Abstract
Extraction of uranium from Egyptian phosphoric acid with synergistic mixture of di-2-ethylhexylphosphoric acid (D2EHPA) and di-butyl butyl phosphonate (DBBP) is reported in this paper. The influence of various factors such as D2EHPA concentration, DBBP concentration, phosphoric acid concentration, contact time, aqueous: organic phase’s ratio (aq:org) and temperature on the degree of extraction has been established. The data on the effect of temperature on the extraction showed that the enthalpy change is −23.12 kJ/mol. Uranium extracted by D2EHPA–DBBP is further subjected to a second cycle of extraction and scrubbing impurities. The uranium is finally converted to a high purity UO3 product using precipitation with hydrogen peroxide and heat treatment at 375 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wet process phosphoric acid (WPA) derived from the dissolution of rock phosphate is an important secondary source of uranium. Exhaustive work in the field of solvent extraction has been carried out for the recovery of uranium from various industrial grades of phosphoric acid employing different synergistic extractant mixtures [1]. Approximately 13.000 tones of U3O8 could be recovered each year and there are many reasons that make the recovery of uranium from WPA attractive, especially in countries without conventional uranium ore bodies [2].At current global rate of consumption, phosphatic uranium can meet the global demand for 440 years as against a life of 86 years for known uranium resources [3]. Recovery from secondary sources is important for conservation of resources. Separation of uranium from fertilizer products also serves the objective of controlling its release into the human environment, including the food chain [4].
Solvent extraction alone has been found to be a successful process for industrial recovery of uranium from phosphates, although other methods such as ion-exchange [5], membrane separation [6] and precipitation [7] have been investigated. Synergistic mixtures of D2EHPA and TOPO or D2EHPA and DBBP have been reported as a suitable for extraction of uranium from WPA [8–10].
In the present work applied and kinetics study for the entrained solvent separation for D2EHPA + DBBP/WPA system has been described. The activation energy, enthalpy of activation and entropy of activation for uranium extraction from concentrated Egyptian phosphoric with D2EHPA + DBBP has been studied.
Experimental procedures
Solutions and reagents
The working phosphoric acid sample used in this study (44% P2O5) was provided by the Abu-Zaabal Co, Egypt. Its average chemical composition is shown in Table 1.
Prior to extraction, the acid was cooled down to a temperature of 40 °C, filtrated for removal of suspended solid particles, treated with activated carbon for removal of soluble organic matter, which is very important factor for the success of uranium recovery and finally oxidized with hydrogen peroxide till EMF >450 mV.
The di-(2-ethylhexyl) phosphoric acid (D2EHPA) used were obtained from indigenous sources while commercially available di-butyl butyl phosphonate (DBBP) of 95% purity were used for the work. The kerosene used was from MISR-Petroleum Ltd. Company, Egypt and had as main properties: specific gravity 0.8; flash point 70 °C; initial boiling point 200 °C; final boiling point 250 °C; aromatics <1%.
The extraction experiments were performed in 100 mL separating funnels, it is very important to note that no third phase or any precipitation was observed during the extraction process and uranium were analyzed in the aqueous phase and the content in the organic phase was calculated by difference. From latter values, the distribution coefficients D u were properly determined where,
Analytical procedures
Uranium was all the time analyzed in the different working aqueous phases by the ArsenazoIII method [11]. Absorbance of the formed uranium ArsenazoIII complex was measured at 650 nm against proper standard solutions. For this purpose, a Lambada3 UV/Vis spectrophotometer (Perkin-Elmer, USA) was used.
Results and discussion
Effect of shaking time
The effect of the shaking time on the uranium extraction efficiency from concentrated Egyptian phosphoric acid, 9.2 M, by 1.0 M D2EHPA + 0.5 M DBBP/kerosene was studied by performing another series of extraction experiments was performed using different shaking times ranging from 1 up to 6 min. In these experiments, the other extraction conditions were fixed at a V org/V aq = 1, T = 25 °C for various time intervals. Figure 1 shows the variation of uranium distribution coefficient (D u) against time. It is clear that 5 min is the minimum time to reach the equilibrium.
Effect of D2EHPA concentration on uranium extraction
In order to study the effect of D2EHPA concentration on the selective of uranium extraction efficiency from the Egyptian concentrated wet process phosphoric acid, a series of extraction experiments was performed using D2EHPA/Kerosene in various concentrations (0.5–2.5 M). In these experiments, the other extraction conditions were fixed at an V org/V aq of 1/1 and using 5 min. shaking time at room temperature. From the obtained results shown in Fig. 2, it is clearly obvious that the uranium distribution coefficient increase with increasing initial D2EHPA concentration.
Effect of DBBP concentration on uranium extraction at constant D2EHPA
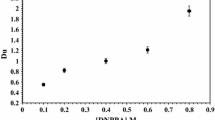
The synergistic effect of DBBP concentration on the extraction percent of uranium from concentrated Egyptian phosphoric acid, 9.2 M, has been investigated. A set of experiments were performed by shaking the treated phosphoric acid with DBBP having concentration ranging from (0.1–0.6 M) at constant D2EHPA concentration (1.0 M) and in V org/V aq ratio equal 1.0 for 5.0 min at room temperature (25 °C). The obtained results show that, the uranium distribution coefficient increase with increase DBBP concentration up to 0.5 M followed by slight increase at higher DBBP concentration. From these results, it is clear that DBBP has a good synergistic effect on the extraction of uranium from commercial concentration phosphoric acid. A plot of log D u versus log [DBBP] at constant D2EHPA concentration of 1.0 M is presented graphically in Fig. 3, shows a slope of ~1, which indicates that 1 mol uranium in organic phase is associated with 1 mol of DBBP.
Effect of D2EHPA concentration on uranium extraction at constant DBBP
To examine the effect of D2EHPA concentration on the extraction of uranium from 9.2 M phosphoric acid solution at constant 0.5 M DBBP concentration, equal volumes (25 mL) of D2EHPA and 0.5 M DBBP/kerosene and 9.2 M phosphoric acid were mixed together. The extraction was carried out by mixing the equilibrated D2EHPA and DBBP/kerosene solutions with the aqueous phases mentioned previously at V org/V aq = 1, DBBP = 0.5 M and T = 25 °C. The results represented in Fig. 4 shows that the uranium distribution coefficient (D u) increases with increasing the D2EHPA concentration.
At a constant D2EHPA/DBBP molar ratio of 2:1, the plot of log D u versus log [D2EHPA] is shown in Fig. 5 and indicates a linear relationship with slope ~1. This contrasts with the results on DNPPA–TBP system where second-order dependence has been observed [12].
Effect of organic/aqueous phase ratio
The effect of the V org/V aq ratio on uranium extraction from concentrated Egyptian phosphoric acid, 9.2 M, was investigated at V aq/V org ratio varying from 1.0 to 4.0. All experiments were performed using D2EHPA and DBBP mixture, 1.0 and 0.5 M, respectively, in kerosene for a mixing time of 5.0 min, T = 25 °C. From the obtained results shown in Table 2, it is clear that by increasing V aq/V org ratio the uranium extraction ratio was decreased.
This decrease can be related to possible increase of the solubility of the extractant in Egyptian phosphoric acid. Subsequently, a relative decrease in the distribution ratio is expected and deviation in the distribution ratio is noticed.
Effect of phosphoric acid concentration
The extraction of uranium from phosphoric acid in the range of (4.06, 5.79, 6.90 and 9.32 M) by 1.0 M D2EHPA + 0.5 M DBBP/kerosene at V org/V aq = 1/1, and at 25 °C was examined. From the obtained data plotted in Fig. 6, it can be shown that uranium distribution coefficient (D u) decrease with increasing the concentration of phosphoric acid.
The log–log plot of D u and phosphoric acid concentration is shown in Fig. 7. The linear relationship with slope of ~−2 indicates that 2 mol of acid are liberated for extraction of 1 mol of uranium. Based on the above findings, a plausible extraction equilibrium, ignoring the complexation by phosphoate ions UO2(H2PO 2−n4 ) n , is postulated as:
where (HX)2 is dimmer of D2EHPA. This is similar to the extraction of hexavalent uranium in D2EHPA–TOPO system [13, 14] and DOPPA–TOPO system [15].
Stability test of the extractant
In two parallel experiments, aliquots of 1.0 M D2EHPA + 0.5 M DBBP/kerosene were mixed with 9.2 M Egyptian phosphoric acid at room temperature and at 70 °C, respectively for 15 days. Samples of the organic phase were withdrawn at intervals and uranium extraction test was carried out. No detectable change in uranium distribution coefficient (D u) was found during this period indicating good stability of the solvent towards strong acid and temperature.
Effect of temperature on uranium extraction
The extraction of uranium from Egyptian concentrated phosphoric acid, 9.2 M, at different temperature was investigated. The extraction experiments were carried out by contacting phosphoric acid with a 1.0 M D2EHPA + 0.5 M DBBP/kerosene for 5 min while the V org/V aq ratio was fixed 1/2 but the temperature was varied.
The results are presented in Fig. 8 as a relation between temperature and uranium distribution coefficient (D u). From the obtained data, it can be noticed that D u is decreased by increasing the temperature which demonstrates the exothermic nature of the extraction process. Therefore the applied temperature was room temperature 25 °C. The effect of temperature on the distribution coefficient can be quantified by making use of the Vant Hoff equation, which relates the chemical equilibrium constant with temperature:
By integration,
And since the distribution ratio D is related by definition to the equilibrium constant K the previous equation can be written as:
The plot of log D u against 1/T yields a straight line equation with slope(x) = −∆H°/2.303R. Figure 9 shows that extraction uranium by 1.0 M D2EHPA + 0.5 M DBBP from Egyptian wet process phosphoric acid decrease with temperature. An enthalpy change of −23.12 kJ/mol was determined by using Eq. 5 in the given range of temperature, which indicates that the extraction is an exothermic process.
Graphical determination of the number of theoretical stages
At equilibrium, only partial transfer of metallic cations occurs. Thus several stages of contact should be used in order to recover the maximum values of these species. In this work, the McCabe–Thiele type construction is used to determine the number of theoretical stages required for achieving this separation. Figure 10 shows the graphical construction based on the 1.0 M D2EHPA + 0.5 M DBBP/kerosene mixture and an organic to aqueous volume ratio of 0.5 at 25 °C. As indicated in this figure, the number of theoretical stages required is three for uranium extraction from Egyptian wet process phosphoric acid.
Stripping of uranium from extract
Uranium stripping from the loaded extract using raffinate is generally preferred in the industry. The raffinate has been subjected to extensive pretreatment whereby ‘humic’ contamination from the phosphate rock has been reduced. However, the phosphate concentration in the raffinate is low and the acid can be used only after concentration by evaporation. Other efficient stripping reagents are ammonium carbonate, ammonium hydrogen fluoride and a HF–H2SO4 mixture. Hydrazine carbonate has also been recently reported as an effective stripping agent [16]; this has the advantage that no additional cations are introduced into the process. Reductive stripping with concentrated phosphoric acid is adopted for the present work since the resulting strip solution, concentrated in uranium, can form the feed for a second cycle of solvent extraction directed at obtaining a pure uranium product.
Uranium is stripped from loaded organic with pure phosphoric acid containing 10 g/L Fe2+ to reduce the less stripped uranium form (VI) to the more stripped uranium form (IV). The different parameters that affected in uranium stripping process were investigated (phosphoric acid concentration, temperature, contact time, V org/V aq phase ratio and the number of stages required for achieving high stripping of uranium from the loaded organic).
The preferred stripping results were found to be; pure phosphoric acid concentration: 8–10 M; temperature: 60–70 °C; contact time: 4 min; V org/V aq phase ratio is equal 20 and five stages were sufficient for stripping about 97.9% of total uranium in loaded organic. The loaded organic containing 366 mg/L U3O8 yielded a product solution containing 3.88 g/L U3O8.
Uranium recovery from strip solution
The strip solution obtained from the first cycle of extraction-stripping was found to contain uranium in a concentrated form, which, however, is not suitable for direct precipitation due to high level of impurities. The strip solution can be processed in a second cycle of extraction-stripping with an additional scrubbing step by sulfuric acid incorporated to obtain a uranium cake of high purity. An important factor in the selection of the solvent for the second cycle is the requirement of high selectivity with regard to the co-extracted rare earths, iron and phosphates. The extractant used in the second cycle was 0.3 M D2EHPA + 0.075 M TOPO, as per earlier reports [17].
From the loaded organic phase, uranium was stripped with 1 M ammonium carbonate solution. The strip liquor was filtered to remove traces of iron precipitate. The uranium tri-carbonate solution contained excess ammonium carbonate and pH was found to be 8.3. Uranium precipitation was carried out using H2O2. To bring down the pH of the solution, an addition of sulphuric acid to the solution with a slight excess of H2O2 was added after the solution had been filtered to remove the traces of iron hydroxide precipitate. The neutralisation was carried out with sulphuric acid. In a pH range of 3–4, the uranium precipitation was complete (<99%). Uranium peroxide hydrate was filtered, washed, dried and calcined at 375 °C to obtain UO3 powder with high purity.
Conclusion
The synergistic extractant D2EHPA + DBBP in kerosene as a diluent can be used for recovery of uranium from concentrated Egyptian phosphoric acid. The solvent is stable and an organic phase composed of 1.0 M D2EHPA and 0.5 M DBBP in kerosene as a diluent at room temperature for 5 min is optimal for simultaneous extraction of uranium. An extraction mechanism for uranium has been postulated based on the results of slope analysis. McCabe–Thiele type graphical construction indicated that the number of theoretical stages required for achieving the overall separation is three using an organic to aqueous volume ratio 0.5. High-purity uranium is recovered from the strip solution by a second cycle of extraction with D2EHPA–TOPO mixture where in scrubbing step has been incorporated and stripping is performed by an alkaline solution. From the resulting alkaline uranium solution, a precipitation process yields high purity uranium peroxide which is filtered, washed, dried and calcined at 375 °C.
Abbreviations
- D2EHPA:
-
Di-2-ethylhexylphosphoric acid
- DBBP:
-
Di-butyl butyl phosphonate
- ∆H :
-
Enthalpy (kJ/mol)
- WPA:
-
Wet process phosphoric acid
- TOPO:
-
Tri-octyl phosphine oxide
- D u :
-
Distribution coefficient
- DOPPA:
-
Di-octyl phenyl phosphoric acid
- DNPPA:
-
Di-nonyl phenyl phosphoric acid
- TBP:
-
Tri-butyl phosphate
- R :
-
Universal gas constant (8.314 J/K mol)
- T :
-
Absolute temperature (K)
- EMF:
-
Electromotive force
- K :
-
Equilibrium constant
- Vorg/Vaq:
-
Volume ratio between organic phase and aqueous phase
References
Gupta CK, Singh H (2003) Uranium resource processing: secondary resources. Springer Verlag, Berlin
Botella T, Gasos P (1989) Recovery of uranium from phosphoric acid: an overview. IAEA-Tecdoc 533, p 69
Nuclear Energy Agency and IAEA, OECD (1999) Environmental activities in uranium mining and milling. Report by OECD, p 29
Haridasan PP, Paul AC, Desai MVM (2001) Natural radionuclides in the aquatic environment of a phosphogypsum disposal area. J Environ Radioact 53:155–165
Kabay N, Demiricioglu M, Yayh S, Gunay E, Yuksel M (1998) Recovery of uranium from phosphoric acid solutions using chelating ion-exchange resins. Ind Eng Chem Res 37:1983–1990
Joshi JM, Pathak PN, Pandey AK, Manchanda VK (2009) Study on synergistic carriers facilitated transport of uranium(VI) and europium(III) across supported liquid membrane from phosphoric acid media. Hydrometallurgy 96:117–122
Weterings K, Jansen J (1985) Recovery of uranium, vanadium, yttrium and rare earths from phosphoric acid by a precipitation method. Hydrometallurgy 15:173–190
Derry R (1981) The Recovery of uranium form phosphatic sources in relation to the EEC (EUR7324EN). Commission of the European Communities, Directorate General for Research, Science and Education, Brussels
Hurst FJ (1989) Recovery of uranium from phosphates: current status and trend. IAEA-Tecdoc 533, pp 9–17
Nazari K, Maragheh MG (2000) Recovery of uranium from phosphoric acid by liquid–liquid solvent extraction. In: 4th international and 5th national chemical engineering congress, Shiraz University, Shiraz, Iran, 24–27 April 2000, pp 2(1)–2(11)
Marczenko Z (1986) Spectrophotometric determination of the elements. Wiley, New York
Singh H, Vijaylakshmi R, Mishra SL (2004) Uranium recovery from phosphoric acid by solvent extraction using a synergistic mixture of di-nonyl phenyl phosphoric acid and tri-n-butyl phosphoate. Hydrometallurgy 73:63–70
Bunus FT, Domocos VC, Dumitrescu P (1978) Synergic extraction of uranium from phosphate solution with di-2-ethyl hexyl phosphoric acid and tri-n-octyl phosphine oxide. J Inorg Nucl Chem 40(1):117–121
Girgin S, Acarkan N, Sirkeci AA (2002) The uranium(VI) extraction mechanism of D2EHPA–TOPO from a wet process phosphoric acid. J Inorg Nucl Chem 251(2):263–271
Krea M, Khalaf H (2000) Liquid–liquid extraction of uranium and lanthanides from phosphoric acid using a synergistic DOPPA–TOPO mixture. Hydrometallurgy 58(3):215–225
Watanbe M, Tatsugae R, Shirahashi K, Morita Y, Kubota M (2001) Back-extraction of uranium(VI) from organophosphoric acid with hydrazine carbonate. J Radioanal Nucl Chem 150(2):377–379
Singh H, Vijaylakshmi R, Mishra SL, Gupta CK (2001) Studies on uranium extraction from phosphoric acid using di-nonyl phenyl phosphoric acid based synergistic mixtures. Hydrometallurgy 59:67–76
Acknowledgment
The authors wish to thank Dr. H. S. Gado Head of phosphoric acid pilot plant for his valuable help in carrying out the test work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Abdel-Khalek, A.A., Ali, M.M., Hussein, A.E.M. et al. Liquid–liquid extraction of uranium from Egyptian phosphoric acid using a synergistic D2EHPA–DBBP mixture. J Radioanal Nucl Chem 288, 1–7 (2011). https://doi.org/10.1007/s10967-010-0964-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-010-0964-z