operating/Discovery grants to AC.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fukushima, M., Matsue, H. & Chatt, A. A feasibility study to measure low levels of boron in selected Canadian and Japanese foods by prompt gamma activation analysis using the JAEA JRR-3 facility. J Radioanal Nucl Chem 302, 1225–1229 (2014). https://doi.org/10.1007/s10967-014-3524-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3524-0