Abstract
Hydration of zirconium diphosphate (ZrP2O7) conduced to formation of active sites in solid/liquid interface. In ZrP2O7/NaClO4 0.5 M suspensions, active sites and their acidity constants are quite determined but the presence of some impurities is now studied. This work was conducted to determine the surface properties changes produced by oxalic and citric acid during the hydration process. Moreover the presence of organic acids with ZrP2O7 modified reveals an increase in uranium sorption constants. The zirconium diphosphate has been characterized using X-ray powder diffraction (XRD), Scanning electron microscopy (SEM) and Particle induced X-ray emission and Neutron (PIXE). Furthermore, the specific surface area, measured by the BET method, was 3.5 m2/g. The pH corresponding to the isoelectric point, determined by Zeta Potential measurements and mass titration was 3.6. The sites density calculated using titration curves was around of 5.37 s/nm2 for NaClO4 0.5 M, 13.71 s/nm2 for NaClO4 0.5 M/citric acid 0.1 M and 7.33 s/nm2 NaClO4 0.5 M/oxalic acid 0.1 M. The surface acidity constants and species distribution in surface were calculated by means of simulation of the titration curves with the FITEQL code (constant capacitance model), for ZrO and PO amphoteric sites of ZrP2O7. The uranyl sorption edge was determined for NaClO4 0.5 M. It spreads between pH 3 and 4.5 for complete sorption according to the previously published results. In the ZrP2O7–citrate modified surface, the uranyl sorption edge begin at pH 2 and was almost complete at pH 3.2 while ZrP2O7–oxalate modified surface edge started at 50% of sorption at pH of 1.5 and was complete at pH 3.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The countries operating nuclear power plants to supply electricity at low prices are very worried about the nuclear wastes produced during the plant operation. Up to now only a temporal storage disposals are operating worldwide, nevertheless, the long term solution is in progress, and, most of the specialists agree that the storage of high-level nuclear waste in deep geological repositories is a safe and final solution [1, 2].
Therefore, it is desirable to improve the chemical characteristics of the engineering barriers surrounding the engineering facilities in which uranium wastes are contained [3, 4]. Permeable reactive barriers have attracted much attention because of their potential in situ treatment of many groundwater contaminants. Uranium typically occurs in the hexavalent form as the mobile aqueous uranyl ion (UO2 2+) [5–7]. Phosphate compounds are expected to play an important role in the safety of underground radwaste repositories because they could be used as a fundamental part of the engineered barriers [1, 8, 9].
Modeling the radioelement migration has been done by many authors, but the effects of organic matter over materials forming engineered barriers have not been entirely studied by now [10].
The reason to study the organic mater is that they form active parts of the barriers constitution. The humic and fulvic acids are very heavy molecules, in which formation of –OH or –COOH ions giving to the molecule a specific cations sorption, forming stable complexes [11].
The decomposition process of humus conduces to smallest molecules, some of them retain –OH or –COOH ions, but the low molecular weight give high solubility and can migrate trough the soil with water streams The surface site density has been mainly characterized in inorganic ground salts, but the effect of organic matter in solution is also of interest because the humic and fulvic acids in ground waters can be present in low concentrations. Due to degradation process, low molecular weight products are produced and their presence may affect the solid/liquid interface. In basis to understand how light organic molecules can modified surface properties, di and tri carboxylic acids were used in this study [12, 13].
This study deals with the surface modification of the zirconium diphosphate (ZrP2O7) with polycarboxylic acids (citric and oxalic). During the hydration process of ZrP2O7 some preferential amphoteric species are formed at the interface solid/liquid that are considered as “active sites” that can sorbed different cations presents in solution. The acidity constants of the substrate are determined by acid–base titration of a well defined suspension, using FITEQL4.0 simulation code (constant capacitance model) to establish the amphoteric species in the distribution diagrams of interface [14, 15]. Moreover, this paper presents the effect of the organic acid on the sorption of uranyl (UO2 2+) ion onto the ZrP2O7 surface.
Materials and methods
Materials
The synthesis of the ZrP2O7 was reported by Drot et al. [16], nevertheless a little improvement was performed in this process. The synthesis was conducted using a chemical condensation reaction of ZrCl4 diluted in fuming HCl, then dropped over H3PO4 (at 130 °C), the mole ratio Zr/PO4.was maintained around of 1:2 with a little excess of H3PO4. After 4 hours of stirring the compound was formed and water was added to dilute H3PO4 excess and to remove the HCl released during the reaction, then, α-Zr(HPO4)2 was separated by centrifugation, washed and finally, calcinated at 800°C for 6 h to obtain the ZrP2O7 [17–19].
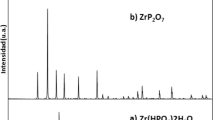
The zirconium diphosphate was characterized by scanning electron microscopy in a PHILIPS model XL-30, for size homogeneity and shape of grains. The X-ray powder diffraction patterns were recorded in a SIEMENS model D500 diffractometer, using CuKα rays (λ = 1.5404 ) matching with the card No. 29-1399 reported by the Joint Committee of Powder Diffraction Standard (JCPDS). No secondary phase was observed in this pattern as shown in Fig. 1. Crystalline diffraction pattern from the transmission electronic microscopy technique showed a polycrystalline material, with good agreement with lattice parameters to ensure the nature and purity of ZrP2O7.
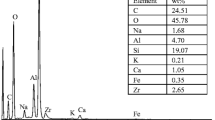
Analysis techniques as particle induced X-ray emission (PIXE) and neutron activation analysis (NAA) [20–22] were used to carry out a very sensitive qualitative and quantitative multi-element analysis. The results showed the presence of zirconium, phosphor and oxygen as major elements, according with the stoichiometric formula, hafnium as minor element and some traces of lanthanum. The rare earth elements are found normally in igneous or metamorphic zircon, which were overlapped in zircon structure during its geological formation, but the element nature and its content are not constant in samples [23]. Then, the ZrCl4 can content some of them during the reagent fabrication.
The specific surface area was determinate from N2 adsorption isotherm, using the BET multipoint method [24] whit a Micromeritics Gemini 2360 apparatus, the specific surface area obtained to this material was 3.5 m2/g.
Methods
Mass titrations
Mass titrations experiments were performed in order to calculate the pH at isoelectric point (pHPIE) for suspensions of ZrP2O7 powder in NaClO4 0.5 m. Increasing quantities of solid were added to 10 mL of a 0.5 M sodium perchlorate solution: 0.1, 1, 2, 5, 10, 15 and 20 wt%. The suspensions were shaken at room temperature for 24 h, after this time equilibrium was reached, then centrifuged and pH of the solution was measured. Sodium perchlorate was considered as supporting electrolyte as it is well established that there is no specific sorption of Na+ and ClO− ions. In such conditions, the isoelectric point (pHIEP) is identical to the point of zero charge (pHZPC) as is usually named [25].
Potentiometric titrations
The potentiometric titration experiments were conducted at 30 °C in a Radiometer Titralab 90 attached to an ABU901 autoburte, under argon atmosphere with continuous stirring to avoid settling. Prior to experiments, 3 sets of solid were hydrated by shaking for 24 h in NaClO4 0.5 M, citric acid 0.1 M and oxalic acid 0.1 M to reach surface equilibrium. Then the samples were centrifuged to remove the supernatant and new fresh NaClO4 0.5 M solution was added. These suspensions were acidified to start titration at low pH value to avoid atmospheric CO2 effect. The potentiometric titrations were carried out with 100 gL−1 aqueous suspensions of solid in studied media. HClO4 and NaOH 0.1 M were used as titrant solutions. Moreover, blanks were also titrated with background electrolyte solutions in similar conditions.
The acid base titration curves were used to estimate the surface site concentration. Comparing the curves of suspensions to corresponding blank allows to determine the proton or hydroxide consumption by the protonated or deprotonated surface sites. The initial linear part of the curves represents the total number of proton added versus the total number of proton in solution. Once the surface site saturation is reached a linear evolution is observed, with equal slope for both suspension and blank solutions [26].
Sorption procedure
The uranium (VI) stock solutions were prepared by dissolving a weighed amount of uranyl nitrate in hot perchloric acid to produce the uranyl perchlorate, then NaClO4 0.5 M solution was added to obtain an 1.0 × 10−2 M uranium solution. The uranium concentration in all studied batches was determined by direct fluorescence analysis of remaining solutions in the Fluorolog3-22 Horiba Jobin–Yvon spectrofluorimeter and compared to standard solutions.
The uranyl sorption experiments were carried out in batch mode, using 15 mL polypropylene centrifuge tubes. Prior to experiments, hydration process of the powder surface was done. Suspensions of 100 mg of ZrP2O7 onto 10 test solutions (NaClO4 0.5 M, citric acid 0.1 M, oxalic acid 0.1 M) were shaken for 24 h at pHIEP. Then, the suspensions were centrifuged to separate the remaining solution. Then 10 mL of fresh UO2(ClO4)2 5 × 10−4 M solutions were added at desired pH value, adjusted with HClO4 or NaOH 0.1 M solutions and shaken again for 24 h at room temperature. Then, it was assumed that the sorption equilibrium was reached and no more reaction was produced. After centrifugation at 3000 rpm for 15 min, the final equilibrium pH value of supernatant was measured and the uptake of uranyl ions was determined as the difference between the initial and final uranium concentrations in solution, determined by means of fluorescence method.
The modeling of both potentiometric titration and of the sorption edges was performed using the FITEQL 4 code [27] and the constant capacitance model (CCM).
Result and discussion
Surface characterization
As the origin of surface charge, developed in solids by the action of background salt solution, depends on surface characteristics due to crystallographic and surface properties, surface characterization is mandatory to calculate acidity constants, surface repartition species and sorption edges.
The surface active sites (≡SOH) are created during the hydration of minerals in solutions when reacting with acid or base to obtain protonation or deprotonation on surface; this process can be described by modeling the acidity constants equilibria as follows.
where F is the Faraday constant, ψ the surface electrostatic potential, R the ideal gas constant, and T temperature expressed in Kelvin. K a and K b acid and basic constants. This model considers a low number of constraints and it is commonly used for rather high ionic strength conditions.
Modeling to estimate the acidity constants were done to assess the type of reaction that occurs on the surface of ZrP2O7 expressed as follows:
The results of the surface characterization are shown in Table 1.
The modification of the ZrP2O7 surface with polycarboxylic acids (oxalic acid and citric acid) may involve the formation of complexes between the carboxyl groups and the phosphorus oxygen zirconium groups (Zr–O–P) of ZrP2O7. The acidity constants in carboxylic media were quite different to the ZrP2O7/NaClO4 one. This material can dissociate with two types of surface sites. One of them is more reactive and the other one can not dissociate easily depending on the hydration time and the nature of ground salt solution.
The Table 2 shows the values of the acidity constants and concentrations of different types of surface sites that are formed at the interface solid/liquid of different hydration systems in hydroxylated and organic acid modified surfaces.
The acidity constant does not change with the presence of organic acid. The surface sites (determined by the solid) are the same. But the acidity behavior of the surface may change because there are others acids (organic acid) sorbed on the surface. The organic acid sorption should be explained as the uranyl sorption and not with a change of the acid–base K values.
The species distribution diagram (Fig. 2) on the surface of ZrP2O7, show the variation of the different surface species sites with the pH. The Fig. 3 shows that the species that dominate in solution at pH 2 are \( {\text{POH}}_{2}^{ + } \) and \( {\text{ZrOH}}_{2}^{ + } \), at pH range 4–8, species that are prevalent are POH and ZrOH, finally PO− and ZrO− are found (in graphs X = ZrO and Y = PO). The neutral species “POH” has a higher concentration as the “ZrOH”; this indicates that the adsorption of uranyl will be in this type of site and the minority species maintains the system in balance.
The hydration systems formed by suspension of ZrP2O7/citric acid (0.1 M) and ZrP2O7/oxalic acid (0.1 M) shown a change in the ZrP2O7/NaClO4 acidity constants values, which indicates that the surface of ZrP2O7 was modified depending on the sorbed organic acid on surface sites during the hydration process.
Moreover, the species diagrams of distribution show that there is an increase in one of the neutral species, according to the pH in the process of titration. The Fig. 3, shows that the predominant species in solid/liquid interface spreads from pH 2 to 10.
In both systems, the species ZrO reacted with organic acids had higher concentration than the other species, this indicates that adsorption of the uranyl will be in these type of complexant species and the minority species maintains the system in balance.
Sorption of uranyl ions on ZrP2O7
The uranium sorption experiments over the ZrP2O7/NaClO4 suspension can be described by the sorption equilibrium as follows [28]:
where n is the number of solid surface sites bound to the uranyl ion. {U.(VI)}.x represents an uranyl species (like \({{\text{UO}}_{2}}^{2 + },\,\mathop {\text{UO}}\nolimits_{ 2} {({\text{OH}})}^{ + } ,\,({\text{UO}}_{2} )_{3} ({{\text{OH}})_{5}}^{ + } ,\) etc.). x is the electric charge of uranyl species. p is the number of protons released during the sorption reaction.
The surface sites developed in presence of organic acids may follows the same general reaction for hydroxyl groups formation in surface, but the organic molecules in solution react with active sites resulting the formation of surface complexes by mechanisms of ligand exchange of surface hydroxyl for the carboxyl group, or by uranyl sorption on the sorbed organic group. The strength of sorption is determined mainly by the degree of dissociation of carbonyl compound and by the position of these groups relative to each other onto surface metal oxide surfaces [29].
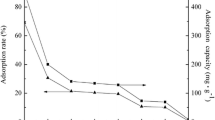
The uranyl sorption edge on ZrP2O7/NaClO4 suspension spreads between pH = 1.5 and 6, the uptake of uranyl was started at 10%, reaching up to 80% at pH 3, and raises again to 100% at pH 6; this fact suggest that bidentate uranium complex is formed with both ≡Zr−O and ≡P–O sites along the pH range, as previously reported [28]. The modification of ZrP2O7 implies that organic acids are present only in surface, because acid solutions were removed after hydration procedure and not more organic acid were present in test solutions. In citric acid modified ZrP2O7, the uranyl sorption at initial time was near of 10% reaching to 80% of sorption at pH 4.5, the final 100% uptake was observed at pH 6, the uranyl–citrate complex species may be responsible of this fact. Finally, in oxalic acid modified ZrP2O7, the initial uranyl uptake in ZrP2O7 was observed immediately in a range of 50%, rising up to 80% adsorption at pH 3, and tends to 100% at pH 6. It may be formed uranyl–oxalate complex species formed in surface (Fig. 4). Sorption reactions at the mineral water interface are one of the types of reactions by which radioisotopes, as UO2 2+, may be immobilized in the ZrP2O7 surface. Previous research indicates that phosphate compounds are strong complexing agents, and among them, the zirconium diphosphate is easily synthesized as a single phase compound. The presence of two different kinds of surface sites have been identified, the ≡Zr−O and the ≡P–O sites. Uranyl sorption test have been carried out using dilute aqueous concentration of ions, ranging from 6.1 to 9.6 × 10 −5 M using 8.75 g of zirconium diphosphate per litre. Maximum sorption occurs at pH 3 and above, as shown in Fig. 4. Then the reaction order was: oxalic acid modified ZrP2O7 > citric acid modified ZrP2O7 > NaClO4–ZrP2O7.
References
Guillaumont R (1994) Radiochemical approaches to the migration of elements from a radwaste repository. Radiochim Acta 66(67):231–242
De Marsily G (1988) Radionuclide migration in the geosphere: an overview Radiochim Acta 44/45:159
Powell RM Puls RW, Blowes DW, Vogan JL, Gillham RW, Schultz D, Powell PP, Sivavic T, Landis R (1998) Permable reactive barrier technologies for contaminant remediation. Technical report, EPA/600/R-98/125. Office of Research and Development, Office of Solid Waste and Emergency Response, U.S. EPA, Washington, DC
Simon FG, Meggyes T (2000) Removal of organic and inorganic pollutants from groundwater using permeable reactive barriers. Pub Land Contam Reclam 8:103–116
Drot R, Simoni E (1999) Uranium(VI) and europium(III) speciation at the phosphate compounds-solution interface. Langmuir 15:4820–4827
Drot R, Simoni E, Alnot M, Ehrhardt JJ (1998) Structural environment of uranium (VI) and europium (III) species sorbed onto phosphate surfaces: XPS and optical spectroscopy studies. J Colloid Interface Sci 205:410–416
Hering JG, Kraemer S (1994) Kinetics of complexation reactions at surfaces and in solution: implications for enhanced radionuclide migration. Radiochim Acta 66/67:63
Finck N, Drot R, Lagarde G, Mercier-Bion F, Catalette H, Simoni E (2008) Temperature effects on the interaction mechanisms between U(VI) and Eu(III) and ZrP2O7: experiment and modeling. Radiochim Acta 96:11–21
Finck N, Drot R, Mercier-Bion F, Simoni E, Catalette H (2007) Temperature effects on the surface acidity properties of zirconium diphosphate. J Colloid Interface Sci 312:230–236
Moulin V (2005) Complexation of radionuclides with humic substances. In: Use of humic substances to remediate polluted environments: from theory to practice, Vol 7, pp 155–173
Institut für Nucleare Entsorgung (2000) Influence of humic acids on the migration behaviour of radioactive and non-radioactive substances under conditions close to nature: Metal-Ion behaviour in water/mineral system. Final Report
Perminova IV, Hatfield K (2005) Remediation chemistry of humic substances: theory and implications for technology. In: Use of humic substances to remediate polluted environments: From Theory to Practice, Vol 1, pp 3–36
Makhfouk ME, Meray ME, Castetbon A, Astruc M (2002) Effect of citrate, oxalate and pyrophosphate ligands on the electrochemical reduction of the uranyl ion in perchloric acid medium. Bull Electrochem 18(2):63–70
Tadanier CJ, Matthew JE (2002) Formulating the charge-distribution multisite surface complexation model using FITEQL. Soil Sci Soc Am J 66:1505–1517
Davis JA, Kent DB (1990) Surface complexation modeling in aqueous geochemistry. In Hochella MF Jr, White AF (eds) Review in mineralogy: mineral water interface geochemistry, Vol 23. Mineralogical Society Americana, Washington, DC, pp 177–260
Drot R, Lindecker C, Fourest B, Simoni E (1998) Surface characterization of zirconium and thorium phosphate compounds. New J Chem 22:1105–1109
Dacheux N, Brandel V, Genet M (2001) Studies on the chemistry of uranium and thorium phosphates. Thorium phosphate diphosphate: a matrix for storage of radioactive wastes. Radiochemistry 43(1):16–24
Masse R, Grenier J-C (1971) Bull Soc Fr Min Cristallogr 94:437
Bjorklund CW (1958) Solid-state synthesis of monazite-type compounds. J Am Chem Soc 79:6347
Markert B (1996) Instrumental element and multi-element analysis of plant samples, methods and applications. Willey & Sons, New York
Woldseth R (1973) X-ray energy spectrometry. Kevex Corporation, Burlingame, California
Friedlander G (1981) Nuclear and radiochemistry. Wiley, New York, pp 208–209
Whitehouse MJ (2003) Geochronology: linking the isotopic record with petrology and textures. Geological Society of London, pp 50
Brunauer S, Emmett PH, Teller E (1938) Adsorption of gases in multimolecular layers. J Am Chem Soc 60:309–319
Noh JS, Schwarz JA (1989) Estimation of the point of zero charge of simple oxides by mass titration. J Colloid Interface Sci 130:157–164
Nagy NM, Kónya J (2007) Study of pH-dependant charges of soils by surface acid-base properties. J Colloid Interface Sci 305:94–100
Herbelin AL, Westall JC (1996) FITEQL: a program for the determination of chemical equilibrium constants from experimental data. Report 96-01. Department of Chemistry, Oregon State University, Corvallis
Lomenech C, Drot R, Simoni E (2003) Speciation of uranium (VI) at the solid/solution interface: sorption modeling on zirconium silicate and zirconium oxide. Radiochim Acta 91:453–461
Balche GU, Georgi A, Woszidlo S, Kopinke FD, Poerchmann J (2005) Utilization of immobilized humic organic matter for in situ subsurface remediation. In: Perminova IV et al (eds) Use of humic substances to remediate polluted environments. Springer, Netherlands, pp 203–232
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Garcia-Gonzalez, N., Ordóñez-Regil, E., Simoni, E. et al. Effect of organic acids on sorption of uranyl ions in solution onto ZrP2O7 . J Radioanal Nucl Chem 283, 409–415 (2010). https://doi.org/10.1007/s10967-009-0406-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-009-0406-y