Abstract
Extraction of europium(III) from nitric acid medium by a solution of tri-n-butylphosphate (TBP) and n-octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide (CMPO) in the room temperature ionic liquid, 1-alkyl-3-methylimidazolium bis(trifluoromethanesulfonyl)imide (amimNTf2 where a = butyl or hexyl or octyl), was studied. The distribution ratio of (152+154)Eu(III) in TBP-CMPO/bmimNTf2 was measured as a function of various parameters such as the concentrations of nitric acid, CMPO and NaNO3. Remarkably large distribution ratios were observed for the extraction of europium(III) when bmimNTf2 acted as diluent. The stoichiometry of metal-solvate in organic phase was determined by the slope analysis of extraction data.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Room temperature ionic liquids (RTILs) are the organic salts molten at temperatures lower than 100 °C [1]. RTILs are receiving increased attention for possible applications in the area of nuclear fuel cycle. The properties such as insignificant vapour pressure, amazing ability to dissolve organic and inorganic compounds, tuning to task-specific form etc. make the RTILs popular for reprocessing applications [2, 3]. RTILs were explored as diluents for nuclear fuel cycle applications since the last decade [4–8]. The results obtained from those studies indicated that the extractants in conjunction with RTIL diluents have provided an unprecedented extraction of target metals from aqueous solution under the condition that gave negligible or meager extraction with customary diluents. This was attributed to the extraordinary solvating ability of RTILs in the extracted phase. Dietz and co-workers [5, 6] studied the extraction behavior of several metal ions of nuclear interest by appropriate neutral extractants present in RTIL medium. Ion exchange between target metal ion and RTIL was reported as one of the prevalent mode of metal transfer in RTIL phase.
After the separation of uranium and plutonium in the PUREX process, the concentrated raffinate is known as high-level liquid waste, which contains significant quantities of trivalent actinides (An(III) and lanthanides (Ln(III)). These are generally partitioned by a solution of 1.2 M tri-n-butyl phosphate-0.2 M n-octyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide (CMPO) in n-dodecane [9–11]. In this context, Nakasima et al. [12] reported the distribution ratio of lanthanides by CMPO/RTIL at various nitric acid concentrations. However the detailed extraction behavior of europium(III) in the solution of CMPO–TBP in RTIL was not reported so far. Therefore, the objective of the present paper is to report the results of the studies on the extraction of europium(III) by CMPO–TBP present in ionic liquid medium namely 1-alkyl-3-methylimidazolium bis(trifluoromethansulfonyl)imide (alkyl = butyl, hexyl, octyl) and to compare the results with that of the conventional TRUEX solution. The distribution ratio of (152+154)Eu(III) in CMPO–TBP-/bmimNTf2 was measured as a function of various parameters such as concentrations of nitric acid, CMPO, NaNO3 and the nature of diluent .
Experimental
Materials
All the chemicals and reagents used in this study were of analytical grade. 1-methylimidazole and 1-chlorobutane were procured from Lancaster, UK. 1-methylimidazole was distilled before use and other chemicals were used without any purification. Tri-n-butylphosphate was obtained from E. Merck, Mumbai. Bis(trifluoromethanesulphonyl)imide lithium salt (Li (NSO2CF3)2; NTf2 = (NSO2CF3)2) was procured from Fluka.
Preparation of amimNTf2
The procedure adopted for the synthesis of amimNTf2 (i.e., bmimNTf2, hmimNTf2, omimNTf2) has been described elsewhere [1]. Yield of ionic liquids were ~85%. Viscosity of bmimNTf2, hmimNTf2 and omimNTf2 were 36 cP, 50cP and 62 cP respectively at 298 K. IR spectra indicated the following bands, 3175, 3122 cm-1 (C–H stretch) imidazole ring, 2964, 2932, 2868 cm−1 (C–H stretch) aliphatic, 1564, 1465, 1168 cm−1 imidazole ring symmetric stretch, 1425, 1378 (MeC–H asymmetric stretch), 1138 cm−1 (SO2 symmetric stretch).
Effect of [HNO3]
All equilibration experiments were conducted in duplicate at 303 K with 1:1 aqueous to organic phase ratio. The experiments involved equilibration of aqueous and organic phases (1 mL each) present in a 10 mL capacity test tube immersed in a constant temperature water bath and rotated in up-side-down manner for about 3 h. However, it was found from the rate of extraction studies that equilibrium was established in 30 min. Tri-n-butyl phosphate solution in amimNTf2 and TBP-CMPO in amimNTf2 (or n-DD) were prepared. The concentration of CMPO was varied depending upon the type of experiment. The organic phase was pre-equilibrated with desired concentration of nitric acid. Extraction of europium(III) as a function of nitric acid concentration was studied by equilibrating 1 mL of organic phase with 1 mL of nitric acid solution containing (152+154)Eu(III) tracer (20 mg/L). The concentration of nitric acid in the test solution was varied from 1 to 5 M. After three hour of equilibration, the radioactivity of (152+154)Eu(III) distributed between organic and aqueous phases was measured using a well-type NaI(Tl) scintillation detector. The distribution ratio (DEu) of europium was determined by using Eq. 1. Similar experiment was also performed with n-DD diluent.
For comparing the extraction trend of (152+154)Eu(III) with 241Am(III) (20 mg/L), the distribution ratio of 241Am(III) was also measured both in ionic liquid and n-DD diluent in a similar manner. Initially, for comparing the extraction of Eu(III) and Am(III) in ionic liquid with those observed in TRUEX solvent (0.2 M CMPO–1.2 M TBP/n-DD), a solution of 0.2 M CMPO–1.2 M TBP in RTIL was used as solvent medium. Later it was found [4] that it was desirable to use 0.05 M CMPO only when ionic liquid acted as diluent as the distribution ratio obtained in this case were comparable with n-DD solution (i.e. TRUEX solvent).
Effect of [CMPO]
Extraction of europium as a function of CMPO concentration in the ionic liquid phase was studied by equilibrating the organic phase and desired concentration of nitric acid solution (1–5 M) spiked with (152+154)Eu(III) tracer. The CMPO concentration in organic phase (CMPO–1.2 M TBP/amimNTf2) was varied from 0.02 to 0.2 M. The radioactivity of europium after equilibration was measured and distribution ratio was determined using Eq. 1.
Effect of [Eu(III)] in aqueous phase
The effect of europium(III) concentration in aqueous phase was studied by equilibrating the organic phase with a solution of Eu(III) nitrate in nitric acid (1–5 M) spiked with (152+154)Eu(III) tracer. The concentration of Eu(III) in aqueous phase was varied from 20 to 100 mg/mL. The loading of europium in organic phase was determined by measuring the radioactivity of organic and aqueous phases.
Effect of [NO3 −]
The concentration of nitrate in the aqueous phase was varied by diluting the desired quantity of NaNO3 solution (concentrated stock) in HNO3. This aqueous phase was equilibrated with organic phase (0.05 M CMPO–1.2 M TBP/bmimNTf2) spiked with (152+154)Eu(III) and the distribution ratio was determined as described above.
Results and discussion
Effect of nitric acid
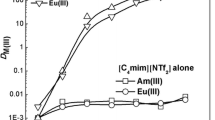
The distribution ratio of europium(III) in 0.2 M CMPO–1.2 M TBP/bmimNTf2 at various concentrations of nitric acid at 303 K is tabulated in Table 1. The data are compared with the distribution ratio of europium(III) in n-dodecane (n-DD) system as well as with Am(III) under similar conditions. Extraction of (152+154)Eu(III) in 1.2 M TBP/bmimNTf2 was determined to be insignificant and it was of the order of ~10−2. However, remarkable enhancement in the distribution ratio is observed in the presence CMPO as shown in Table 1. The D values of (152+154)Eu(III) in 0.2 M CMPO–1.2 M TBP/bmimNTf2 decrease from 1040 to 160 with increase in the concentration of nitric acid from 1.0 to 5.0 M. In contrast, a maximum D value of ~15 is obtained for the extraction of europium(III) and americium(III) in n-DD system. This could be attributed to the extraordinary solvating capability of ionic liquid in organic phase, which seems to facilitate the mass transfer of metal ions. Table 1 also shows the comparison of the separation factor (SF = DAm/DEu), achieved with the use of ionic liquid and n-DD diluent. It is observed that the SF are similar in both cases even though the distribution ratio achieved in case of ionic liquid diluent is much higher. Moreover, the D value decreases with increase in the concentration of nitric acid and the separation factor also decreases with increase in nitric acid concentration from 1 to 5 M.
Effect of [CMPO]
Several authors [9–11] reported the metal-solvate stoichiometry of various metal ions by slope analysis of the extraction data. The stoichiometry of trivalent actinides and lanthanides in CMPO/n-DD system was reported [10, 11] as 1:3. In RTIL diluent, Nakasima et al. have reported the stoichiometry of 1:3 for Ln–CMPO complex [12]. We also studied the metal-solvate stoichiometry of Am(III) in CMPO–TBP/bmimNTf2 and reported the variation of slope from 3.0 at 1 M nitric acid to 2.1 at 5 M nitric acid [4].
CMPO being a neutral ligand, extracts europium(III) in the form of neutral europium(III) nitrate as shown in Eq. 2. Figure 1 shows the plot of log DEu against log [CMPO]. It is seen that DEu value increases with increase in the concentration of CMPO. A slope value of 3.0 is obtained for the extraction of europium(III) from 1.0 M nitric acid medium. Thus, the metal-solvate stoichiometry for the extraction of europium(III) by CMPO–TBP/bmimNTf2 resembles that of TRUEX system at 1.0 M nitric acid medium, wherein three molecules of CMPO are involved in co-ordination with europium in organic phase. A similar 1:3 complex (Ln3+:CMPO) was reported for the extraction of some trivalent lanthanides from neutral solution by CMPO/RTIL [12]. In 3.0 M nitric acid medium, the slope decreases to 2.8 (~3) and at 5.0 M nitric acid medium the value decreases to 2. A similar observations was noticed in the extraction of Am(III) in CMPO–TBP/bmimNTf2 [4], indicating more studies are needed to understand the extraction behavior of trivalents (Ln(III) and An(III)) in ionic liquid diluent.
Effect of Eu(III) loading
The effect of europium loading in the aqueous phase on the extraction is shown in the Table 2. The europium concentration in aqueous phase was varied from 20 to 100 mg/mL. Interestingly it is observed that there is no third phase formation even at the maximum europium(III) loading i.e., 100 mg/mL. In conventional n-DD system, the limiting organic concentration (LOC) of europium in organic phase was 9 mg/mL and the equilibrium aqueous concentration was 6 mg/mL. However, the present study indicates that by using ionic liquid diluent, bmimNTf2, the undesirable third phase formation can be easily avoided. More detailed studied are published elsewhere [8].
Effect of diluents
The extraction of (152+154)Eu(III) in CMPO–TBP in amimNTf2 (a = alkyl (butyl, hexyl and octyl)) as a function of nitric acid concentration was studied and the results are shown in Fig. 2. It is observed that the distribution ratio of (152+154)Eu(III) decreases with increase in the chain length of alkyl group attached to the cation part of the ionic liquid. This observation is not new to ionic liquid systems. Chun et al. [7] and Dietz et al. [5, 6] have reported the decrease in distribution ratio with increase in the hydrophobicity (due to increase in alkyl chain length) of ionic liquids. This was attributed to the decrease in the ability of ionic liquids to undergo ion exchange with increase of hydrophobicity. However the correct reason for the decrease in distribution ratio observed in the present study is not clear and more studies are needed to understand this behavior.
Effect of [NO3 −]
The effect of nitrate ion concentration on the extraction of Eu(III) in 0.05 M CMPO–1.2 M TBP/bmimNTf2 is displayed in the Table 3. It is noticed that the variation in the distribution ratio is insignificant irrespective of the concentration of NO3 − ion in the aqueous phase. Thus it can be concluded that the salting out effect by NaNO3 is insignificant.
Conclusions
Remarkable extraction of europium(III) was achieved with the use of ionic liquid diluent. The extraction stoichiometry of Eu(III)-CMPO solvate was 1:3 at 1 M nitric acid and decreased to 1:2 at 5 M nitric acid. Increasing the chain length of alkyl group attached to imidazolium ring decreased the distribution ratio of Eu(III). No third phase formation was observed up to the loading of 25 mg/mL in organic phase.
References
Welton T (1999) Room temperature ionic liquids. Solvents for synthesis and catalysis. Chem Rev 99(8):2071–2083
Dai S, Ju YH, Barnes CE (1999) Solvent extraction of strontium nitrate by crown ether using room—temperature ionic liquids. J Chem Soc, Dalton Trans 1201–1202
Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2009) A review on the electrochemical applications of room temperature ionic liquids in nuclear fuel cycle. J Radiochem Nucl Sci 10(1):R1–R6
Rout A, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2010) Extraction of americium(III) from nitric acid medium by CMPO-TBP extractant in ionic liquid diluent. Radiochim Acta 97(12):719–725
Dietz ML, Stepinski DC (2008) Anion concentration-dependent partitioning mechanism in the extraction of uranium into room-temperature ionic liquids. Talanta 75(8):598–603
Dietz ML, Dzielawa JA (2001) Ion-exchange as a mode of cation transfer into room-temperature ionic liquids containing crown ethers: implications for the ‘greenness’ of ionic liquids as diluents in liquid–liquid extraction. Chem Commun 2124–2125
Chun S, Dzyuba SV, Bartsch RA (2001) Influence of structural variation in room-temperature ionic liquids on the selectivity and efficiency of competitive alkali metal salt extraction by crown ether. Anal Chem 73(15):3737–3741
Rout A, Venkatesan KA, Srinivasan TG, Vasudeva Rao PR (2010) Extraction and third phase formation behavior of Eu(III) in CMPO-TBP extractants present in room temperature ionic liquid. Sep Purif Technol 76(3):238–243
Horwitz EP, Kalina DG, Kaplan L, Mason GW, Diamond H (1982) Selected alkyl(phenyl)-N,N-dialkylcarbamoylmethylphosphine oxides as extractants for Am(III) from nitric acid media. Sep Sci Technol 17(10):1261–1279
Mathur JN, Murali MS, Natrajan PR, Badheka LP, Banerji A (1992) Extraction of actinides and fission products by cotyl(phenyl)-N,N-diisobutylcarbamoylmethylphosphine oxide from nitric acid media. Talanta 39(5):493–496
Belair S, Labet A, Mariet C, Dannus P (2005) Modeling of the extraction of nitric acid and neodymium nitrate from aqueous solutions over a wide range of activities by CMPO. Solvent Extr Ion Exch 23(4):481–499
Nakashima K, Kubota F, Maruyama T, Goto M (2003) Ionic liquids as a novel solvents for lanthanide separation. Anal Sci 19(8):1097–1098
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rout, A., Venkatesan, K.A., Srinivasan, T.G. et al. Room temperature ionic liquid diluent for the extraction of Eu(III) using TRUEX extractants. J Radioanal Nucl Chem 290, 215–219 (2011). https://doi.org/10.1007/s10967-011-1234-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1234-4