Abstract
The objective of this study is to evaluate the use of titanium dioxide nanoparticles which were prepared by novel sonochemical method as an ion exchange material for the removal of Sr from aqueous solution. The pH effect on the Sr2+ sorption was investigated. The data obtained have been correlated with Freundlich, Temkin and Dubinin–Radushkevich (D–R) isotherm models. Thermodynamic parameters fort he sorption system have been determined at four temperatures. Simple kinetic models have been applied to the rate and isotherm sorption data and the relevant kinetic parameters were determined from the graphical presentation of these models at 298°K. Results explained that the pseudo second-order sorption mechanism is predominant and the overall rate constant of sorption process appears to be controlled by chemical sorption process. The value of sorption energy E = 13 kJ/mol at 298°K and the value of Gibbs free energy ∆G° = 3,222 kJ/mol at 298°K prove that the sorption of strontium on titanium dioxide nanoparticles is an endothermic and non-spontaneous process.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Use of fission reactors for production of energy generates considerable amounts of radioactive wastes in almost all phases of the nuclear fuel cycle [1]. The advent of nuclear industries, various approaches and technologies such as chemical precipitation, ion exchange, and evaporation have been developed and adopted for the disposal and immobilization of radioactive aqueous wastes generated at different stages of the nuclear fuel cycle [2, 3].
The treatment process based on adsorption/ion exchange phenomenon plays an important role in preconcentration/separation of toxic radio-nuclides from aqueous waste. A variety of natural or synthetic (inorganic/organic) sorbents/ion exchangers and their application in the treatment of diverse types of radioactive aqueous waste [4–8].
Due to the high adsorption capacity, irradiation resistance, thermal and chemical stability, inorganic exchangers have found wide use in the treatment of aqueous nuclear wastes [9, 10]. Zirconium phosphate (Zr–P), ammonium molybdophosphate (AMP) and crystalline silicotitanate, hydrous titanium dioxide are proposed as the promising inorganic sorbents for the efficient separation of 137Cs and polyantimonic acid for 90Sr from acid solution [5].
Titanium dioxide (TiO2) is used as model mineral because of its high chemical stability, negligible solubility over a wide pH range and its ideal point of zero charge (PZC = 7), which makes it possible to study adsorption on positively and negatively charged surfaces of TiO2 over a broad pH range. Because of these properties several authors have studied as photocatalyst to deal with environmental pollution, water purification, wastewater treatment, hazardous waste control, and air purification [11, 12].
There are various methods to produce titania powders. For example, sol–gel method [12] hydrolysis of titanium compounds are some of [4] the important examples. The particle sizes and crystal structures of prepared TiO2 vary remarkably with different preparative methods.
In this study, TiO2 nanoparticles were prepared a novel sonochemical method at low temperature for a short time and these nanoparticles are used as an ion exchange material to the removal of Sr from aqueous solution in batch operation. The data obtained have been correlated with Freundlich, Temkin and Dubinin–Radushkevich (D–R) isotherm models. The kinetics of the sorption process has been evaluated in the light of current known theoretical models and the relevant parameters were determined.
Experimental
Reagents
The following commercial reagents were used without further purification. Titanium(IV)-iso-propoxide, [Ti(OPri)4], (97%, Alpha) was used as starting precursor for synthesizing crystalline TiO2 particles. Hydrochloric acid (HCl) (37%, Merck) was used as a catalyst for alkoxide hydrolysis. 2-Propanol (99%, Merck) was used as a solvent. Deionized water was used for the hydrolysis of Ti(OPri)4 and Sr(NO3)2·6H2O was purchased from Merck and was used to prepare Sr(II) stock solutions.
TiO2 nanoparticles preparation
In the present work, TiO2 nanometer particles were prepared via sonochemical method [9]. Titanium (IV) isopropoxide was dissolved in propanol. This solution called precursor solution. After stirring vigorously for 5 min, a mixture of HCl and propanol was added drop wise to the above solution with a burette under stirring. The mixture was stirred for 30 min. After stirring, a mixture of water and propanol which called hydrolysis solution was added to alkoxide solution. After adding the hydrolysis solution, the mixture was stirred for about 4 h at 70 °C in ultrasonic bath. And a gel product was obtained. The gel product was separated by centrifuging and dried at 25 °C for a night. The dried product was then heat treated at different temperature for 2 h.
Identification and characterization
TiO2 nanoparticles was characterized by X-ray diffraction analysis using a Philips Panalytical X’Pert-Pro diffractometer (CuKα radiation λ = 1.5418 Å at 45 kV/40 mA). The specific surface area was measured by the BET method using a High Speed Surface Area Analyzer (Micrometrics 2200 model). The chemical analyses were made using a Perkin-Elmer Optima 2000DV model Inductively Coupled Plasma Optical Emission Spectrometer (ICP-OES). All pH measurements were made by 654 Model Metrohm pH meter.
Adsorption studies
The adsorption experiments of strontium ion were conducted by batch process. Stock solution of the Sr2+ was prepared by dissolving adequate amount of Sr(NO3)2·6H2O in distilled water. TiO2 nanoparticles calcined at 450 °C were used as adsorbent in all experiments. The adsorption studies with strontium synthetic solutions were performed in the following sections.
The effect of pH
To investigate the effect of pH on the strontium adsorption, each 50 mL of 100 ppm strontium nitrate solutions were prepared at different pH values (pH 2–11). Different pH values of these solutions were adjusted with 1 M NaOH and 1 M HNO3 and following the addition of 0.200 g of titanium dioxide, the samples were shaken at 298°K for 2 h. The supernate solutions were filtered and the concentrations of strontium were determined by ICP-OES. The adsorption percentage of strontium on the mixed oxide gel spheres was calculated. The adsorption percentage of strontium on the titanium oxide was calculated. The amount of metal ion retained in the solid phase, q e (μmol/g), was calculated from the expression:
where C 0 and C e are the initial and equilibrium concentrations (M) of metal ion in solution. V is the volume (L) and W is the weight (g) of the solid.
Isotherm studies
Fifty milliliters of strontium nitrate solutions at different concentrations (10–200 ppm) were prepared and the pH values of these solutions were adjusted to 10.6 with 1 M NaOH. 0.200 g of the TiO2 nanoparticles were added to each sample. The samples were treated in a thermostatically controlled shaker at 298 ± 1°K for 2 h. The supernates were filtered and the concentrations of strontium were determined by ICP-OES. The quantity of adsorbed strontium on the TiO2 was calculated as the difference between initial and final concentration at equilibrium. The results are then analyzed in terms of Temkin and Dubinin–Radushkevish (D–R) isotherms.
The effect of temperature
The experiments were carried out at 288, 298, 308, and 318°K. The other parameters were kept constant. In all experiments, 50 mL of 100 ppm strontium nitrate solution was shaken with 0.200 g TiO2 at pH 10.6 for 2 h. The strontium concentration was determined by ICP-OES.
Kinetic studies
The experiments were carried out at 15, 30 and 60 min. The other parameters were kept constant. In all experiments, 50 mL of 100 ppm strontium nitrate solution was shaken with 0.200 g TiO2 at pH 10.6 at 298 K. The strontium concentration was determined by ICP-OES.
Results and discussion
The effect of pH
Figure 1 shows the effect of pH on the adsorption efficiency of strontium on TiO2 nanoparticles. The plot shows a marked influence with a gradual rise in the strontium uptake with the increase in pH from 2 to 6, then it becomes nearly constant between the pH range of 8–12, followed by a sharp increase with a further rise in the pH. The increase in the adsorption of Sr(II) ions on titanium dioxide with increasing pH of the aqueous solution is explicable on the basis of enhanced dissociation of surface hydroxyl groups of the hydrous oxide. At a lower pH, the oxide surfaces will have positive character and less affinity for Sr2+, on the other hand at higher pH, the oxide will behave as negatively charged surface, as a result of which the uptake is a maximum in basic solutions. This is confirmed by the present results.
Isotherms studies
The equilibrium adsorption isotherm is fundamental in describing the interactive behavior between adsorbates and adsorbent, and is important in the design of adsorption systems [10]. In this study, we tried to use the isotherm equations given by Freundlich, Langmuir, Temkin and Dubinin–Radushkevich (D–R) to fit the revealed experimental data for Sr adsorption. The isotherms were obtained from the logarithmic form of the isotherms equations.
Figure 2 shows the relationship between the quantity of strontium adsorbed per unit mass of the TiO2 nanoparticles and the equilibrium concentrations at pH 10.6.
The analysis of the relationship between titanium dioxide adsorption capacity and strontium concentration was performed using the Freundlich equation [13]:
and linearised form
where K F is the constant indicative of the relative ion exchange capacity of the adsorbent (μmol/g) and 1/n is the constant indicative of the intensity of the ion exchange, q e the amount of strontium ions adsorbed per unit weight of TiO2 nanoparticles (mol/g) and C e is the equilibrium concentration of strontium ions in solution (mol/L). From the slope and intercept of the linear graph of log q e versus log C e, the values of Freundlich constants and the value of linear correlation coefficient (R 2) are given in Table 1.
The Temkin isotherm equation assumes that the heat of adsorption of all the molecules in layer decreases linearly with coverage due to adsorbent–adsorbate interactions and that the adsorption is characterized by a uniform distribution of the bonding energies, up to some maximum binding energy [11].
The Temkin isotherm is given as [12]:
where a T (L/g) is the equilibrium binding constant, corresponding to the maximum binding energy, b T is a constant related to the heat of adsorption, T is the temperature (K), and R is the ideal gas constant (8.315 J/mol/K).
From plotting q e versus log C e numerical values of the Temkin constants a T and b T and the correlation coefficient R 2 can be determined as shown in Table 1.
In order to study the nature of the sorption processes, the D–R isotherm was also verified in the form [13]:
where q max is the maximum concentration on the solid phase, i.e., ion exchange capacity (mmol/g), q e is the concentration in solid at equilibrium (mol/g), K is the constant of the adsorption energy (mol2/kJ2), and ε is the Polanyi potential (kJ/mol) and it is equal to
where R is the gas constant (kJ/mol/K), T is the absolute temperature in degrees Kelvin, and C e is the concentration in solution at equilibrium (mol/L).
The D–R isotherm can be linearized as:
The D–R parameters, evaluated for sorption of studied ion at different temperatures, are presented in Table 1.
The mean sorption energy E (kJ/mol), defined as the free energy change when 1 mol of ion is transferred to the surface of the solid from infinity in the solution, is calculated according to the following equation:
The value of E is used to estimate the reaction mechanism occurring. If E is in the range of 8–16 kJ/mol sorption is governed by ion exchange. In the case of E < 8 kJ/mol, physical forces may affect the sorption mechanism. On the other hand, if E > 16 kJ/mol sorption may be dominated by particle diffusion [13]. The calculated value for Sr sorption is 13 kJ/mol, so sorption is governed by ion exchange.
Thermodynamic studies
The adsorption efficiency of strontium at different temperatures is demonstrated in Fig. 3. It is clearly that the adsorption increases with increasing temperature and equilibrium is attained after 318°K.
The distribution coefficients (K D) were calculated from the following equation [2]
where C 0 and C e are the initial and equilibrium concentrations of Sr ion in solution (mg/L), V the solution volume (mL) and m is the mass of sorbent (g). The values of ∆H° and ∆S° are calculated from the slope and intercept of the linear variation of ln K D with reciprocal temperature, 1/T, using the relation:
where K D is the distribution coefficient (mL/g), ∆S° standard entropy, ∆H° standard enthalpy, T the temperature and R is gas constant (kJ/mol/K).
The free energy of specific adsorption ∆G° is calculated using the equation:
The values of and were calculated from the slope and intercept of the linear variation of ln K D with reciprocal temperature, 1/T as shown in Fig. 4. The values of ∆S°, ∆H° and ∆G° are given in Table 2.
The results of ∆H° and ∆G° at 298°K indicated that adsorption of strontium onto TiO2 nanoparticles is endothermic and non-spontaneous process.
Kinetic studies
Chemical reaction-based kinetic models
It is well recognized that the characteristic of the sorbent surface is a critical factor that affect the sorption rate parameters and that diffusion resistance plays an important role in the overall transport of the ions. To describe the changes in the sorption of metal ions with time, three simple kinetic models were tested. The rate constant of strontium ion removal from the solution by titanium dioxide nanoparticles was determined using pseudo first-order, pseudo second-order rate models and Elovich model.
Pseudo first-order kinetic model
The Lagergren pseudo first-order expression is written as [14].
where q e and q t (mg/g) are the amount of strontium ion sorbed onto titanium dioxide nanoparticles at equilibrium and at time t, respectively and k 1 is the pseudo first order rate constant (min−1). The slopes and intercept of the plots of log(q e − q t ) versus t, as showing in Fig. 5 were used to determine the first order rate constant (k 1) and the theoretical equilibrium sorption capacities (q e), respectively. The calculated values of k 1 and q e with the values of the linear correlation coefficients (R 2) of each plot are presented in Table 3.
The pseudo second-order kinetic model
The pseudo second-order rate model is expressed as [15, 16]:
where k 2 is the rate constant of pseudo second-order equation (g/mg min). The kinetic plots of t/qt versus t for Sr2+ ion sorption at 298.15 K temperature is presented in Fig. 6.
The Elovich equation
The Elovich equation is written as:
where α is the initial adsorption rate (mg/g/min), β is the desorption constant (g/mg) during any one experiment. Figure 7 shows that the Elovich equation can be used to interpret the sorption of strontium ion onto TiO2 nanoparticles. The constants of Elovich equation and linear regression coefficient (R 2) presented in Table 3.
The relation is linear, and the correlation coefficient (R 2), suggests a strong correlation between the parameters and also explains that the sorption process of strontium ion follows pseudo second-order kinetics. The correlation coefficient R 2 has an extremely high value (R 2 = 1), and its calculated equilibrium sorption capacity (q e) is close the experimental data. These results explain that the pseudo second-order sorption mechanism is predominant and that the overall rate constant of each sorption process appears to be controlled by the chemical sorption process [16].
Identification and characterization
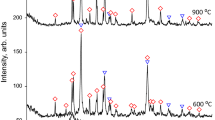
Figure 8 shows XRD pattern of the heat-treated TiO2 at different temperatures. It was indicated that TiO2 powders were mainly in the amorphous phase until 250 °C. However, when the raising heating temperature from 250 to 450 °C, TiO2 nanoparticles were transformed into the anatase phase.
Average crystallite sizes of TiO2 samples were measured from X-ray line broadening analysis using the Debye–Scherrer equation:
where β1/2 is the width of the XRD peak at half peak-height in radian (2θ = 25.3), α the wavelength of the X-ray in nanometer (α = 1.54056 Å), θ the angle between the incident and diffracted beams in degree and D p is the average crystallite size of the powder sample in nanometer. The average crystallite sizes of TiO2 nanoparticles were calculated approximately 17 nm.
The BET specific surface area for TiO2 nanoparticles at different temperature as shown in Fig. 9. It is cleary that the BET specific surface area for TiO2 nanoparticles decreases with increasing temperature.
Conclusion
Titanium dioxide nanoparticles prepared by novel sonochemical method at 70 °C for 4 h. Then the particles were tested as inorganic ion exchange material for the removal of strontium ions from aqueous nitrate solutions. Equilibrium isotherms have been determined and tested for different isotherm expressions and the sorption data were modeled using Freundlich, Temkin and Dubinin–Radushkviech (D–R) approaches. The D–R model expression, the maximum sorption capacity and the mean free energy of the studied ions have been determined. Thermodynamic parameters were calculated for sorption process. The values of sorption energy and gibbs free energy are indicated that the sorption of strontium ion is an endothermic process and non-spontaneous reaction. The kinetics of strontium ion was experimentally studied and the obtained rate data were analyzed using simple kinetic models. Results explained that the pseudo second-order sorption mechanism is predominant and the overall rate constant of sorption process appears to be controlled by chemical sorption process. These reported results showed that titanium dioxide nanoparticles which prepared via sonochemical method is an efficient ion exchange material for the removal of strontium ion from aqueous and waste water solution.
References
Koivula R (2004) Inorganic ion exchangers for decontamination of radioactive wastes generated by the nuclear power plants. Report Series in Radiochemistry
Mishra SP, Tiwary D (1999) Appl Radiat Isot 51:359
El-Rahman KM, Abd El-Sourougy MR, Abdel-Monem NM, Ismail IM (2006) J Nucl Radiochem Sci 7:21
Shabana EI, El-Dessouky MI (2001) J Radioanal Nucl Chem 253:281
Kumar SS, Sivaiah MV, Venkatesan KA, Krishna RM, Murty GS, Sasidhar P (2003) J Radioanal Nucl Chem 258:321
İnan S, Tel H, Altaş Y (2006) J Radioanal Nucl Chem 267:615
Bilgin B, Altun G, Keçeli G (2001) J Radioanal Nucl Chem 250:323
Galamboš M, Kufčáková J, Rosskopfova O, Rajec P (2010) J Radioanal Nucl Chem 283:803
Metwally E, El-Zakla T, Ayoub RR (2008) J Nucl Radiochem Sci 9:1
Debnath SU, Ghosh C (2009) Chem Eng J 152:480
Konstantinou M, Pashalidis I (2008) Colloids Surf A: Physicochem Eng Aspects 324:217
Guo W, Lin Z, Wang X, Song G (2003) Microelectron Eng 66:95
Rodrigues LA, Maschio LJ, Silva RE, Silva MLCP (2010) J Hazard Mater 173:630
Oladoja NA, Aboluwoye CO, Oladimeji YB (2008) Turk J Eng Environ Sci 32:303
Münir K, Yusuf M, Noreen Z, Hameed A, Hafeez FY, Faryalı R (2010) Pak J Bot 42:593
Gürboğa G, Tel H (2005) J Hazard Mater B120:135
Acknowledgment
The authors appreciate the generous financial support of this work by the Scientific and Technological Research Council of Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kasap, S., Tel, H. & Piskin, S. Preparation of TiO2 nanoparticles by sonochemical method, isotherm, thermodynamic and kinetic studies on the sorption of strontium. J Radioanal Nucl Chem 289, 489–495 (2011). https://doi.org/10.1007/s10967-011-1090-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-1090-2