Abstract
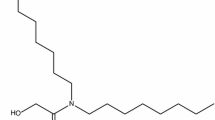
In this paper a quaternary ammonium based room temperature ionic liquid (IL) trioctylmethylammonium hydrogen phthalate has been reported as promising extractant for separation of U(VI) from other metal ions from aqueous media. The IL was synthesized via metathesis route and characterized using various techniques such as hydrogen nuclear magnetic resonance, electron spray ionization mass spectrometry and infra red etc. The newly synthesized IL was evaluated for extraction of U(VI), Th(IV), La(III), Y(III), Nd(III) and Fe(III) from aqueous solutions and follow the order: U(VI) > Th(IV) > Fe(III) > Y(III) >> Nd(III) ~ La(III).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Separation, recovery and purification of metal ions by liquid–liquid extraction from various aqueous media play an important role in separation science and purification technology [1–4]. The main advantages of the process are their processing capability of a large volume of aqueous solutions in a very small time using well established industrial scale equipments such as pulse column and mixer- settler unit [5–10]. However, the conventional liquid–liquid extraction using organic solvents have a large number of limitations in connection with environmental safety issues [11, 12]. The conventional organic extractants in combination with different diluents are toxic in nature and their disposal to the environment is of great concern [13, 14]. Ionic liquids (ILs), a class of low melting organic salt have become alternative extractants for metal ions removal from various aqueous media, since the first report published by scientist [15]. ILs can be considered as organic salt containing bulky organic cations with a wide variety of anions that are liquid at room temperature. Whereas, classical solvents are neutral organic compounds, ILs are made up of large ions which are held together by electrostatic forces. Because of these interactions, the properties of ILs are different from the classical organic solvents. They have a wide liquid range, very low vapor pressure, environmental friendly nature and wide chemical and electrochemical windows. Their excellent properties make them alternative solvent for recovery of valuable metal ions from different waste streams [16–20]. Generally liquid–liquid extraction of valuables metal ions using ILs can be carried out in two ways. First option is based on the use of conventional organic extractants in ILs media and extraction of metal ions from aqueous phase to ILs phase [21–24]. Second route is based on the use of functionalized ILs which can extract metal ions from aqueous media to ILs phase [25–30]. The second types of ILs are often known as task-specific ILs (TSILS). A large number of ILs as well as TSILs containing disulfide-, thioether-, urea-, or hydroxyl benzyl amine group as extracting agent for metal ions have been reported in literature [31–33]. Luo et al. [34] synthesized a imidazolium cation based TSIL containing a monoaza-crown ether fragment for extraction of Cs+ and Sr2+ from aqueous solutions with enhance selectivity. Separation of Ag+ and Pb2+ ions from aqueous medium has been studied using imidazolium based IL [28]. Various parameters such as effect of anions type and effect of their structure on extraction efficiency of Ag+ and Pb2+ has been evaluated in detail [28]. Separation of heavy metal ions such as Hg2+ and Cd2+ from aqueous medium has been investigated using imidazolium cation based TSILs containing different functional groups [30]. Harijani et al. have evaluated extraction and complexation properties of Cu(II), Ni(II) and Co(II) in aqueous medium with imidazolium cation based TSILs containing appended aninodiacetic acid moieties as di-tert-butyl ester as functional group [35, 36]. Liquid–liquid extraction of non ferrous metal ions has been investigated in detail using TSILs [37]. In addition to that, tetraalkyl ammonium cation based TSILs having different functionalized anions as counter ions draw attention as emerging solvents for metal ion recovery in recent past [38–46]. Further, various types of anions such as aromatic and aliphatic carboxylate anions, thiosalicylate anions as well as unsaturated aliphatic carboxylate are incorporated to prepare tetraalkyl ammonium cation based TSILs of desired functionality [31, 32, 37]. Literature survey indicated that there are very little work available tetraalkyl ammonium hydrogen phthalate based IL [47]. Further, no literatures are available on extraction of metal ions such as U(VI), Th(IV) and Ln(III) ions from aqueous medium using tetraalkyl ammonium hydrogen phthalate based TSIL.
In the present paper we report on synthesis and characterization of TSIL, trioctylmethyl ammonium hydrogen phthalate (TOMAHP) and its application on recovery of metal ions from various aqueous media using liquid–liquid extraction method for the first time. The TOMAHP was synthesized by metathesis route using Aliquat®336 and potassium hydrogen phthalate as starting material in water–toluene mixture at room temperature. The TSIL was purified and characterized using hydrogen nuclear magnetic resonance (1H-NMR), electron spray ionization mass spectrometry (ESI-MS) techniques. The TSIL was evaluated for extraction of metal ions U(VI), Th(IV), Y(III), Nd(III), La(III) and Fe(III) from various aqueous streams using liquid–liquid extraction technique and it was observed that TOMAHP is a better extractant for U(VI) than other metal ions. The presence of one free carboxylic acid in TOMAHP is acts as a functional group to bind with metal ions at various aqueous phase acidities. Based on the liquid–liquid extraction studies it is observed that the extraction order of different metal ions varies with the following order: U(VI) > Th(IV) > Fe(III) > Y(III) >> Nd(III) ~ La(III).
Experimental
Chemicals and solutions
Aliquat®336 (S.D.Fine Chemicals; trioctylmethylammonium chloride, MW: 404.17), potassium hydrogen phthalate (Aldrich; >99 % purity), toluene (Analytical grade) were used without further purification. Transition metal salt, Fe2(SO4)3 was prepared by dissolving required amount of salt in distilled water. Nuclear grade pure U3O8 (Obtained from Uranium Extraction Division, Mumbai, India) was dissolved in HNO3 and converted to sulphate and phosphate medium by fuming with H2SO4 and H3PO4. Rare Earths (REEs) oxides (Y2O3, Nd2O3 and La2O3 >99 % purity) were procured from Indian Rare Earths Ltd. India. The REEs solutions were prepared by dissolving oxides in HNO3 medium and diluting to required concentration with distilled water. Th(NO3)4 (A.R. grade, obtained from S. D. Fine Chemicals) was dissolved in distilled water to prepare its stock solution.
Synthesis
IL i.e. TOMAHP was synthesized by metathesis route. Equimolar amount of Aliquat®336 and potassium hydrogen phthalate were dissolved in 1:1, water/toluene mixture and stirred for a period of 5 h at room temperature. A viscous pale yellow liquid was obtained after purification of organic phase. Detailed synthesis of TOMAHP can be found in the Electronic supplementary information (ESI).
Analysis
Analysis of IL synthesized in our laboratory was performed using a 200 MHz Bruker nuclear magnetic resonance (NMR) spectrometer for 1H NMR. The molecular weight and structure of the IL was determined using mass spectrometric technique, ESI-MS. ESI-MS spectra was recorded using micrOTOF II Electrospray Ionization Mass Spectrometer from Bruker. In the ESI-MS spectra, cationic peak at mass number 368.41 is due to trioctylmethylammonium cation and anionic peak at mass number 165.01 is due to phthalate anion. The Infrared spectrum of IL was recorded with JASCO FTIR 4100 Infrared Spectrometer using KBr pallet as window. The nature of cations and anions present including impurities such as Cl− were determined using Dionex make ion chromatography (IC, Model No. DIONEX, ICS-1600). The sample was prepared in 40 % acetonitrile–water mixture at room temperature. The density and viscosity of the IL were measured using an Anton Paar SVM 3000/G2 type stabinger viscometer with an uncertainty of ±0.0005 g/cc for density, ±0.005 mPa.s for the viscosity and ±0.01 K for temperature. The melting temperature measurement was carried out using differential scanning calorimeter (DSC) Mettler Toledo make (Software DSC 823 e-Services), with a scan rate of 10 °C/min, a sensitivity of 0.1 mW and temperature precision of ±0.1 °C. Similarly decomposition temperature was measured with a Mettler Toledo make thermographic analysis (TGA) with a scan rate of 10 °C/min. The pK a value as well as water content of IL was determined by potentiometric and Karl Fischer titration using Metrohm make potentiometer with Karl Fischer as attachment, Model 798 MPT tritrino. The pK a value was found to be 3.2.
Solvent extraction experiments
The solvent extraction experiments were performed by equilibrating 1 mL aqueous and 1 mL pre-equilibrated IL in a 5 mL extraction vial for a period of 20 min. This time was found to be sufficient to reach the equilibrium. The organic phase and aqueous phase were separated by centrifuge, (REMI, research centrifuge) for a period of 10 min. The aqueous phases were analyzed for metal ion content using Horiba, Jobin–Yvon make inductively couple plasma spectrophotometer (ICP-AES), model No. JY238. The ICP-AES has a detection limit of 3 ppb for transition metal and 2 ppb for REEs and uranium with an uncertainty of 2–5 % below 50 ppm. The concentrations of metal ions in organic phase ware calculated by the difference of the metal ions concentrations in the aqueous phase before and after extraction. The distribution ratio (D M) of metal ions was calculated as:
where, [U]org,eq and [U]aq,eq refer metal ion concentrations in organic and aqueous phases, respectively, under equilibrium condition.
Percentage extraction (%E) of metal ions was defined as:
where, [M]aq,in initial metal ion concentration in the aqueous phase, [M]aq.eq metal ion concentration in the aqueous phase at equilibrium.
Results and discussion
Evaluation of physiochemical properties of IL
For the synthesis of IL i.e. TOMAHP, potassium hydrogen phthalate and Aliquat®336 were used in a metathesis reaction. The synthesis of IL was carried out at room temperature (25 °C) by just mixing the reactants in water–toluene mixture and stirred for a period of 5 h. The physical properties of IL were listed in Table 1. Literature survey indicated that the most of the tetraalkylammonium based IL with carboxylic acid as anion have viscosity (η) in the range 100–1,500 mPa.s and it is mostly a function of cationic structures [43, 44]. The present studies indicate that the IL (TOMAHP) has lower viscosity (542.75 mPa.s) and density (0.974 g/cm3) as compared to other tetraalkyl ammonium cation based IL containing various anions as counter ions. The decrease in viscosity is due to presence of potassium hydrogen phthalate anion in TOMAHP. Presence of impurities such as water, chloride anion in IL can alter the physical properties drastically [45]. In the present case the water content of IL before and after equilibration with water are 3,000 and 6,550 ppm but chloride content is <5 ppm. Based on differential scanning calorimetry (DSC) the melting point of IL was evaluated as 7.08 °C (Fig. 1a). Further, Fig. 1b showed the thermal gravimetric analysis (TGA) curve of the IL. The decomposition temperature (T d) was determined from the intersection of the base line and tangent line in the TGA curve [46] and the T d value was found to be 251.35 °C (Table 1).
Extraction of various metal ions using IL
The synthesized IL was evaluated for metal ion extraction such as actinides (U(VI), Th(IV)), lanthanides (La(III), Nd(III)), Y(III)) and transition metal ions Fe(III) from a HNO3 solution at various pH. The concentration of each metal ion was maintained at 2 × 10−3 M. During the extraction of U(VI) from aqueous phase, a distinct color change was observed in the organic (IL) phase (Fig. 2). Figure 3 showed the pH isotherm of extraction of different metal ion from HNO3 acidic aqueous media. With increase in pH of the aqueous phase there is an increase in extraction efficiency of U(VI), Th(IV) and Fe(III) where as very little or no extraction was observed in case of Y(III), Nd(III), and La(III) metal ions. These results indicate that the IL could be a potential green solvent for selective separation of U(VI) from an aqueous solution containing Th(IV), Fe(III), Ln(III) as impurities.
Extraction of U(VI) from various acidic medium
Further, extraction of U(VI) using IL was investigated from different acidic media (HNO3, H2SO4 and H3PO4) in the pH range of 0.1–2 and the results are listed in Fig. 4. With increase in pH of the aqueous phase there is an increase in U(VI) extraction for all the three acidic media indicating the cation exchange nature of extraction mechanism where cation is exchanged with proton present in carboxylic group of the IL according to the following equation:
where HIL represents the TOMAHP.
Similar mechanism was reported on extraction of transition metal ions using trioctyl methyl salicylate as extractant [33]. At lower pH (<1) the extraction of U(VI) from different acidic media using IL varies with the nature of acids (HNO3 > H2SO4 > H3PO4) where as at higher pH (>1) more than 99 % extraction was observed from all acidic solutions. This could be explained in terms of complexing ability of corresponding anions with UO2 2+ in aqueous media. Further experiments were carried out to investigate the effect of U(VI) concentration in aqueous (HNO3) solution on D U using IL. It was observed that with increase in U(VI) concentration in aqueous solution from 0.5 to 23 g/L there is a decrease in D U from 1.86 to 1.18 as well as in %E from 65.03 to 54.13 (Table 2). With increase in U(VI) concentration in aqueous medium there is an increase of U(VI) concentration in IL phase, but the ratio of U(VI) concentration decrease due to increase in U(VI) concentration in denominator.
Separation of U(VI), Th(IV), Fe(III) and lanthanides
The selectivity of IL for U(VI) extraction from HNO3 medium over other actinides, lanthanides and transition metal ions such as Th(IV), La(III), Nd(III), Y(III) and Fe(III) has been investigated from an aqueous solution of pH: 0.1. The concentration of each metal ion was kept constant at 2 × 10−3 M. Table 3 showed the extraction of binary mixture using U(VI) as a common metal ions using IL. It is observed that except U(VI) and Th(IV) no other metal ions get extracted under that conditions. Similar results were obtained for separation of U(VI) from an aqueous solution of pH: 0.1 over a multi-mixture of U(VI), Th(IV), La(III), Nd(III), Y(III) and Fe(III) using IL as extractant (Table 4).
Conclusions
TOMAHP was synthesized from Aliquat®336 and potassium hydrogen phthalate via metathesis route. The IL was characterized using 1H-NMR, ESIMS spectrometry. The IL synthesized has a lower viscosity and water content compared to the other Aliquat®336 based IL reported in literature. These make the new IL attractive for faster mass transfer during extraction of metal ions. The newly synthesized IL was evaluated for liquid–liquid extraction of transition, lanthanides and actinides metal ion from aqueous solutions. The extraction data indicate that the TOMAHP is a green solvent for selective extraction of U(VI) from aqueous medium containing Th(IV), Fe(III) and Ln(III) as impurities.
References
Gupta CK, Singh H (2003) Uranium resource processing: secondary resources. Springer, Germany
Singh H, Gupta CK (2000) Min Process Ext Met Rev 21:307–347
Mowafy EA, Aly HF (2001) Solv Extr Ion Exch 19(4):629–641
Suresh A, Srinivasan TG, Vasudeva Rao PR, Rajagopalan CV, Koganti SB (2005) Sep Sci Technol 39(10):2477–2496
Suresh A, Srinivasan TG, Vasudeva Rao PR (1994) Solv Ext Ion Exch 12(4):727–744
Koladkar V, Dhadke PM (2002) J Radional Nucl Chem 253:297–302
Mathur JN, Choppin GR (1998) Solv Extr Ion Exch 16:459–469
Mathur JN (1983) Solv Extr Ion Exch 1:349–412
Sato T (1965) J Inorg Nucl Chem 27:1853–1860
Mukherjee TK, Singh H (2006) Recovery of uranium and thorium from secondary resources. In: Raj B, Vasudeva Rao PR (eds) Nuclear fuel cycle technologies—closing fuel cycles. Board of Research in Nuclear Sciences, Mumbai, pp 70–85
Deqian L, Yong Z, Shulan M (2004) J Alloys Comp 374:431–433
Shoun RR, Thompson MC, Schulz WW, Navratil JD, Talbot AE (eds) (1984) Chemical properties and reactions, science and technology of tributyl Phosphate, vol 1. CRC Press, Boca Raton, p 137
Barney GS, Cooper TD (1994) The chemistry of tributyl phosphate at elevated temperature in the plutonium finishing plant process vessels Report WHC-EP-0737, Washington
Huddleston JG, Willauer HD, Swatloski RP, Visser AE, Rogers RD (1998) Chem Commun 44:1765–1766
Abbott AP, Frisch G, Hartley J, Ryder KS (2011) Green Chem 13:471–481
Cocalia VA, Holbrey JD, Gutowski KE, Bridges NJ, Rogers RD (2006) Tsinghua Sci Technol 11(2):188–193
Visser AE, Swatloski RP, Griffin ST, Hartman DH, Rogers RD (2001) Sep Sci Technol 36(5–6):785–804
Visser AE, Rogers RD (2002) J Solid State Chem 171(1–2):109–113
Sun X, Ji Y, Guo L, Chen J, Li D (2011) Sep Purif Technol 81(1):25–30
Dai S, Ju YH, Barnes CE (1999) Dalton Trans 417:1201–1202
Dietz ML, Jakab S, Yamato K, Bartsch RA (2008) Green Chem 10:174–176
Dietz ML, Dzielawa JA, Laszak I, Young BA, Jensen MP (2003) Green Chem 5:682–685
Dietz ML, Stepinski DC (2005) Green Chem 7:747–750
Wei G-T, Yang Z, Chen C-J (2003) Anal Chim Acta 488:183–192
Hsu SCN, Su C-J, Yu F-L, Chen W-J, Zhuang D-X, Deng M-J, Sun I-W, Chen P-Y (2009) Electrochim Acta 54:1744–1751
Kidani K, Hirayama N, Imura H (2008) Anal Sci 24:1251–1254
Hirayama N (2008) Bunseki Kagaku 57(12):949–959
Domańska U, Rękawek A (2009) J Solut Chem 38:739–751
Martinis EM, Olsina RA, Altamirano JC, Wuilloud RG (2009) Talanta 78:857–862
Visser AE, Swatloski RP, Reichert WM, Davis JH Jr, Rogers RD, Mayton R, Sheff S, Wierzbicki A (2001) Chem Commun 135:135–136
Kalb RS, Kotschan MJ (2005) Aldrich Chem Files 5(6):1–19
Kogelnig D, Stojanovic A, Galanski M, Groessl M, Jirsa F, Krachler R, Keppler BK (2008) Tetrahedron Lett 49:2782–2785
Egorov VM, Djigailo DI, Momotenko DS, Chernyshov DV, Torocheshnikova II, Smirnova SV, Pletnev IV (2010) Talanta 80:117–1182
Luo H, Dai S, Bonnesen PV, Buchanan AC (2006) J. Alloy Compd 428:195–199
Harjani JR, Friscic T, MacGillivray LR, Singer RD (2008) Dalton Trans 34:4595–4601
Messadi A, Mohamadou A, Boudesocque S, Dupont L, Guillon E (2013) Sep Purf Technol 107:172–178
Guo-cai T, Jian L, Yi-xin H (2010) Trans Nonferrous Met Soc China 20:513–520
Nockemann P, Thijs B, Parac-Vogt TN, Hecke KV, Meervelt LV, Tinant B, Hartenbach I, Schleid T, Ngan VT, Nguyen MT, Binnemans K (2008) Inorg Chem 47:9987–9999
Park SH, Demberelnyamba D, Jang SH, Byun MW (2006) Chem Lett 35:1024–1025
Liu H, Wang H-Z, Tao G-H, Kou Y (2005) Chem Lett 34:1184–1185
Nockemann P, Thijs B, Pittois S, Thoen J, Glorieux C, Hecke KV, Meervelt LV, Kirchner B, Binnemans K (2006) J Phys Chem B 110:20978–20992
Parmentier D, Metz SJ, Kroon MC (2013) Green Chem 15:205–209
Hallett JP, Welton T (2011) Chem Rev 111(5):3508–3576
Seki S, Kobayashi T, Kobayashi Y, Takei K, Miyashiro H, Hayamizu K, Tsuzuki S, Mitsugi T, Umebayashi Y (2010) J Mol Liq 152(1–3):9–13
Seddon KR, Stark A, Torres M (2000) J Pure Appl Chem 72(12):2275–2287
Fredlake CP, Crosthwaite JM, Hert DG, Kaki SNV, Brennecke JF (2004) J Chem Eng Data 49:954–964
Jessen SM, Küppers H (1991) J Mol Struct 263(2):247–265
Acknowledgments
The authors thank Dr. S.K. Aggarwal, Head Fuel Chemistry Division, Bhabha Atomic Research Centre, India for analysis of mass of TSIL (TOMAHP) using Electron Spray Ionization mass spectrometry (ESIMS). The authors also thank Dr. D.K. Singh, Rare Earths Development Section, Bhabha Atomic Research Centre, India for his kind help for determination of water content in TOMAHP using Karl Fischer Titrator.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Biswas, S., Rupawate, V.H., Roy, S.B. et al. Task-specific ionic liquid tetraalkylammonium hydrogen phthalate as an extractant for U(VI) extraction from aqueous media. J Radioanal Nucl Chem 300, 853–858 (2014). https://doi.org/10.1007/s10967-014-3063-8
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-014-3063-8