Abstract
The basic strategic aims in the field of managing high-level radioactive waste and liquidation of nuclear power plants are all contained in the Energy policy of the Slovak Republic. Its aim is to resolve the concept of the backside of the nuclear energetics fuel cycle—long-term deposition of high-level radioactive waste and spent nuclear fuel (SNF). The most important form of high-level radioactive waste and SNF long-term deposition is their deposition in deep geological formations created by natural as well as engineering barriers used to isolate the long-lived radionuclides from the biosphere. The basic components of these barriers are clays, of which bentonite is generally referred to as the most suitable clay material. There are a few significant bentonite deposits in the Slovak Republic: Jelšový potok, Kopernica, Lastovce, Lieskovec, Dolná Ves. The review article summarizes the information on geotechnical properties of Slovak bentonites published up-to-date, which is inevitable to know for the intention of their use. It highlights the advantages and shows drawbacks of five Slovak deposits. It suggests further research direction, to draw a thorough hydraulical, microbial and radiation profile of Slovak bentonites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The basic strategic aims in the field of managing high-level radioactive (HLRW) waste and liquidation of nuclear power plants are all contained in the Energy policy of the Slovak Republic. Its aim is to resolve the concept of the backside of the nuclear energetics fuel cycle—long-term deposition of HLRW and spent nuclear fuel (SNF).
The review article summarizes the information on geotechnical properties of Slovak bentonites published up-to-date, which is inevitable to know for the intention of their use in deposition of HLRW and SNF. It highlights the advantages and shows drawbacks of five Slovak deposits. It suggests further research direction, to draw a thorough hydraulic, microbial and radiation profile of Slovak bentonites.
The most important form of HLRW and SNF long-term deposition is their deposition in deep geological formations created by natural as well as engineering barriers used to isolate the long-lived radionuclides from the biosphere. The basic components of these barriers are clays, of which bentonite is generally referred to as the most suitable clay material [1–5].
Significant producers of HLRW and SNF in Slovakia are nuclear power plants located in Bohunice and Mochovce [6]. In the Slovak energetics as fuel mainly uranium dioxide is used, enriched by uranium-235 radioisotope, that the average enrichment in the fuel cartridge is 3.82%. One reactor of 1,000 MW power annually produces about 30 t of SNF. Because the fuel has a high density, it represents the volume of only about 1.5 m3 [7]. One WWPR 440 block annually produces about 220 m3 of low-level, 90 m3 of medium-level radioactive waste and 10 t of SNF. It is assumed that blocks of individual nuclear power plants in Slovakia will produce during their project operation period 2,500 t of SNF and 3,700 t of RW, which under current legislation will not be able to be deposited in the Mochovce RR RW surface type (this estimate includes also the production of RW from NPP A-1 Bohunice) but will have to be deposited in a deep geological repository (DGR).
Deep repository development in Slovakia
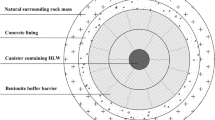
The preparatory and development work on building DGR in Slovakia began in 1996 [8]. DGR should be placed below the ground surface (about 300–700 m) in a stable geological formation, which will form one of the most important barriers of a multi-barrier system (a combination of technical and natural barriers, i.e. geological barrier) [9].
The role of geological barrier as a longest-acting one in the multi-barrier system of repository protection is to isolate the surrounding environment from harmful effects of radiation. Its insulation and protective function must be maintained and sufficient for a period of 100,000 years, till the radiation level has dropped to an acceptable level.
Slovak DGR should be put into operation no later than 2037. Based on preliminary evaluation of existing geological data have been identified 12 territories, potentially suitable for DGR. Further evaluation led to a reduction of this number to five territories in two potential host environments (granitoids and sediments) that have been proposed for more detailed research: Tribec Mts., Veporske vrchy Mts., Stolicke vrchy Mts., Rimavska kotlina Basin, Cerova vrchovina Upland [8].
Preparation of models of potentially suitable localities for DGR placement includes extensive multidisciplinary research of interactions between host environment and engineering barriers [10–12]. To construct DGR is needed homogeneous block of geological rock (measuring approx. 1 km × 1 km × 1 km), which behaviour and properties is important to know especially in the considered depth [13].
Currently an option of building an international DGR, where various countries would store would impose their HLRW and SNF seems to be a realistic solution.
Bentonite
Bentonite and other clay rocks are of paramount importance in the field of environmental and waste management [14–32]. Bentonites are a group of natural nanomaterials composed predominantly of crystalline mineral particles from the group of dioctahedral smectites—montmorillonite [33, 34].
Bentonites from Slovak deposits should be used as part of multi-barrier system in DGR for SNF and HLRW [35–41]. This requires extensive multidisciplinary research a comprehensive and detailed characterization of bentonites [42–57]. The use of bentonite rocks, or bentonite backfills in the repository is destined to their mineralogical, erosion and rheological properties [33, 34, 58–65, 144], and their favourable adsorption and retardation behaviour to the activation and corrosion products and the fission products of U [66–83]. Study of those products is the subject of still ongoing research [84–103]. Adsorption properties of bentonites are determined by their chemical and mineralogical composition, value of cation exchange capacity (CEC) and specific surface area (SSA) [104–135]. Slovak bentonites are subjected to basic [136–143] and applied [144–157] research for several decades. The first results our department has been published already in 1966 [158] The purpose of the multidisciplinary study of bentonites is to assess comprehensively in the long term the geotechnical properties and to simulate conditions expected in DGR for SNF and HLRW [159–174].
Bentonite deposits in Slovakia
In the Slovak Republic there are several important bentonite deposits [8, 9, 17, 25, 33–39, 41]. There is the most popular and long mined bentonite deposit formed by Al–Mg-montmorillonite in the Slovak upland in the locality Stará Kremnička: Jelšový potok. In this area, there is one more a partially mined deposit of andesitic bentonite formed by Fe-montmorillonite: Lieskovec. In the eastern neovolcanic area, there are two currently mined deposits of rhyolitic bentonites: Kuzmice and Lastovce (Al–Mg-montmorillonite). Moreover, in this area there are currently not mined deposits Nižný Hrabovec, Fintice, Nižný Žipov, Vel’aty and Hliník. Deposit Dolná Ves (illite–smectite) is a part of Jastrabská formation.
Genetically are these deposits partly different, even though their common essential characteristic is that they arose as a product of volcanic activity and by subsequent action of alternating processes.
From the perspective of the qualitative composition of the Slovak bentonites can be earmarked two basic groups of Slovak deposits [20, 22, 33, 34, 36, 65]:
-
I.
Smectite deposits—an essential component forming a clay proportion of rock are smectites:
-
(a)
High smectite—smectite content above 60% (Jelšový potok labelled as J, Kopernica labelled as K, Lieskovec labelled as L),
-
(b)
Low smectite—smectite content about 40% (Lastovce labelled as LA).
-
(a)
-
II.
Illite–smectite deposit—an essential component forming a clay proportion of rock is mixed-layer mineral illite–smectite (Dolná Ves labelled as DV).
The properties of natural chemically modified and irradiated forms from the above-mentioned five Slovak deposits (J, K, L, LA, DV) were studied and compared. The fractions of bentonites grounded under 15, 45 and 250 μm were monitored.
From the perspective of the crystal-chemical specifications of the main clay component structure itself can be deposits further classify into [33, 34, 36–39, 41, 43–45]:
-
Al–Mg-montmorillonite—smectite is identified as montmorillonite and its octahedral structure is composed mainly of Al and Mg (J, K, LA).
-
Fe-montmorillonite—smectite is identified as montmorillonite and its octahedral structure is composed mainly of Fe (L).
-
Illite–smectite—the main clay component is mixed-layer illite–smectite (DV).
J, K and L have similar qualitative mineral composition. J and K are also close quantitatively. L has a significantly lower content of smectite (by about 20%), whose presence is mainly replaced by kaolinite and cristobalite. LA differ significantly from the other smectite deposits, especially by very low content of smectite, high content of cristobalite and unavoidable presence of calcite.
Performance requirements for bentonite barriers
The utilization of bentonites in multi-barrier system inevitably requires assessing a long-term stability of bentonite barrier behaviour, to characterize it from various geotechnical and physico-chemical aspects.
The focus is on:
-
Mineralogical properties [52, 55, 64, 140, 159, 161, 166, 167, 169, 171, 175–181]: mineralogical composition, the presence of hazardous materials (K-feldspar and biotite, calcite, anthropogenic carbonates), hypergene processes, illitization, kaolinization, interaction of bentonite barrier and the environment, mineral, thermal and microbial stability, technological processing.
-
Physico-chemical properties [20, 40, 53, 154–156, 165, 168, 170, 173, 182–216]: chemical composition, CEC, adsorption and retardation properties, radiation stability, interaction of metal Fe, buffering capacity.
-
Physico-mechanical properties [16, 33, 36, 40, 42, 44, 52, 56, 60, 136, 139, 162, 163, 175, 191, 192, 217, 218]: expansion, SSA, plasticity, grain size, hydraulic properties, permeability, compactibility, high strength at the pressure and shear, low compression, high deformation modulus, thermal conductivity, density, consistency limits, strength.
Mineralogical composition and stability of bentonite
Mineralogical composition
The use of bentonites is largely conditioned by the content of smectites, their crystal-chemical composition and by the admixtures of another clay and other minerals [2, 10, 15, 37, 38, 45, 54, 55, 58, 120]. From other clay minerals, kaolinite occurs most frequently, especially in the deposits of hydrothermal origin [33]. Halloysite also in the case of bentonite belongs to accessory present clay minerals and is not crucial to its properties and use [34]. Illite may appear on sedimentary deposits and adversely affect them [46, 47, 50]. From other minerals forming an admixture in bentonite has dominance cristobalite, belonging most often to opal. It appears in all genetic types of bentonite, but mainly in the volcanic-sedimentary deposits, where is formed by the alteration of volcanic glass [36, 41, 50]. Its admixture sometimes reaching up to 40%. It cannot be effectively removed by any treatment and, therefore, significantly limits the use of bentonite in particular for its demanding applications [60, 65].
Hypergene processes
The most important process of hypergen sphere is weathering [12, 33, 166, 167, 171]. It is a spontaneous process generated as a result of lack of balance between exogenous conditions influenced by the interaction between atmosphere, biosphere, hydrosphere, and rocks forming the lithosphere [36, 38]. Clays and clay minerals belong among the most important products of weathering [22, 34, 40, 139]. Weathering processes are conditioned especially by original weathering material, climate and landscape morphology [140, 161, 166]. Based on the composition of the attacking solution can be these processes divided into acidolysis, salinolysis, alkalinolysis and hydrolysis [60].
Illitization
One of the processes leading to the loss of the required properties of bentonite barriers is a mineral conversion of smectite into illite—illitization [12, 33, 36, 52, 53, 166, 169]. This is an irreversible process that causes an irreversible fixation of K atoms in smectite inter-layer area, a loss of hydrated exchangeable cations from inter-layer space and Al for Si substitution in the structure of tetrahedrons [34, 40, 46, 47]. The conversion of smectite into illite via the mixed-layer mineral illite–smectite at elevated temperature is processing not by impacts or saltation, but gradually, depending on the temperature. As a result of dehydration and K fixation in the inter-layer the collapse of the layers occurs, which is accompanied by the loss of smectite expanding ability, the significant reduction of the SSA, adsorption and CEC, i.e. the total loss of the bentonite retention properties [60, 65, 106, 160, 171–173, 176, 186–192].
Kaolinization
Kaolinization process is processing in acidic conditions of acidolysis [30, 33, 36, 60, 196]. Advanced acidolysis is characterized by the higher activity of Al, which is released by octahedron decomposition and migrates into the inter-layer of expanding clay minerals [169]. In slightly acidic conditions (pH 4.3–5.1) of temperate climate zone, there occurred to smectite dissolution, which was accompanied by the disintegration of smectite crystals during cambisol pedogenesis on the contact of Al–Mg-montmorillonite Stará Kremnička deposit (deposit J) [34]. At about 2 m gross profile involving soil and bentonite affected by weathering the degradation up to about 60% of the original smectite occured. A part of amorphous material probably became the source for neoformation of kaolinite, which representation increases towards the surface.
Thermal stability
A weakness of bentonites, compared e.g. with natural zeolites (containing mainly mordenite), is greater possibility of distortion of their structure at higher temperatures, which may occur a collapse of smectite structures and a loss of initial adsorption, expanding and other, from a geotechnical perspective significant, properties [25, 29, 36–40, 49, 114, 123, 139, 150, 166, 169, 176]. Bentonite loses the expansion already at temperatures above 150 °C [33, 34, 196, 198, 206, 210, 215]. During the water saturation of bentonite backfill may occur to its evaporation by the decay heat from HLRW and SNF, and thereby to an increase of salt content, which reduces expansion [122, 123, 171, 172]. Similarly smectite illitization can reduce the expansion and adsorption properties of this material [46, 47, 50, 186–192].
Microbial stability
Microbial destruction of alumosilicate minerals, could negatively affect some of their basic properties [176–180]. Pore size of microbes on average generally ranges from 0.1 μm up to several micrometers. The mechanism of microbial destruction by bacteria of the genus Bacillus is not stoichiometrically described and fully elucidated yet, but it is known that its effect is a reduction of Si content, the extraction of Al, Ti, U, Au and other elements of silicates and alumosilicates [179].
Research on microbial activity in the Canadian type depository, which included the radionuclide microbial transport, the production of gas and microorganism influence on the corrosion of the containers was confirmed that the compact clay barriers will limit or fully prevent the transport of radionuclides by microbes in view of their small pores [178, 180]. On the container itself, there will not be appropriate conditions for their existence (temperature—drying, radiation). Surviving bacteria will be inactive as long as the environment keeps being dry. After water percolation into the system their re-activation could happen, but retarded by physical conditions of compressed clay barrier.
Physico-chemical properties
Chemical composition
Bentonites J and K have a very similar chemical composition [43–45, 58, 106, 137, 161]. The most significant difference is the higher Mg content in the J bentonite, which is except of removable positions strongly represented also in montmorillonite octahedrons. Bentonites L have a significantly higher Fe content compared with the previous two bentonites. This is due to a different parent rock—andesite [52, 64, 78, 79, 134, 167]. When comparing the chemical composition of bentonite fractions with different grain size (15, 45 and 250 μm) from one deposit no significant differences have been shown [186–190]. When comparing J and K bentonites the differences are minimal. At L bentonite samples can be observed minor differences.
Cationic exchange capacity
Cation exchange capacity is determined by the ability to adsorb cations and to keep them in the exchangeable positions [12, 33, 34, 60]. In smectites it is called inter-layer space. Quantitative analysis of individual exchangeable cations confirmed the predominance of Ca and Mg over Na and K elements [55, 78, 161, 165, 167, 168]. Distribution of individual initial cations indicates the correlation between the J, K and L, with the difference that Lieskovec has a slightly higher content of Na. DV bentonites form a separate group, which dominates in the content of exchangeable Na and K. CEC values for individual bentonites deposits decrease in the following order: J > K > L > La > DV [186–190]. The lowest CEC values, which were measured for the DV were up to 4-times lower than in the case of J bentonites.
Radiation stability
One of the basic prerequisites for the bentonite utilization as engineering barriers is their stability against exposure of ionizing radiation from radionuclides present in the irradiated fuel cells and HLRW [6, 12, 36, 65, 165, 193–216].
Most activity during the first 100 years falls on the activity of 137Cs. It is expected that in a box during the first 100 years since fuel cell removal from the reactor is 3 × 1013 Bq 137Cs. Dose rate for 137Cs with this activity at a distance of 0.5 m is 26.6 Gy hod−1. To calculate the absorption of radiation in the 30 cm bentonite layer is used half-width value, which is the same as for concrete, i.e. 3.7 cm. For the 30 cm bentonite layer is then ray photon absorption 99.6%. The annual dose absorbed by the bentonite should be considered with a value of 74 MGy for a year [191, 192].
Interaction of γ-radiation at dose interval of 105, 106, 107 and 3 × 107 Gy with various clay minerals such as kaolinite, montmorillonite—dioctahedral Mg-smectite, palygorskite and muscovite was observed in order to detect a change in their physico-chemical properties [209]. There was observed the impact on the type and concentration of the radiation-induced structural defects (in tetrahedrons Si–O− and octahedrons AlVI–O–AlVI bond), the degree of the deuterization in consequence of D2O interaction, the change in the SSA, adsorption and CEC and the effect on the solubility of clay minerals irradiated in heavy water. An important finding was that the degree of accumulation of defects in the structure is a function of the content tetrahedral structural cells (Si4O10). In addition to chemical composition, formation of structural defects is significantly affected by the content of H2O adsorbed on the mineral surface and in the inter-layer space. In general, high water content was slowing down the formation of defects. With increasing radiation dose, there was in the montmorillonite increased the loss waiter of Si4+ and decreased the loss waiter of Al3+ from the structure. In kaolinite the situation was reversed. The radiation dose increase from 0 to 3 × 107 Gy resulted in the increase of smectite SSA about 8% (for montmorillonite), furthermore in the adsorption capacity increase (15%) and the solubility change.
Adsorption and retardation properties
The basic cause of the bentonite adsorption behaviour is determined by the presence of negative charge on the smectite particle surface, which is directly related to the ability to receive and exchange in the inter-layer space so-called exchangeable cations most often Na, Ca, Mg and K) [12, 33, 34, 60, 66]. Bentonite adsorption properties are determined by the bentonite chemical and mineralogical composition, CEC and the SSA [54, 55, 58, 61, 62, 82, 107, 108, 110].
Research of bentonite adsorption properties is an essential step for developing a radionuclide migration model [15, 20, 31, 122, 123, 126]. The radionuclide adsorption is the most frequently studied by the radio-indicator methods, using batch and diffusion techniques [3, 5, 16, 29, 73, 84, 174]. The experiments are carried out under aerobic and anaerobic conditions, in static or dynamic experiment configuration so that the experimental environment approaches as close as possible to the conditions in the deep repository geoenvironment [120, 132, 133, 176, 216–218]. The traditional way of characterization of radionuclide sorption behaviour on the bentonite are sorption isotherms (most often Fruendlich, Langmuir, Dubbin-Radushkevitch) [4, 75, 104, 109, 111]. If the experimental isotherms cover a wide interval of concentrations, Langmuir model is the prime candidate to study the sorption behaviour of radionuclides on bentonite.
The focus is on the kinetics of adsorption and the influence of various factors affecting the bentonite barrier adsorption processes, which are applied in the vicinity of stored HLRW in deep geological conditions [2, 65, 69, 70, 129, 135, 159, 173].
There were experimentally studied the adsorption rate, the impact of pH environment change, ionizing radiation, the presence of competing ions, complexing organic agents, synthetic and natural organic ligands on Slovak bentonite adsorption processes in relation to the radionuclides [78–80, 106, 112, 114, 116, 134, 158, 182–192].
Adsorption kinetics
Adsorption of 137Cs and 85Sr radionuclides in bentonites is relatively quick [68, 182, 184]. The equilibrium of the bentonite sample J was reached almost immediately, within 1 min from the beginning of the contact between solid and liquid phase [186, 188]. Comparable values of distribution ratios and percentage adsorption were obtained at interval of 1–480 min. Instantaneous adsorption can be explained by the rapid adsorption process and the cation exchange on the adsorbent surface. At higher concentrations of Sr the capture may also be due to precipitation of Sr in the carbonate form on the bentonite surface [69, 187].
pH environments
At the deep environment the changes of pH environment value may occur [2, 4, 6, 69, 127, 135, 161, 171]. Bentonites are characterized by two types of “functional” groups that are in the literature known as “edge sites” and “layer sites” [12, 187]. For the “edge sites”—surface groups, which are usually labelled by symbol XOH (X = Al, Si, Fe…), is characteristic that on them, depending on the pH value, is in the acidic area processing a protonation with the formation of XOH2+. In alkaline area there occur to deprotonation with the formation of XO−. To the sorption, respectively desorption occurs on the protonized, as well as on the deprotonized form with the fact that the character of the reaction mechanism may be complexing (especially XO−) as well exchangeable (substitution), depending on the type of component that interacts with that group. The effect of the pH value change on the Sr-adsorption in the values range of pH 2–8, in various rates of solid (bentonites J, K and L) and liquid phase (eight different concentrations of Sr) confirmed, that the adsorption of Sr decreases in the order: pH = 8 > pH = 6 > pH = 4 > pH = 2 [187, 189, 191]. The values of adsorption percentage and distribution ratios rates increase with increasing pH value (towards the alkaline area also with decreasing initial concentration of Sr in the solution. The value of adsorption percentage close to 99% was reached on the adsorption of Sr-cations on bentonites J, K and L from pH = 8. From above it can be concluded that in addition to the basic adsorption mechanism, which is cationic exchange, there are processing at higher pH values the complexing reactions with surface groups of bentonite. The increase of the adsorption percentage value can be attributed to “hydrolytic” adsorption because of the reaction between Sr(OH)+ and OH− groups and competition of H+ ion is suppressed.
At pH = 2 there were at the whole studied interval of concentrations observed low values of adsorption percentage, distribution ratio and adsorbed amount of strontium, which is attributable to significant competitive effect of hydrogen ions and disturbed bentonite structure.
Influence of γ-irradiation
On the radiation stability of bentonite barrier are placed high demands because the bentonites will be exposed to the long-term, continuous and immediate effects of ionizing radiation [40, 165, 193, 195]. The initial surface dose rates from γ-ray and neutron radiation are estimated at around 2 Gy h−1. The interaction effect of ionizing radiation and bentonites from J and L deposit have been studied after sample irradiation by cobalt source (60Co) with energy of 1.17 and 1.33 MeV for a period of about 50 days, with a moderate dose rate of 0.092 Gy s−1 [186, 187]. Total absorbed dose was 390 kGy. At the irradiated samples of bentonites J250 and L250 there were detect higher sorbed amount of Cs and Sr than at their non-irradiated forms. Currently there is being conducted a research of Cs and Sr adsorption on bentonites from five Slovak deposits (J, K, L, LA, DV, native and natrified forms, grain size 15, 45, 250 μm), that were irradiated in a wider range of doses by γ-source 60Co with the highest reached dose of 1 MGy. The radiation dose increase resulted in the increase of the SSA, the change of the clay minerals solubility and the possible loss of the initial adsorption properties due to the radiolysis of sorbed water [195, 198–215].
Competition of cations
Metal cations, which may be present in groundwater, significantly affect the adsorption of radionuclides [2, 5, 12, 15, 18, 33, 66, 73, 78]. The effect can be explained by ion-exchange competing reactions and occupying the active adsorption centres of bentonites. The adsorption is suppressed more by the presence of bivalent cations than by univalent cations. The cause of the different observed effect is in bentonite ability to prefer cations with low hydration energy and small ionic radius. They preferably come into their inter-layer. According to the received ion size the distance between the layers is variably. Smectite inter-layer increases its size by the ion, which is into its structure received. After losing the received ion the structure is changed again. This unique property—expandability is characteristic only for smectites and partially for vermiculites.
The effect of competing cations (Na+, K+, NH4 +, Ca2+, Ba2+, Mg2+) on the Cs and Sr adsorption confirmed that the higher distribution ratio values are achieved in the presence of univalent as well in the presence of bivalent cations [182, 186, 187, 189, 191]. Values of the distribution ratios increase with decreasing initial concentration of competing cations in the solution. The Sr-adsorption is the most suppressed by the presence of Ba2+ cation.
The presence of organic complexing agents, synthetic and natural organic ligands
Organic compounds such as EDTA, oxalic and citric acid, humic acids and fulvic acids that are the part of the decontamination solutions or are ranged among the organic components of soil and water suppress the adsorption of radionuclides [12]. The effect of EDTA, oxalic and citric acids on adsorption of Cs has been studied in the concentration interval of 1 × 10−5–5 × 10−2 mol dm−3 [190]. The results confirmed that the presence of the studied organic and natural ligands significantly affects the adsorption of Cs. By increasing their initial concentration the values of percentage adsorption and distribution rates drop. The adsorption of Cs on samples J15, K15 and L15 is significantly suppressed at the ligand concentration of 5 × 10−2 mol dm−3: EDTA > citric acid > oxalic acid. The adsorption reducing effect can be explained by formation of soluble complex ions, which are too large to enter the available structural positions of bentonite [69, 190, 191].
Interaction with metallic iron
The prognosis of the long-term stability of bentonite barriers in DGR includes studying the interaction of bentonite-metallic Fe and bentonite-surrounding rock system [40, 55, 64, 126, 133, 134, 154–156, 193, 196]. The processes processing in the repository were simulated to determine the stability of the Slovak bentonites in the presence of metallic Fe, pyrite and Fe(III)-oxide-hydroxides [168, 170]. The results show a partial destabilization of the smectite structure. The smectites with the highest Fe content in its structure in the presence of metallic Fe have shown as the least stable. It follows that the bentonite barriers that will be contacted with iron structural elements of depository should be without structural Fe.
Physico-mechanical properties
Expansion
It allows in contact with water and polar liquids to expand in volume up to twelve-times [33]. It is important at sealing joints and cracks when a contact of bentonite barrier and water (self-healing) [34, 40, 159]. Expanding pressure of the bentonite barrier may not adversely affect the function of the container and building elements of engineering barrier, respectively the function of natural barrier [10, 36, 60]. Expanding pressure is low order in the case of K-bentonites from DV as well in the smectite type bentonites [34, 65, 192].
Specific surface area
The SSA, which characterizes the adsorbent active surface area allows high reactivity with the environment in which the adsorbent—bentonite is [33, 34, 36, 60, 139]. The comparison of physico-chemical properties of the Slovak bentonites showed that natural J and K bentonites have a significantly higher SSA than bentonites from L and LA deposits [44, 65]. The same applies to the bentonite hydration ability. Those properties achieved the lowest values at DV bentonites, which fully correlated with their mineral composition [187, 188].
When comparing the chemically activated samples (natrification), the largest SSA have the natrified bentonites from the deposit of Jelšový potok, when are the changeable positions in the inter-layer saturated by Na+ cations [189, 191]. Partially lower values were achieved by K samples and natural J samples. L bentonites showed the lowest values. Natrification allows the best separation of the basic smectite layers, which leads to the obtaining of a new area for adsorption. The decrease of the SSA from the finest to the coarsest fraction (15 → 45 → 250 μm) was moderate and consistent for all samples.
Plasticity
The plasticity is in geotechnical sciences defined as the moisture range between the value of the plasticity limit and fluidity limit [33, 34, 36, 51]. It is expressed by the plasticity index. For the light self-healing of areas of discontinuity it is appropriate to the plasticity index value was the highest. High plasticity index value means that even at large moisture increase over the plasticity limit soil remains plastic and does not pass into the liquid state. In the plastic state bentonite keeps sealing effects. The large increase of plasticity was observed due to the bentonite natrification [65, 188, 191, 192]. Smectitic bentonites of Central Slovakia (J, K, L) achieved the results of plasticity comparable with internationally tested bentonites (Montigel a MX80). LA and DV bentonites show significantly worse properties than the above three deposits. K-bentonite from DV has the worst physical and mechanical parameters at all indicators. Short-term treatment of temperature on the non-natrified bentonite (7 days at 90 °C) had no noticeable effect on the bentonite plasticity.
Permeability
Imperfect preparation of bentonite, thermal and mechanical stress of barrier material may cause the area discontinuity, then the increase of the material permeability [36, 56, 65]. Among the studied Slovak deposits (J, K, LA, L, DV) the samples of K-bentonites DV had the worst hydraulic permeability parameters [162, 163, 192].
Modification of bentonites
Bentonite can be used in a natural, physically or chemically modified form [25, 37–39, 65, 80, 82, 105, 114, 144, 146, 154, 156]. Modification is made to improve their physical and chemical properties.
Physical modification is a basic mechanical treatment of bentonites, which consists of drying and milling [33, 44]. By milling are bentonites fined down to the desired grain size. When comparing the Slovak bentonite fractions milled under 250, 45 and 15 μm has been shown that it does not make sense to work with the finer grain sizes than 250 μm [187–192]. At the finer fractions (45 and 15 μm) there is no significant improvement of the physico-chemical properties and technological process of bentonite barrier treatment would be pointlessly financially encumbered.
Drying reduces the original moisture content of bentonite (when surface mining of Na-bentonite around 30%, Ca-bentonite around 25%) to the required 7–8% [12, 33, 36, 38, 49, 52, 60, 136]. Drying temperature ranges from 100 to 200 °C at the input and up to 800 °C at the output, depending on the desired application. Smectite structural properties get significantly worse if when is the temperature exceeded.
More exacting bentonite treatment method, nowadays already quite common, is based on the preparation of the chemically modified forms [63].
At the chemical treatment the natural bentonite is cultivated by chemicals for various types of finished products, usually directly in the mining plant [33, 34]. Chemical modification is allowed by a large SSA with active sorption centres, the presence of molecular water and exchangeable cations in the smectite inter-layer space area. There are prepared mono-ion forms, of that is the most often used the protonation (the changeable positions in the inter-layer are saturated by H+ cations) or natrification (the changeable positions in the inter-layer are saturated by Na+ cations) [57, 60, 73, 103, 189]. As a nitrificant salt is almost exclusively used bicarbonate Na2CO3 because of its financial ease and the significant exchange rate. The amount of soda ingredient varies with the intended use and the type of bentonite. Preferable is the wet method of activation, by which is all bentonite transformed into Na-bentonite. Natrification allows the best separation of the basic smectite layers, what leads to the obtaining of a new area for the cations exchange and to an increasing of the full adsorption capacity. The utilization of artificial natrified Slovak bentonites as bentonite barriers in DGR was not excluded yet [189, 191]. The main problem is the technological process, which significantly shifts the pH to alkaline area. On the adsorption of Cs+ and Sr2+ the formation of colloids occurred that could not be removed even by repeated centrifugation at high speed. Natrification may also cause undesirable excessive pressure from the expansion of the clay barriers. Specific and more complicated method of chemical modification is a intercalation and pillaring of the smectite structure [42, 49, 142, 150, 205, 219, 220]. In the first case, is concerned the process of intercalation by organometallic and organic cations, in which are prepared so-called organobentonites. Pillaring respectively reinforcing of the smectite structure is made due to their very low thermostability. Into the smectite inter-layer area is inserted e.g. Al2O3 molecule that performs there a function of the pillar, defending to the smectite structure collapse.
Conclusion
Bentonites from the Slovak deposits (Jelšový potok, Kopernica, Lastovce, Lieskovec) meet most of the geotechnical requirements for that type of barriers. They can be used as filler, damping, respectively sealants materials in the vicinity of a container of radioactive waste and SNF.
The deposit Dolná Ves showed the lack of geotechnical, especially adsorption and retarder properties of bentonite. From the long-term barrier stability point of view the Lieskovec bentonites with high content of structural bound Fe appear to be unusable.
Smectite bentonites jastrabská formation—Jelšový potok and Kopernica can be identified as the most suitable material for practical use when depositing high-level radioactive waste and SNF. Bentonites of these deposits are stable material guarantees a long-term stability.
Hydraulic properties require attention in the further study of the matter as well as radiation and microbial stability of Slovak bentonites. The authors of the article would be extremely grateful to the workplace which could on the studied Slovak bentonites reach absorbed doses of ionising radiation of 10–100 MGy.
References
Slovenské elektrárne, as. (2001) Hlbinné geologické úložisko pre vyhorené jadrové palivo a rádioaktívne odpady. http://www.seas.sk/_cms_/_files/1183/HU_72dpi_WWW.pdf. Accessed 27 Dec 2010
Jedináková-Křížová V, Zeman J, Vinšová H, Hanslík E (2010) Bentonite stability, speciation and migration behaviour of some critical radionuclides. J Radioanal Nucl Chem 286(3):719–727
Filipská H, Štamberg K (2005) Experimental study and mathematical modeling of Cs(I) and Sr(II) sorption on bentonite as barrier material in deep geological repository. Acta Geodyn Geomater 2(2):77–84
Vokál A, Filipská H, Jedináková-Křížová V, Štamberg K, Vopálka D (2006) Radiochemical studies in the development of deep geological repository in the Czech Republic. Czechoslov J Phys 56(4):D63–D71
Prvakova S, Duran J, Necas V (2005) Evaluation of radionuclide migration in the homogeneous system of a geological repository. Int J Nucl Energy Sci Technol 1(4):265–273
Chmielewská E, Kuruc J (2008) Odpady. Nakladanie s tuhým neaktívnym a rádioaktívnym odpadom. Univerzita Komenského, Bratislava
Drábová D (2004) Hlubinné úložište radioaktívního odpadu a vyhořelého jaderného paliva (od emocí k hledání řešení). Urbanizmus a územní rozvoj VII/5:8–11
Slaninka I, Hók J, Franzen J (2007) Status and development of deep geological repository in Slovak republic from geological point of view. Acta Montan Slovava 1(12):17–23
Bauer V, Šofranko M, Stavnikovič M (2007) Research of the multibarrier system for an underground deposition of radioactive wastes. Acta Montan Slovava 1(12):217–225
Prváková S, Pospíšil M, Ďúran J (2004) Geological disposal of spent nuclear fuel in clay host formation in Slovakia, 199–202. In: Stability and buffering capacity of the geosphere for long-term isolation of radioactive waste, OECD, Paris, p 241
Bartko M, Éhn L (2007) Safety barriers and complex operational monitoring system of the radioactive waste deposit of Slovakia RÚ RAO in Mochovce including its perspective and extension. Acta Montan Slovava 12(1):9–16
Jenne EA (1998) Adsorption of metals by geomedia. Academic Press, San Diego
Slaninka I, Frankovská J, Kordík J (2008) Výber lokalít pre hlbinné geologické úložisko rádioaktívnych odpadov v SR. Enviromagazín 5:12–13
Styra B, Butkus D (1990) Geophysical problems of Krypton-85 in the atmosphere. Hemisphere Publishing Corporation, New York, 153 pp
Adamcová R, Madsen FT (1996) Heavy metal retardation in some Slovak clays and clayey soils. Geologica Carpathica Clays 5(1):13–20
Hiller E (2000) Stručná teória difúzie a jej aplikácia v environmentálnej geochémii. Geochémia 2000, Bratislava, pp 103–107
Jesenák K (2001) Prírodné sorbenty a ich využitie pri ochrane životného prostredia. Biológia Ekológia Chémia 6:28–33
Čipáková A (2002) Imobilizácia rádionuklidov a kadmia v pôdach aplikáciou zeolitu [The immobilisation of radionuclides and cadmium in soils using application of zeolite]. J Hydrol Hydromech 50(4):320–340
Lichner L’, Čipáková A (2002) Cadmium distribution coefficients and Cd transport in structured soils. Rostl Výr 48:96–100
Adamcová R (2002) Retardation capability of mineral linings of waste disposal sites. Acta Geol Univ Comen 57:5–33
Obut A (2005) Sedimentation Characteristics of Kaolin and Bentonite in Concentrated Solutions. Acta Montan Slovava 10(1):145–150
Jesenák K (2006) Čo sú to fylosilikáty. Biológia Ekológia Chémia 11(1):18–23
Lichner L, Dlapa P, Sir M, Cipakova A, Houskova B, Fasko P, Nagy V (2006) The fate of cadmium in field soils of the Danubian lowland. Soil Tillage Res 85:154–165
Jablonská K, Štyriaková I (2007) Application possibility of bentonite and zeolite in bioremediation. Adv Mater Res 20–21:295–298
Kraus I (2008) Nové trendy a možnosti využívania nerudných surovín na Slovensku. Mineralia Slovaca 40:175–182
Pusch R, Kasbohm J, Pacovsky J, Cechova Z (2007) Are all smectite clays suitable as “buffers”? Phys Chem Earth 32:116–122
Hiller E, Šutriepka M (2008) Effect of drying on the sorption and desorption of copper in bottom sediments from water reservoirs and geochemical partitioning of heavy metals and arsenic. J Hydrol Hydromech 55(1):45–58
Kadlečíková M, Breza J, Jesenák K, Pastorková K, Luptáková V, Kolmačka M, Vojačková A, Michalka M, Vávra I, Križanová Z (2008) The growth of carbon nanotubes on montmorillonite and zeolite (clinoptilolite). Appl Surf Sci 254(16):5073–5079
Mockovčiaková A, Matik M, Orolínová Z, Hudec P, Kmecová E (2008) Structural characteristic of modified natural zeolite. J Porous Mater 15(5):559–564
Barany S, Kozakova I, Marcinova L, Skvarla J (2010) Electrokinetic potential of bentonite and kaolin particles in the presence of polymer mixtures. Colloid J 5:595–601
Olu-Owolabi BI, Popoola DB, Unuabonah EI (2010) Removal of Cu2+ and Cd2+ from aqueous solution by bentonite clay modified with binary mixture of goethite and humic acid. Water Air Soil Pollut 211(1–4):459–474
Čipáková A, Hiller E, Lichner L’ (2011) Interaction and fractionation of added cadmium in some typical soils of the Danubian Lowland. J Radioanal Nucl Chem 287(1):157–165
Gregor M, Číčel B (1969) Bentonit a jeho použite. SAV, Bratislava
Šucha V (2001) Íly v geologických procesoch. Acta Geologica, Universitas Comenianae, Bratislava
Hraško L’ (1999) Zhodnotenie vhodných geologických štruktúr na definitívne ukladanie rádioaktívnych a toxických odpadov na území SR. Geologická služba SR Bratislava. Manuskript, Archív Geofondu, p 266
Adamcová R, Haasová Z (2005) Vybrané fyzikálne vlastnosti bentonitu pre úložisko rádioaktívneho odpadu. Mineralia Slovaca 37(3):387–389
Jesenák K (2006) Prehl’ad priemyselných a environmentálnych aplikácií fylosilikátov. Partikulárne látky vo vede, priemysle a v životnom prostredí, Košice, pp 90–94
Jesenák K (2007) Stručný prehľad vlastností a použitia fylosilikátov. In: VII. Conference preparation of ceramic materials. Technická, Košice, pp 58–61
Jesenák K (2009) Anorganické pórovité látky prírodného pôvodu. Silikátnik 2009:21–26
Uhlík P, Osacký M, Stríček I, Šucha V, Honty M (2006) Bentonit ako bariéra v hlbinných úložiskách rádioaktívneho odpadu. Súčasné trendy a problémy vo výskume nerudných nerastných surovín. ŠGUDŠ, Bratislava, pp 68–71
Jesenák K (2010) Exkurzia po miestach ťažby a spracovania anorganických surovín na Slovensku. Univerzita Komenského, Bratislava
Krajčovič J, Kubranová M, Horváth I, Grejtak F (1992) Reversible hydration of Al pillared an Na-montmorillonite. Geologica Carpathica Clays 1(1):43–46
Fajnor V, Kuchta L’, Jesenák K (1992) Is montmorillonite Jelšový Potok a mixture of two types? In: 12th Conference on Clay mineralogy and petrology, Bratislava, 27 pp
Jesenák K, Kuchta L’, Fajnor V (1992) Granulometric analysis of bentonite from Jelšový Potok. In: 12th Conference on Clay mineralogy and petrology, Bratislava, p 47
Jesenák K, Šucha V (1992) Trace elements in bentonite Jelšový Potok—Stará Kremnička. In: 12th conference on Clay mineralogy and petrology, Bratislava, p 46
Šucha V, Kraus I, Mosser C, Hroncová ZA, Sobolieva K, Siranová V (1992) Mixed-layer illite/smectite from the Dolna Ves hydrothermal deposit, the Western Carpathians Kreminica mts. Geologica Carpathica Clays 1(1):13–19
Šucha V, Kraus I, Gerthofferová H, Petes J, Sereková M (1993) Smectite to illite conversion in bentonites and shales of the East Slovak Basin. Clay Miner 28(2):243–253
Butkus D, Krenevičius R (1996) Influence of radioactive noble gases on the air ionization variation in the environment of nuclear power plants and nuclear fuel reprocessing plants. Atmos Phys 18(2):43–49
Hlavatý V, Fajnor VŠ (2002) Thermal stability of clay/organic intercalation complexes. J Therm Anal Calorim 67(1):113–118
Honty M, Uhlík P, Šucha V, Čaplovičová M, Francu J, Clauer N, Biroň A (2004) Smectite-to-illite alteration in salt-bearing bentonites (The East Slovak Basin). Clays Clay Miner 52(5):533–551
Adamcová R, Haasová Z, Schügerl R, Magulová B (2006) Alternatívne metódy pre laboratórne stanovenie vybraných vlastností bentonitov. Geológia a životné prostredie 5, ŠGÚDŠ
Adamcová R, Valter M, Füri B (2008) Poznatky o termofyzikálnych aspektoch vybraných slovenských bentonitov. In: Zb. Geológia životné prostredie 6, ŠGÚDŠ Bratislava, pp 75–80
Adamcová R, Valter M, Ploetze M (2008) The response of two bentonites to an acid and/or thermal attack. Mineralogia—Special Papers 33:35
Andráš P, Nagyová I, Melichová Z (2008) Separácia a identifikácia ílových minerálov z haldových polí ložiska L’ubietová pre účely štúdia ich sorpčných vlastností. Chem Listy 102(8):684
Andrejkovičová S, Madejová J, Czímerová A, Galko I, Dohrmann R, Komadel P (2006) Mineralogy and chemistry of Fe-rich bentonite from the Lieskovec deposit (Central Slovakia). Geologica Carphatica 57(5):371–378
Frankovská J, Andrejkovičová S, Janotka I (2010) Effect of NaCl on hydraulic properties of bentonite and bentonite–palygorskite mixture. Geosynth Int 17(4):250–259
Bayram H, Önal M, Yılmaz H, Sarıkaya Y (2010) Thermal analysis of a white calcium bentonite. J Therm Anal Calorim 101(3):873–879
Číčel B, Komadel P, Bednáriková E, Madejová J (1992) Mineralogical composition and distribution of Si, Al, Fe, Mg, and Ca in the fine fractions of some Czech and Slovak bentonites. Geologica Carpathica Clays 1(1):3–7
Madejová J, Komadel P, Číčel B (1992) Infrared spectra of some Czech and Slovak smectites and their correlation with structural formulas. Geologica Carpathica Clays 1(1):9–12
Jesenák K (2002) Environmentálna anorganická chémia. Nadácia Jana Husa, Bratislava, PriF UK
Kónya J, Nagy NM, Nemes Z (2005) The effect of mineral composition on the sorption of cesium ions on geological formations. J Colloid Interface Sci 290(2):350–356
Nemes Z, Nagy N, Komlosi A, Kónya J (2006) The effect of mineral composition on the interaction of strontium ions with geological formations. Appl Clay Sci 32(3–4):172–178
Komadel P, Madejová J (2006) Acid activation of clay minerals, chap 7.1. Handb Clay Sci 1:263–287
Komadel P, Anastácio AS, Andrejkovičová S, Stucki JW (2008) Iron phases identified in bentonite from the Lieskovec deposit (Slovakia) by variable-temperature Mössbauer spectroscopy. Clay Miner 43(1):107–115
Adamcová R, Frankovská J, Drmeková T (2009) Engineering geological clay research for a radioactive waste repository in Slovakia. Acta Geologica Slovaca 1(2):71–82
Misaelides P, Godelitsas A (1999) Interaction of actinides with natural microporous materials: a review. Czech J Phys 49(1):167–174
Marinin DV, Brown GN (2000) Studies of sorbent/ion-exchange materials for the removal of radioactive strontium from liquid radioactive waste and high hardness groundwaters. Waste Manag 20:545–553
Altas Y, Tel H, Yaprak G (2003) Sorption kinetics of cesium on hydrous titanium dioxide. Radiochim Acta 91:603–606
Khan SA (2003) Sorption of the long-lived radionuclides cesium-134, strontium-85 and cobalt-60 on bentonite. J Radioanal Nucl Chem 258(1):3–6
Nemes Z, Nagy NM, Kónya J (2005) Kinetics of strontium ion adsorption on natural clay samples. J Radioanal Nucl Chem 266(2):289–293
Gurboga G, Tel H, Altas Y (2005) Sorption studies of cesium on TiO2–SiO2 mixed gel spheres. Sep Purif Technol 47(3):96–104
Gurboga G, Tel H (2005) Preparation of TiO2–SiO2 mixed gel spheres for strontium adsorption. J Hazard Mater 120(1–3):135–142
Vejsada J, Vokál A, Vopálka D, Filipská H (2006) Study of cesium sorption on Na and Ca-Mg bentonites using batch and diffusion experiments. Czech J Phys 56(4):D73–D79
Inan S, Tel H, Altas Y (2006) Sorption studies of strontium on hydrous zirconium dioxide. J Radioanal Nucl Chem 267:615–621
Mockovčiaková A, Orolínová Z (2009) Adsorption properties of modified bentonite clay. Cheminé Technologija 1(50):47–50
Chegrouche S, Mellah A, Barkat M (2009) Removal of strontium from aqueous solutions by adsorption onto activated carbon: kinetic and thermodynamic studies. Desalin 235(1–3):306–318
Ulevičius V, Butkus D, Plauškaite K, Girgždys A, Byčenkiene S, Špirkauskaite N (2009) Impact of Krypton-85 beta radiation on aerosol particle formation and transformation. Lith J Phys 49(4):471–478
Andrejkovičová S, Pentrák M, Jankovič L’, Komadel P (2010) Sorption of heavy metal cations on rhyolitic and andesitic bentonites from Central Slovakia. Geologica Carpathica 61(2):163–171
Melichová Z, Hromada L, Nagyová I (2010) Štúdium sorpcie olova na prírodný neupravovaný bentonit z ložiska Lieskovec. Anorganická chémia v tret’om tisícročí: seminár venovaný 45. výročiu výučby a výskumu na KACH PF UPJŠ, pp 40–41
Vereš J, Orolínová Z, Mockovčiaková A, Jakabský Š, Bakalár T (2010) Removal of nickel by natural and magnetically modified bentonite. In: Water treatment technologies for the removal of high-toxicity pollutants. NATO Science for Peace and Security Series C: Environmental Security, Earth Environ Sci, pp 289–294
Gao L, Yang Z, Shi K, Wang X, Guo Z, Wu W (2010) U(VI) sorption on kaolinite: effects of pH, U(VI) concentration and oxyanions. J Radioanal Nucl Chem 284(3):519–526
Mockovčiaková A, Orolínová Z, Škvarla J (2010) Enhancement of the bentonite properties. J Hazard Mater 180:274–281
Butkus D, Kleiza J (2011) Adsorption of 85Kr radioactive inert gas into hardening mixtures. J Radioanal Nucl Chem 287(1):247–254
Chmielewská-Horváthová E, Lesný J (1992) Adsorption of cesium and barium ions on Slovak natural zeolites. Geologica Carpathica Clays 1(1):47–50
Vajda N, Ghods-Esphahani A, Cooper E, Danesi PR (1992) Determination of radiostrontium in soil samples using a crown ether. J Radioanal Nucl Chem Articles 162:307–323
Hiller E, Jurkovič L’, Kordík J, Slaninka I, Jankulár M, Majzlan J, Gottlicher J, Steiniger R (2009) Arsenic mobility from anthropogenic impoundment sediments—consequences of contamination to biota, water and sediments, Poša, Eastern Slovakia. Appl Geochem 24(11):2175–2185
Kočiová M, Pipíška M, Horník M, Augustín J (2005) Bioaccumulation of radiocesium by lichen Hypogymnia physodes. Biologia 60:655–660
Pipíška M, Kočiová M, Horník M, Augustín J, Lesný J (2005) Influence of time, temperature, pH and inhibitors on bioaccumulation of radiocesium—137Cs by lichen Hypogymnia physodes. Nukleonika 50:29–37
Závodská L, Lesný J (2006) Comparison of Zn2+, Cd2+ and Sr2+ sorption characteristics for clinoptilolite and mordenite. In: Szilágyi M, Szentmihályi K (eds) Trace elements in the food chain: proceedings: international symposium on Trace elements in the food chain, Budapest, pp 135–139
Pipíška M, Vrtoch L, Horník M, Augustín J, Lesný J (2007) Uptake of 137Cs and 60Co by bryophytes and lichens. Cereal Res Commun 35:929–932
Horník M, Pipíška M, Kočiová M, Augustín J, Lesný J (2008) Influence of chelating agents on 60Co uptake by fresh water algae. Cereal Res Commun 36:419–422
Pipíška M, Horník M, Vrtoch L’, Augustín J, Lesný J (2008) Biosorption of Zn and Co ions by Evernia prunastri from single and binary metal solutions. Chem Ecol 24:181–190
Wallová G, Wallner G (2008) Isolation and measurement of strontium-90 and lead-210 in environmental samples using a strontium-specific resin and liquid scintillation counting. In: Eikenberg J, Ja¨ggi M, Beer H, Baehrle H (eds) International conference on advances in liquid scintillation spectrometry. Radiocarbon, Tuscon, Davos, Switzerland, pp 367–373
Hiller E, Jurkovič L’, Šutriepka M (2010) Metals in the surface sediments of selected water reservoirs, Slovakia. Bull Environ Contam Toxicol 84(5):635–640
Marešová J, Horník M, Pipíška M, Augustín J (2010) Sorption of Co2+, Zn2+, Cd2+ and Cs+ ions by activated sludge of sewage treatment plant. Nova Biotechnol 10–1:53–61
Tel H, Altas Y, Eral M, Sert S, Cetinkaya B, Ínan S (2010) Preparation of ZrO2 and ZrO2–TiO2 microspheres by the sol–gel method and an experimental design approach to their strontium adsorption behaviours. Chem Eng J 161:151–160
Wallová G, Acharya KK, Wallner G (2010) Determination of naturally occurring radionuclides in selected rocks from Hetaunda area, central Nepal. J Radioanal Nucl Chem 283:713–718
Vajda N, Kim Ch-K (2010) Determination of radiostrontium isotopes: a review of analytical methodology. Appl Radiat Isot 68(12):2306–2326
Yaron B, Dror I, Berkowitz B (2010) Contaminant geochemistry—a new perspective. Naturwissenschaften 97(1):1–17
Bear JJ, Cheng HDA (2010) Modeling contaminant transport. Theory Appl Transp Porous Media 1(23):341–523
Wallová G, Kandler N, Wallner G (2010) Determination of 90Sr and 210Pb in deer bone samples by liquid scintillation counting after ion-exchange procedures. J Radioanal Nucl Chem 286(2):429–433
Vrtoch L’, Pipíška M, Horník M, Augustín J, Lesný J (2010) Sorption of cesium from water solutions on potassium nickel hexacyanoferrate-modified Agaricus bisporus mushroom biomass. J Radioanal Nucl Chem. doi:10.1007/s10967-010-0837-5
Valderrama César (2010) Transport of strontium through a Ca-bentonite (Almería, Spain) and comparison with MX-80 Na-bentonite: experimental and modelling. Water Air Soil Pollut 214(1–4):1–8
Petrovič P, Sykorová J, Slosiariková H (1992) Sorption of vanadium(V) aqua complex on montmorillonite surface. Geologica Carpathica Clays 1(1):41–42
Hrobarikov J, Komadel P (2002) Sorption properties of reduced-charge montmorillonites. Geologica Carpathica Clays 53(2):93–98
Kufčáková J, Jesenák K, Rajec P et al (2003) Sorption of lead and cesium on bentonite and soils. Separation of ionic solutes. In: Abstracts of the 10th international conference SIS’03, Bratislava, 64 pp
Vejsada J, Jelínek E, Řanda Z, Hradil D, Přikryl R (2005) Sorption of cesium on smectite-rich clays from the Bohemian Massif (Czech Republic) and their mixtures with sand. Appl Radiat Isot 62:91–96
Vejsada J, Hradil D, Řanda Z, Jelínek E, Štulík K (2005) Adsorption of cesium on Czech smectite-rich clays—a comparative study. Appl Clay Sci 30(1):53–66
Vejsada J (2006) The uncertainties associated with the application of batch technique for distribution coefficients determination—a case study of cesium adsorption on four different bentonites. Appl Radiat Isotop 64(12):1538–1548
Klika Z, Kraus L, Vopálka D (2007) Cesium uptake from aqueous solutions by bentonite: a comparison of multicomponent sorption with ion-exchange models. Langmuir 23:1227–1233
Chmielewská E, Sabová L, Jesenák K (2008) Study of adsorption phenomena ongoing onto clinoptilolite with the immobilized interfaces. J Therm Anal Calorim 92(2):567–571
Orolínová Z, Mockovčiaková A, Vereš J (2008) Sorpcia t’ažkých kovov na kompozitných materiáloch bentonit-maghemit. Situácia v ekologicky zat’ažených regiónoch Slovenska a Strednej Európy, XVII. vedecké sympózium s medzinárodnou účast’ou, Hrádok, 23–24 október 2008, pp 63–67
Kruglov SV, Anisimov VS, Anisimova LN, Aleksakhin M (2008) Specific 137Cs-sorption capacity parameters of soils and mineral sorbents. Eurasian Soil Sci 41(6):608–617
Vereš J, Orolínová Z (2009) Study of the treated and magnetically modified bentonite as possible sorbents of heavy metals. Acta Montan Slovava 14(2):152–155
Galunin E, Alba DM, Aviles MA, Santos MJ, Vidal M (2009) Reversibility of La and Lu sorption onto smectites: implications for the design of engineered barriers in deep geological repositories. J Hazard Mater 172(2–3):1198–1205
Orolínová Z, Mockovčiaková A (2010) Effect of magnetic modification on the sorption properties of natural bentonite. In: Water treatment technologies for the removal of high-toxicity pollutants. NATO Science for Peace and Security Series C: Environmental Security, Earth Environ Sci, pp 295–300
Vieira MGA, Neto Almeida AF, Gimenes ML, da Silva MGC (2010) Sorption kinetics and equilibrium for the removal of nickel ions from aqueous phase on calcined Bofe bentonite clay. J Hazard Mater 177(1–3):362–371
Zhang W, Ding Y, Boyd SA, Teppen BJ, Li H (2010) Sorption and desorption of carbamazepine from water by smectite clays. Chemosphere 81(7):954–960
Ye WM, Chen YG, Chen B, Wang Q, Wang J (2010) Advances on the knowledge of the buffer/backfill properties of heavily-compacted GMZ bentonite. Eng Geol 116(1–2):12–20
Lee JO, Kang IM, Cho WJ (2010) Smectite alteration and its influence on the barrier properties of smectite clay for a repository. Appl Clay Sci 47(1–2):99–104
Vieira MGA, Neto Almeida AF, Gimenes ML, da Silva MGC (2010) Removal of nickel on Bofe bentonite calcined clay in porous bed. J Hazard Mater 176(1–3):109–118
Přikryl R, Weishauptová Z (2010) Hierarchical porosity of bentonite-based buffer and its modification due to increased temperature and hydration. Appl Clay Sci 47(1–2):163–170
Ouhadi VR, Yong RN, Goodarzi AR, Safari-Zanjani M (2010) Effect of temperature on the re-structuring of the microstructure and geo-environmental behaviour of smectite. Appl Clay Sci 47(1–2):2–9
Holzer L, Munch B, Rizzi M, Wepf R, Marschall P, Graule T (2010) 3D-microstructure analysis of hydrated bentonite with cryo-stabilized pore water. Appl Clay Sci 47(3–4):330–342
Lange K, Rowe RK, Jamieson H, Flemming RL, Lanzirotti A (2010) Characterization of geosynthetic clay liner bentonite using micro-analytical methods. Appl Geochem 25(7):1056–1069
Savage D, Watson C, Benbow S, Wilson J (2010) Modelling iron–bentonite interactions. Appl Clay Sci 47(1–2):91–98
Guerra DL, Oliveira HCP, Correa da Costa PC, Viana RR, Airoldi C (2010) Adsorption of chromium(VI) ions on Brazilian smectite: effect of contact time, pH, concentration, and calorimetric investigation. Catena 82(1):35–44
Galunin E, Alba MD, Santos MJ, Abräo T, Vidal M (2010) Lanthanide sorption on smectitic clays in presence of cement leachates. Geochim Cosmochim Acta 74(3):862–875
Jansson M, Jonsson M, Mohlén J (2010) Kinetic evaluation of sorption and desorption. Adsorption 16(3):155–159
Milyutin VV, Kononenk OA, Mikheev SV, Gelis VM (2010) Sorption of cesium on finely dispersed composite ferrocyanide sorbents. Radiochemistry 52(3):281–283
Hu J, Xie Z, He B, Sheng G, Chen Ch et al (2010) Sorption of Eu(III) on GMZ bentonite in the absence/presence of humic acid studied by batch and XAFS techniques. Sci China Chem 53(6):1420–1428
Palágyi Š, Štamberg K, Vidčková H (2010) Transport and sorption of 85Sr and 125I in crushed crystalline rocks under dynamic flow conditions. J Radioanal Nucl Chem 283(3):629–636
Vinšová H, Jedináková-Křížová V, Ožanová M (2009) Interaction of 99Tc onto bentonite and influence of Fe(II) and (Ca, Mg) ion exchange in its interlayers. J Radioanal Nucl Chem 281(1):75–78
Melichová Z, Hromada L, Brtáňová A (2010) Štúdium sorpčných vlastností bentonitu z ložiska Lieskovec. Acta Universitatis Matthiae Belli Ser Chem 12:15–23
Wang YQ, Fan QF, Li P, Zheng XB, Xu JZ, Jin YR, Wu WS (2011) The sorption of Eu(III) on calcareous soil: effects of pH, ionic strength, temperature, foreign ions and humic acid. J Radioanal Nucl Chem 287(1):231–237
Fajnor VŠ, Kuchta L’ (1995) Effect of degradation of montmorillonite by vibration grinding on the dTA curves in the range 20–1500 °C. J Therm Anal Calorim 45(3):481–489
Jesenák K, Fajnor V (1995) Distribúcia stopových prvkov v bentonite Stará Kremnička—Jelšový potok. Mineralia Slovaca 27(3):221–224
Fajnor VŠ, Jesenák K (1996) Differential thermal analysis of montmorillonite. J Therm Anal Calorim 46(2):489–493
Jesenák K, Kuchta L’, Guller L et al (1997) Physico-chemical properties of bentonite “Stará Kremnička—Jelšový potok” I: particle size distribution. Mineralia Slovaca 29(6):439–442
Stríček I, Šucha V, Uhlík P et al (2006) Zvetrávanie smektitu na ložiskách bentonitu. Mineralia Slovaca 38:337–342
Hrachová J, Vargová M, Fajnor VŠ (2007) Vysokoteplotná klasifikácia montmorillonitu “Kunipia”. ChemZi 3(1):188
Hrachová J, Billik P, Fajnor VŠ (2010) Influence of organic surfactants on structural stability of mechanochemically treated bentonite. J Therm Anal Colorim 101(1):161–168
Štyriaková I, Jablonovská K, Mockovčiaková A, Bekéniyová A, Štyriak I, Kraus I, Osacký M, Lovás M (2010) Dissolution of iron from quartz sands by basin bioleaching under static in situ conditions. Hydrometallurgy 104(3–4):443–447
Janek M, Lagaly G (2001) Proton saturation and rheological properties of smectite dispersions. Appl Clay Sci 19(1–6):121–130
Ji YQ, Black L, Weidler PG, Janek M (2004) Preparation of nanostructured materials by heterocoagulations interaction of montmorillonite with synthetic hematite particles. Langmuir 20:9796–9806
Zemanová M, Link G, Takayama S, Nuesch R, Janek M (2006) Modification of layer charge in smectites by microwaves. Appl Clay Sci 32:271–282
Janek M, Emmerich K, Heissler S, Nuesch R (2007) Thermally induced grafting reactions of ethylene glycol and glycerol intercalates of kaolinite. Chem Mater 19:684–693
Andrejkovičová S, Janotka I, Komadel P (2008) An investigation into the use of blends of two bentonites for geosynthetic clay liners. Appl Clay Sci 38(3–4):297–303
Andrejkovičová S, Rocha F, Janotka I, Komadel P (2008) An investigation into the use of blends of two bentonites for geosynthetic clay liners. Geotext Geomembr 26:436–445
Jóna E, Sapietová M, Šnircová S, Pajtášová M, Ondrušová D (2008) Characterization and thermal properties of Ni-exchanged montmorillonite with benzimidazole. J Therm Anal Calorim 94(1):69–73
Zitnan M, Szocs V, Janek M, Bugar I, Bdzoch J, Palszegi T, Link G, Velic D (2009) Fluorescence dynamics of coumarin C522 on reduced-charge montmorillonite in aqueous dispersion. Langmuir 25(12):6800–6807
Janek M, Bugár I, Lorenc D, Szocs V, Velič D, Chorvát D (2009) Terahertz time-domain spectroscopy of selected layered silicates. Clays Clay Miner 57(4):416–424
Janek M, Matejdes M, Szocs V, Bugár I, Gaál A, Velič D, Darmo J (2010) Dielectric properties of micaceous clays determined by terahertz time-domain spectroscopy. Philos Mag 90(17):2399–2413
Orolínová Z, Mockovčiaková A, Feldhoff A, Menzel D (2009) The role of iron oxides in composites with bentonite. Cheminé Technologija 1(50):42–46
Orolínová Z, Mockovčiaková A (2009) Structural study of bentonite/iron composites. Mater Chem Phys 14(2–3):956–961
Orolínová Z, Mockovčiaková A, Feldhoff A, Menzel D (2010) Influence of amount of Iron oxide and temperature of synthesis on their particle size in composites with bentonite. J Basic Princ Diffus Theory Exp Appl Diffus Fundam 12:80–81
L’aliková S, Pajtášová M, Ondrušová D, Bazyláková T, Olšovský M (2010) Thermal and spectral properties of natural bentonites and their applications as reinforced nanofillers in polymeric materials. J Therm Anal Calorim 100(3):745–749
Dillinger M, Valent A, Macašek F (1966) Sorpcia cézia (Cs-137) a stroncia (Sr-90) na bentnite z Fintíc (Sorption of radiocesium and radiostrontium on Fintice bentonite). Silikaty 180–184
Přikryl R, Ryndová T, Boháč J, Weishauptová Z (2003) Microsstructures and physical properties of “backfill” clays: comparison of residual and sedimentary montmorillonite clays. Appl Radiat Isot 23:149–156
Kolaříková I, Přikryl R, Hanus R, Jelínek E (2005) Thermal loading of smectite-rich rocks: natural processes vs. laboratory experiments. Appl Clay Sci 29:215–223
Stríček I, Šucha V, Uhlík P (2006) Weathering of smectite at Kopernica deposit. Acta Mineralogica-Petrographica. Abstract series, Szeged, 5, p 110
Jesenák K, Vargová M, Bakošová B (2006) Hydraulický odpor jemných frakcií bentonitu Stará Kremnička—Jelšový potok priemyselných a environmentálnych aplikácií fylosilikátov. Partikulárne látky vo vede, priemysle a v životnom prostredí, Košice, pp 96–101
Jesenák K, Vargová M, Bakošová B (2007) Hydraulický odpor ílových substrátov s vysokým obsahom montmorillitu. In: VIII. vedecká konferencia SF TU, Košice, pp 117–122
Pacovský J, Svoboda J, Zapletal L (2007) Saturation development in the bentonite barrier of the Mock-Up-CZ geotechnical experiment. Phys Chem Earth 32:116–122
Stríček I, Šucha V, Uhlík P (2008) Gamma-irradiation effects on smectite properties. In: 4th Mid-European clay conference, Zakopane, Poland, Abstracts, Mineralogia—Special papers, 33, p 157
Stríček I, Šucha V, Uhlík P (2008) Bentonite stability testing using the mock-up experiment, IX. International Geological Conference of Ph.D. Students and Young Scientists—Extended abstracts, Zawoja-Herl’any, 93
Stríček I, Šucha V, Uhlík P, Madejová J et al (2009) Mineral stability of Fe-rich bentonite in the Mock-Up-CZ experiment. Geologica Carpathica 60(5):431–436
Osacký M, Honty M, Madejová J, Bakas T, Šucha V (2009) Experimental interactions of Slovak bentonites with metallic iron. Geologica Carpathica 60(6):535–543
Honty M, De Craen M, Wang L, Madejová A, Czímerová A, Pentrák M, Stríček I, Van Geet M (2010) The effect of high pH alkaline solutions on the mineral stability of the Boom Clay–Batch experiments at 60 °C. Appl Geochem 25(6):825–840
Osacký M, Šucha V, Czímerová J, Madejová J (2010) Reaction of smectites with iron in a nitrogen atmosphere at 75 °C. Appl Clay Sci 50(2):237–244
Kolaříková I, Švandová J, Přikryl R, Vinšová H, Jedináková-Křižová V, Zeman J (2010) Mineralogical changes in bentonite barrier within Mock-Up-CZ experiment. Appl Clay Sci 47(1–2):10–15
Kaufhold S, Dohrmann R (2010) Effect of extensive drying on the cation exchange capacity of bentonites. Clay Miner 45(4):441–448
Ferníndez AM, Villar MV (2010) Geochemical behaviour of a bentonite barrier in the laboratory after up to 8 years of heating and hydration. Appl Geochem 25(6):809–824
Vokál A, Vopálka D, Večernák P (2010) An approach for acquiring data for description of diffusion in safety assessment of radioactive waste repositories. J Radioanal Nucl Chem 286(3):751–757
Andrejkovičová S, Janotka I, Komadel P (2008) Evaluation of geotechnical properties of bentonite from Lieskovec deposit, Slovakia. Appl Clay Sci 38(2–4):297
Vinšová H, Jedináková-Křížová V, Kolaříková I, Adamcová J, Přikryl R, Zeman J (2008) The influence of temperature and hydration on the sorption properties of bentonite. J Environ Radioact 99(2):415–425
Pedersen K (2000) Microbial processes in radioactive waste disposal. Technical Report TR-00-04, Swedish Nuclear Fuel and Waste Management Co., Stockholm, Sweden
Stroes-Gascoyne S, Lucht LM, Oscarson DW, Dixon DA, Hume HB, Miller SH (1998) Migration of bacteria in compacted clay-based material. Whiteshell Laboratories, Pinawa, Man
Groudev SN (1990) In: The 5th European congress on biotechnology: workshop on the aluminosilicate minerals biodegradation, Copenhagen, Denmark
Groudeva VI, Groudev SN (1984) Travaux ICSOBA Congress: Bauxite dressing by means of Bacillus cirkulans. Zagreb 13(8):257
Vašíček R, Hynková E (2003) Termofyzikální vlastnosti bentonitových směsí. Chem Listy 10P-07, 97:818
Rajec P, Mátel L, Orechovská J, Šúcha J, Novák I (1996) Sorption of radionuclides on inorganic sorbents. J Radioanal Nucl Chem Art 208(2):477–486
Shaban IS, Macášek F (1998) Influence of humic substances on sorption of cesium and strontium on montmorillonite. J Radioanal Nucl Chem 229(1–2):73–78
Macášek F, Shaban IS (1998) Cesium speciation in solid matrices and its specific ion adsorption by soils. J Radioanal Nucl Chem 229(1–2):79–83
Macášek F, Shaban SI, Mátel L (1999) Cesium, strontium, europium(III) and plutonium(IV) complexes with humic acid in solution and on montmorillonite surface. J Radioanal Nucl Chem 241(3):627–636
Kufčáková J, Galamboš M, Rajec P (2005) Sorption of strontium on selected group of bentonites. ChemZi 1(1):270
Galamboš M, Kufčáková J, Rajec P (2009) Sorption of strontium on Slovak bentonites. J Radioanal Nucl Chem 281(3):347–357
Galamboš M, Kufčáková J, Rajec P (2009) Adsorption of cesium on domestic bentonites. J Radioanal Nucl Chem 281(3):485–492
Galamboš M, Kufčáková J, Rosskopfová O, Rajec P (2010) Adsorption of cesium and strontium on natrified bentonites. J Radioanal Nucl Chem 283(3):803–813
Galamboš M, Paučová V, Kufčáková J, Rosskopfová O, Rajec P, Adamcová R (2010) Cesium sorption on bentonites and montmorillonite K10. J Radioanal Nucl Chem 284(1):55–64
Galamboš M, Rosskopfová O, Paučová V, Rajec P, Adamcová R (2010) Sorpčné vlastnosti bentonitových bariér. Bezpečnost jaderné energie 18(56)1/2:37–39
Galamboš M, Rosskopfová O, Rajec P (2011) Geotechnické kritéria na bentonitové bariéry. Bezpečnost jaderné energie 19(57)1/2:38–44
Reed DT, Scott DD, Weiner MF (1987) Gamma and alpha radiation levels in a basalt high-level waste repository: potential impact on container corrosion and packing properties. In: Tsang C (ed) Coupled processes associated with nuclear waste repositories. Academic Press, Orlando, pp 325–338
Kawano M, Tomita K (1991) Dehydration and rehydration of saponite and vermiculite. Clays Clay Miner 39(2):174–183
Dran J-C (1993) Radiation effects in radioactive waste storage materials. Solid State Phenom 30(31):367–378
Pusch R, Karnland O (1996) Physico/chemical stability of smectite clays. Eng Geol 41(1–4):73–85
Weber WJ, Ewing RC, Angell CA, Arnold GW, Cormack AN, Delaye JM, Griscom DL, Hobbs LW, Navrotsky A, Price DL, Stoneham AM, Weinberg MC (1997) Radiation effects in glasses used for immobilization of high-level waste and plutonium disposition. J Mater Res 12:1946–1978
Meunier A, Velde B, Griffault L (1998) The reactivity of bentonites: a review: an application to clay barrier stability for nuclear waste storage. Clay Miner 33:187–196
Weber WJ, Ewing RC, Catlow CRA, Diaz de la Rubia T, Hobbs LW, Kinoshita C, Matzke H, Motta AT, Nastazi M, Salje EHK, Vance ER, Zinkle SJ (1998) Radiation effects in crystalline ceramics for the immobilization of high-level nuclear waste and plutonium. J Mater Res 13(6):1434–1484
Allard T, Muller J (1998) Kaolinite as an in situ dosimeter for past radionuclide migration at the Earth’s surface. Appl Geochem 13(6):751–765
Pushkareva RA, Litovchenko AS, Plastinina MA, Pushkarev AV, Kalinichenko EA (1999) Infrared spectroscopic study of γ-radiation-induced hydrogen isotope exchange in clay minerals. Radiochemistry 41(6):599–603
Bray HJ, Redfern SAT (1999) Kinetics of dehydration of Ca-montmorillonite. Phys Chem Miner 26(7):591–600
Wang SX, Wang LM, Ewing RC (2000) Electron and ion irradiation of zeolites. J Nucl Mater 278:233–241
Karakassides MA, Gournis D, Ssmopoulos T, Petridis D (2000) Mössbauer and infrared study of heat-treated nontronite. Clays Clay Miner 48(1):68–74
Gournis D, Mantaka-Marketou AE, Karakassides MA, Petridis D (2000) Effect of γ-irradiation on clays and organoclays: a Mössbauer and XRD study. Phys Chem Miner 27:514–521
Gu BX, Wang LM, Minc LD, Ewing RC (2001) Temperature effects on the radiation stability and ion exchange capacity of smectites. J Nucl Mater 297:345–354
Gournis D, Mantaka-Marketou AE, Karakassides MA, Petridis D (2001) Ionizing radiation-induced defects in smectite clays. Phys Chem Miner 28:285–290
Negron A, Ramos S, Blumenfeld AL, Pacheco G, Fripiat JJ (2002) On the structural stability of montmorillonite submitted to heavy γ-irradiation. Clays Clay Miner 50:35–37
Pushkareva R, Kalinichenko E, Lytovchenko A, Pushkarev A, Kadochnikov V, Plastynina M (2002) Irradiation effect on physico-chemical properties of clay minerals. Appl Clay Sci 21:117–123
Plötze M, Kahr G, Hermanns SR (2003) Alteration of clay minerals—gamma irradiation effects on physicochemical properties. Appl Clay Sci 23:195–202
Wang LM, Chen J, Ewing RC (2004) Radiation and thermal effects on porous and layer structured materials as getters of radionuclides. Curr Opin Solid State Mater Sci 8(6):405–418
Farnan I, Cho H, Weber WJ (2007) Quantification of actinide alpha-radiation damage in minerals and ceramics. Nature 445(7124):190–193
Sorieul S, Allard T, Wang LM, Grambin-Lapeyre C, Lian J, Calas G, Ewing RC (2008) Radiation-stability of smectite. Environ Sci Technol 42(22):8407–8411
Allard T, Calas G (2009) Radiation effects on clay mineral properties. Appl Clay Sci 43:143–149
Fourdrin C, Allard T, Monnet I, Menguy N, Benedetti M, Calas G (2010) Effect of radiation-induced amorphization on smectite dissolution. Environ Sci Technol 44(7):2509–2514
Kudrnáčová I, Hynková E, Čechová Z (2003) Testování material na bázi bentonitu. Chem Listy 10P-06, 97:818
Hynková E, Sádovská G (2003) Thermal and calorimetric analyses of bentonites. Chem Listy 10P-04, 97:817
Čechová Z, Kudrnáčová I, Hynková E (2003) Research on strength parameters of bentonite. Chem Listy 10P-05, 97:817
Bakhmatyuk BP, Grygorchak II, Pidluzhna AY, Ripetskii EI (2007) Intercalation of bentonite: thermodynamics, kinetics, and practical applications. Inorg Mater 43(5):537–540
Vaughan DEW (1988) Pillared clays—a historical perspective. Catal Today 2:187–198
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galamboš, M., Rosskopfová, O., Kufčáková, J. et al. Utilization of Slovak bentonites in deposition of high-level radioactive waste and spent nuclear fuel. J Radioanal Nucl Chem 288, 765–777 (2011). https://doi.org/10.1007/s10967-011-0987-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-011-0987-0