Abstract
Developing a better understanding of xenon transport through porous systems is critical to predicting how this gas will enter the atmosphere after a below ground nuclear weapons test. Radioxenon monitoring is a vital part of the Comprehensive Nuclear-Test-Ban Treaty (CTBT) International Monitoring System. This work details the development of prompt gamma activation analysis for measuring the diffusion rates of xenon and argon gases through a porous medium. The University of Texas at Austin maintains a prompt gamma activation analysis facility with a peak neutron flux of ~1.5 × 107 cm−2 s−1 and a beam diameter of 1 cm. Due to the relatively large prompt gamma cross sections of many stable xenon isotopes at thermal and sub-thermal neutron energies, prompt gamma activation analysis is a suitable technique for in situ non-destructive analysis of natural xenon. A test chamber has been designed and constructed to utilize prompt gamma activation analysis to measure xenon and argon diffusion through geological materials (e.g., sand, soil, etc.). Initial experiments have been conducted to determine the detection limits for stable gas measurements. The results from these experiments will be utilized to benchmark parts of a xenon transport model that is being used to determine diffusion coefficients for xenon and argon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The International Monitoring System of the Comprehensive Nuclear-Test-Ban Treaty (CTBT) Organization uses seismic, hydroacoustic, infrasound, and radioxenon detection technologies to monitor for clandestine nuclear explosions. However, only the detection of radionuclides can definitely characterize an event as nuclear. More precisely, 131mXe, 133Xe, 133mXe, and 135Xe are of particular interest for the International Monitoring System [1]. In 2006, measurements of airborne 133Xe and 133mXe in the Republic of Korea were used to confirm the fissile nature of weapons test that was conducted by the Democratic People’s Republic of Korea [2].
Before xenon can be detected in the air after a below ground nuclear test, the gas must first migrate to the surface. An important parameter in modeling the transport of xenon in the subsurface is its diffusion coefficient. Importantly, because xenon isotopes have different masses, they should also have different diffusion coefficients. However, measuring this difference requires the ability to distinguish between different isotopes of xenon within a sample. Prompt gamma activation analysis is anticipated to be well suited for this purpose as the energy spectrum of every xenon isotope is unique and this distinction makes it possible to differentiate between them. Importantly, the cross sections for 125Xe, 129Xe, 130Xe, and 131Xe (165, 29, 26, and 85 b respectively) are high enough that prompt gamma activation analysis should detect differences in isotopic diffusion rates. The current study details work that has been done to develop prompt gamma activation analysis for the purpose of measuring xenon diffusion through porous media.
Methodology
The Nuclear Engineering Teaching Laboratory at the University of Texas at Austin houses a prompt gamma activation analysis facility that is coupled with the Texas Cold Neutron Source [3–6], Fig. 1. A neutron guide with a parabolic focusing element is used to increase the flux at the sample position. When the cold neutron source is not operating, the facility has a thermal equivalent neutron flux of approximately 1.5 × 107 cm−2 s−1 at the sample location [5].
The prompt gamma system uses a p-type ORTEC high purity germanium detector with a 65% relative efficiency and a resolution of 1.95 keV at 1,332 keV. The detector is covered with a combination of shielding materials with lead constituting the primary gamma shield and an arrangement of borated foam and cadmium sheets are utilized for neutron shielding. A Tennelec TC 244 spectroscopic amplifier and a 16k Canberra 8713 ADC are used to process the signals, and Genie 2000 is employed for the spectrum acquisition and analysis.
The number of events detected at a particular energy in a prompt gamma activation analysis facility is proportional to the concentration of the sought for nuclide within a sample. As in other radiation detection techniques, the statistical precision of prompt gamma activation analysis can be improved by increasing the number of prompt gammas that are detected. This could be done by increasing the analysis time (s), the thermal neutron flux Φ (cm−2 s−1), or the molar concentration of the nuclide of interest C (mol cm−3). Equation 1 gives an estimate of the prompt gamma count rate R (1/s) for an effective sample volume V eff (cm−3), isotopic cross section (b), and a total detection efficiency ε:
The detector efficiency, as a function of energy, for the prompt gamma activation analysis facility was obtained using a 152Eu calibrated source from the National Institutes of Standards and Technology. Equation 2 shows the least squares curve fit for a third order polynomial of the detector efficiency from the 152Eu analysis. An energy calibration process was conducted using the same radioactive source.
The counting rates for samples of natural xenon and argon at 101.3 kPa and 290 K were estimated using Eq. 1, assuming an effective gas volume of 16 cm3 and Ideal Gas behavior, and a thermal neutron flux of 5.3 × 106 cm−2 s−1 (reactor operating at 950 kW) [4]. Natural xenon was used in these proof of concept studies as a surrogate for radioxenon because of its availability and easy to use within the laboratory. The effective volume was estimated by considering a neutron beam of 2.5 cm by 1 cm cross section [4] impinging on a sample container with a 6.5 cm diameter. Table 1 shows the five largest counting rates predicted for 129Xe, 131Xe and 40Ar. These isotopes were selected for analysis because of their large natural abundance and relatively prompt-gamma cross sections.
Experiments were conducted to measure the count rates for 131Xe, 129Xe and 40Ar in samples of natural xenon and high purity argon. Two steel sample cylinders were evacuated to 0.67 kPa and placed into the prompt gamma activation facility for 60 min in order to obtain a background spectrum. Then, each cylinder was filled to 101 kPa with natural xenon, or high purity argon, and placed in the prompt gamma facility for another 60 min. The spectra obtained were then analyzed using the Analyze tools and Genie2000 Software. Gamma peaks were located using a Peak Locate Unidentified 2nd Diff. toll with a peak significant threshold of 3.5. Peak areas were obtained using the Sum/Non-Linear LSQ_Fit tool with a continuum of 1 channel.
Figure 2 shows part of the xenon spectrum obtained as described above. As can be seen, the largest counts for natural xenon correspond to those at 667 keV. The counting rates obtained from the analysis of the bottle filled with xenon and argon are also shown in Table 1 with reasonable correlation to predicted values. The differences between predicted and measured count rates likely arise from differences between the actual and assumed effective volume, as well as the attenuation of the neutron beam as it passes through the sample cylinder. Despite this discrepancy, the results obtained confirm the feasibility of using prompt gamma activation analysis to analyze for natural xenon and argon.
Design of an experimental set-up to evaluate the transport of Xe through a porous media
In order to evaluate the possibility of conducting xenon transport experiments using prompt gamma activation analysis, an analytical model for noble gas diffusion through a semi-infinite porous media was developed. The formulation for 1-D diffusion of xenon and argon (initially at a molar concentration C o and within a volume V, of cross sectional area A) is shown in Eq. 3. Equations 4–6 show the appropriate initial and boundary conditions employed [7] for diffusion of a finite volume in a semi-infinite porous media. The diffusion coefficients for xenon and argon isotopes in nitrogen were calculated using the correlation given in Bird et al. [8] for binary gas mixtures at low pressure shown in Eq. 7. The effect of the porous media on the transport of these noble gases was taken into account by calculating an effective diffusion coefficient as proposed by Matyka et al. [9]. At 101.3 kPa, 290 K and assuming a media porosity of 0.3, the effective diffusion coefficients for xenon in nitrogen is 0.0186 cm2 s−1.
Equation 8 shows the solution for Eqs. 3–6 [7]:
Using this result, and the estimated counting rates tabulated in Table 1, it was concluded that the analysis of the variation of xenon and argon concentrations with respect of time would be most suitable conducted at x = 0. Figure 3 presents the variation of the concentration of xenon and argon with respect of time at x = 0. This figure shows that after 10 h of diffusion of these noble gases in nitrogen, the original noble gas concentrations would drop by about 80%. Therefore, the prompt gamma counting rates for the after-diffusion samples will also decrease by 80% to about 1 count/s for xenon and 0.2 count/s for argon.
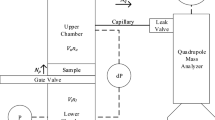
From these results, a 1-D diffusion experiment was designed and constructed. As shown in the schematic in Fig. 4, the experimental set-up consists of a body, along with inlet and analysis chambers. The body is a 1 m long, 10 cm inner diameter steel tube filled with 20/30 mesh Ottawa sand. Both chambers are single end open aluminum cylinders of 10 cm long, 10 cm inner diameter, and 3 mm wall thickness. The purposes of these chambers are to have a uniform diffusion front through the whole steel tube, and to provide an analysis chamber of low prompt gamma-background because of the material it is made of. Finally, two differential pressure transducers of model PX653-05D5V from OMEGA® are connected to the aluminum chambers. These transducers determine the pressure gradient present during the experiment in order to ensure a diffusion dominated transport process.
Figure 5 shows the gamma spectrum of a high purity argon sample before and after a 24 h diffusion and 3 h counting experiment. A comparison of the counting rates of these two spectra at 164.3 and 1,188.0 keV show a variation of molar concentration by 32 and 33% respectively. The data were generated by performing prompt gamma activation analysis on an argon filled bottle that had been attached to the porous medium as shown in Fig. 4. These data confirm that prompt gamma activation analysis can be used to study noble gas diffusion in porous systems.
Conclusions
In order to accurately predict the radionuclide concentrations released to the surface from an underground nuclear weapons test, the transport of xenon through porous media needs to be properly understood. Furthermore, isotopic fractionation during subsurface transport could affect above ground xenon signals. Isotope fractionation could be influenced by the dependence of the diffusion coefficient on the isotopic mass, as suggested by both Kinetic Gas Theory and the experimental correlations shown in Eq. 7. From the prompt gamma results obtained to date, a test chamber for analysis of isotopes fractionation in geological media was designed and constructed. Variation in the prompt gamma activation analysis spectrum of a high purity argon sample after a 24 h diffusion experiment shows the viability of using this nuclear technique to measure noble gas diffusion in porous media. This work confirms experimentally the feasibility of using prompt gamma activation analysis to measure how concentrations of xenon and argon vary as a result of diffusion through a porous media. Furthermore, the proven capability of distinguishing different isotopes of the same nuclide will allow investigation of diffusive fractionation and evaluation of its importance for CTBT monitoring. Future work will be done to measure the binary diffusion coefficient of argon and xenon isotopes through a porous media. Also, a numerical model that will allow the prediction of noble gas concentrations in a non-semi-infinite media will be developed to analyze other ways to study the transport of xenon through a porous media.
References
Wernsperger B, Schlosser C (2004) Noble gas monitoring within the international monitoring system of the comprehensive nuclear test-ban treaty. Radiat Phys Chem 71(3–4):5. doi:10.1016
Ringbom A, Elmgren K, Lindh K, Peterson J, Bowyer TW, Hayes JC, McIntyre JI, Panisko M, Williams R (2009) Measurements of radioxenon in ground level air in South Korea following the claimed nuclear test in North Korea on October 9, 2006. J Radioanal Nucl Chem 282(3):7. doi:10.1007/s10967-009-0271-8
Biegalski SR, Green TC, Alvarez E, Aghara S (2007) Background characterization of The University of Texas PGAA Facility. J Radioanal Nucl Chem 271(2):413–417. doi:10.1007/s10967-007-0224-z
Rèvay Z, Harrison RK, Alvarez E, Biegalski SR, Landsberger S (2007) Construction and characterization of the redesigned PGAA facility at The University of Texas at Austin. Nucl Instrum Methods Phys Res A 577(3):611–618
Biegalski SR, Green TC, Sayre GA, Charlton WC, Dorsey DJ, Landsberger S (2005) Flux weighted efficiency calibration of The University of Texas at Austin PGAA Facility. J Radioanal Nucl Chem 265(2):303–308. doi:10.1007/s10967-005-0825-3
Unlu K, Rios-Martinez C, Wehring BW (1994) The University of Texas cold neutron source. Nucl Instrum Methods Phys Res A 353(1–3):397–401
Takeda M, Nakajima H, Zhang M, Hiratsuka T (2008) Laboratory longitudinal diffusion tests: 1. Dimensionless formulations and validity of simplified solutions. J Contam Hydrol 97(3–4):117–134
Bird RB, Stewart WE, Lightfoot EN (2007) Transport phenomena, 2nd edn. Wiley, New York
Matyka M, Khalili A, Koza Z (2008) Tortuosity-porosity relation in porous media flow. Phys Rev E 78(2). doi:10.1103/PhysRevE.78.026306
Acknowledgments
The authors would like to thank the staff of the Nuclear Engineering Teaching Laboratory for their collaboration in this project and to the Department of Energy for funding this project under the contract No. DE-A-09NA28608.
Funding
This project was founded by the Department of Energy under the contract No. DE-A-09NA28608.
Disclaimer
This report was prepared as an account of work sponsored by an agency of the United States Government. Neither the United States Government, nor any agency thereof, nor any of their employees, makes any warranty, expressed or implied, or assumes any legal liability or responsibility for the accuracy, completeness, or usefulness of any information, apparatus, product, or process disclosed, or represents that its use would infringe privately owned rights. Reference herein to any specific commercial product, process, or service by name, trademark, manufacturer, or otherwise does not necessarily constitute or imply its endorsement, recommendation or favoring buy the United States Government or any agency thereof. The views and opinions of authors expressed herein do not necessarily state or reflect those of the United States Government or any agency thereof.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Rios Perez, C.A., Lowrey, J.D., Biegalski, S. et al. Xenon diffusion studies with prompt gamma activation analysis. J Radioanal Nucl Chem 291, 261–265 (2012). https://doi.org/10.1007/s10967-011-1328-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1328-z