Abstract
The development of self-healing polymeric materials was inspired by biological systems wherein damage initiates an autonomic healing response and can automatically repair the internal cracks/ damages without the need for external intervention. This is a new and fascinating field of research that has the potential to improve service life of the materials. These have attracted the attention of many scientists/researchers due to their wide range of applications. The self-healing materials have been broadly classified in to two categories: (1) extrinsic self-healing materials, wherein, the repairing agent is pre-embedded in to the resin matrix and no human involvement is needed to start the healing process, and (2) intrinsic self-healing materials, which do not have an embedded healing agent and an external-stimuli is essential to initiate the healing process. The current review article summarizes a state-of-art in terms of self-healing ability of the polymeric materials. It also provides comprehensive comparison of healing efficiencies, advantages and challenges for the future development, and potential applications of such materials in numerous fields, such as aerospace, coatings and paints, electronics energy, etc.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Polymeric materials and polymer composites have a widespread range of applications in engineering fields since these possess the advantages of good processibility, lightweight and chemical stability. For structural applications, long-term durability and reliability becomes a prerequisite for polymeric materials. Exposure of such materials to astringent environmental conditions may lead to damage at micro-level (scratches) which may further progress on a meso-level (micro-cracks) and ultimately cause a macro-level damage [1]. Recyclability and frequent use are the quintessential requirements in polymeric materials. In the present scenario, with the evolution of technologies, the human desire to develop materials that could possess properties such as reliability, quality, and service lifetime has channeled the efforts of the scientist to explore the avenue of self-repairing in such materials. These materials are inspired by biological systems and are presumed to replicate the ability of biotic materials to enclose the wound and prevent it from dehydration and infection by microorganisms and germs, and can also further provide time for subsequent repair of the injury along with wound closure and healing. The self-healing techniques are designed to provide the proficiency to seize the crack reproduction at initial stages. In case of small repairs, no manual interference and no detection of the site of damage is needed. The ability to self-heal not only reestablishes structural integrity but also can manage the structural life by repairing at the right site and at the right time. This can bring down the maintenance cost as well as eliminate the risk of catastrophic collapse of the structure [2].
Nearly all kinds of polymers such as elastomers [3,4,5], thermoplastics [6,7,8,9,10], and thermosets [11] can be designed to possess the self-healing ability. A scratch in case of thermoplastic polymers can be healed simply via heating the polymer above its glass transition temperature. The mechanism of the healing process in such case goes through five phases i.e., surface rearrangement, surface approach, wetting, molecular inter-diffusion, and surface randomization [12]. Thermoplastics, however, due to thermal instability and low stiffness, have limited structural applications. Thermosetting polymers on the other hand are most widely used in coatings, lightweight, structural, and high-end price applications since these possess good mechanical properties as well as good corrosion resistance. These polymers like any other materials are also liable to damage on exposure to various environmental conditions. Moreover, the cracks formed in case of thermosets can’t be healed easily by visco-elastic reflow. Thus, the self-mending of thermosets has become topic of interest these days for scientists and much attention is being paid to design different routes for the same.
Self-healing may be autonomic or non-autonomic. Autonomic self-repairing takes place without any external intervention and non-autonomic healing requires human involvement or external trigger. Depending upon the type of healing, the self-healing polymers are classified into two main categories: Extrinsic and intrinsic self-healing. In both the cases, the presence of a mobile phase that could fill the crack during healing is a prerequisite. This mobile phase can be made available, in case of intrinsic self-healing, through the presence of reversible covalent bonds or, by the flow of incorporated catalyst or thermoplastic additives, for extrinsic self-healing. Apart from these techniques few articles also report the introduction of a secondary phase which can impart self-healing ability and impact the engineering performance of such material [13, 14].
The metal surfaces are secured from exposure to harsh environments and corrosion by applying a thin barrier layer of polymeric coating materials on their surfaces. The damages on the micro-level are formed under the surface of the coating material where it becomes difficult to perceive and repair these damages in the initial stages. These may proliferate until the coating material weakens and breaks, resulting in undesired modifications and a reduction in performance. The self-healing coatings can safeguard the underlying substrate by providing unaided reconstruction of the damaged coating. The balance between the rate of destruction and the rate of repair regulates the effectiveness of healing. Recent article by Yuan et al. [15] reports the synthesis of a coating material that possesses self-healing ability and exhibits polymerization induced phase separation (PIPS) morphology to supervise the repair and mechanical properties of the coatings. They synthesized a bio-based epoxy resin by mixing n-alkyl esters of diphenolic acid (DGEDP) with thermoplastic polyurethane (TPU) prepolymer. The molecular weight of TPU prepolymer was used to regulate the extent of phase separation. At the time of curing, the TPU prepolymers having lower molecular weights can phase mix with epoxy, resulting in a relatively flexible epoxy network. The mobility of TPU prepolymer was reported due to lower epoxy network crosslinking. The mechanical performance along with glass transition temperature decreases but the mending functionality increases.
The insertion of the self-healing concept into the fiber-reinforced polymer composites [16] can result in the development of an impairment tolerant design and reconstruction of the crippled area. The fiber-reinforced polymer composites with self-mending ability can possess high strength, impact resistance, corrosion resistance, low specific weight, along with autonomic crack repair. In polymer composites the thin brittle layer lie in contact with the deformable layer and thus cracking is a critical problem. Lin et al. [17] used Halloysite nanotubes (HNTs) in a polyurethane-based resin system to create a self-healing polymer composite. HNTs are multi-walled nanofillers with a tubular shape that can be used as containers for healing chemicals in self-healing systems. When 1wt% HNTs was added to the resin system, the thermal stability and mechanical properties of the nanocomposite, such as tensile strength and Young’s modulus, were significantly improved. However, when the content of HNTs were raised to 2wt%, the nanocomposite’s strength reduces due to agglomeration and weak H-bonding interactions between the HNTs and polyurethane chain ends. Furthermore, the inclusion of HNTs had no apparent influence on polymer chain mobility, and thus had no effect on self-healing efficiency. The nanocomposite system may be remolded thermally, and healing efficiencies were reported to be 90%. Yuan et al. [18] developed an epoxy composite system with self-healing ability. They used 2,4,6-tris (dimethylaminomethyl) phenol (DMP 30) as a catalyst, EPON 828 as a polymer matrix, and dual encapsulated epoxy/mercaptan as healant. The hardener used was a mixture of mercaptan and tertiary amine catalyst. The composite system was thermally stable when exposed to high temperatures and possess autonomous restoration of the fracture and mechanical properties. The self-healing efficiency of the composite system was found to be about 72–86% even at a high temperature of 250 °C. Lee et al. [19] made use of computer simulation and found that on the addition of nano-particles to the polymer then these particles get localized at nano-scale injuries, form patches similar to blood clotting in biological systems, and can repair the cracked areas. The properties of such systems in the intact, impaired, and repaired states were checked out through micromechanics simulations, and the optimal conditions for nano-particles to act as self-assembled, responsive patches were determined. The nanoparticle may flow to the damage site on appearance of new crack in the composite and this process could occur multiple times until the nanoparticle concentration get depleted in the bulk material.
Although immense research work is being reported on the self-healing technology since it finds its application in various fields such as electronics, mechanics, robotics, etc., the TRL (technology readiness level) shows that still more efforts are needed to lift the technology to the operational level. Frei et al. [20] used the existing TRL (technology readiness level) method to determine the technical maturity of the technology throughout its research, development, acquisition, and implementation phases. TRL was reported for nearly every type of self-healing system, including software, electronics, and so on. The TRL of the intrinsic polymer healing mechanism is level 4 (component and/or breadboard validation in a laboratory environment), the capsule-based mechanism is level 5 (component and/or breadboard validation in a particular environment), and the vascular layer mechanism is also at level 4. Furthermore, some applications such as ‘liquid inside tires’ are at advanced TRL level 9 (actual system “flight proven” through successful mission operations) are well known technologies and are available in the market [20].
This article reviews the newly developed discrete self-healing techniques most of which are inspired by biological systems. Here we target self-healing thermoset polymers which find application in coatings and fiber-reinforced polymer composites.
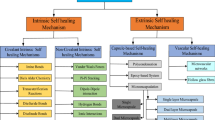
Extrinsic self-healing
In this type of self-healing, the matrix resin is not repairable by itself. The healing agent has to be stored in micro-containers that are dispersed in the polymer matrix in advance. When exposed to damage, the crack ruptures the micro-containers, and the repairing agent due to capillary action gets released into the cracked plane. The healing agent heals the crack and the surface integrity is restored by curing [21]. The healing agent must be stable within the polymer matrix, must flow into the site of damage, react immediately to offer healing in a reasonable time. The extrinsic self-healing approach leads to a single healing event and repeated healing of the repaired crack is not possible [22]. In this technique, the research is focused on many factors such as mending agents, fracture processes, kneading processes, and material formulation techniques. The healing performance and mechanical properties of self-healing materials also depend upon structural factors such as size, shape, and pattern of micro-containers, and dynamic factors such as flow and incorporation of healing agents. Depending upon the kind of micro-containers, the self-healing methods can be classified into the following three main types.
-
1.
Microcapsules approach.
-
2.
Microvascular approach.
-
3.
Hollow glass tubes/fibers approach.
Microcapsules approach
In the microcapsule-based self-healing system, the healing agent is confined in discrete capsules and these capsules are dispersed within a polymer matrix. In this approach, the self-repairing process is initiated by crack formation. Whenever the damage occurs, the microcapsules break, and the healing agent within the capsules is released which reacts with the catalyst and initiates a polymerization reaction due to which the crack is sealed [23, 24].
In microencapsulation techniques, all the involved materials must be wisely engineered, that is the healing agent should not be reactive towards the shell wall of the microcapsule. The walls of microcapsules should be resistant towards the processing conditions of the matrix, but at the same time it should be weak enough to be ruptured easily at the time of damage, and the monomer viscosity should be low so that it can flow easily. Moreover, during the long shelf-life of the composite matrix, the liquid healing agent should not diffuse out of the shell wall of capsules. The emulsion polymerization technique (i.e., oil in water dispersion mechanism) was the most used technique for the synthesis of these polymeric microcapsules. This technique can be applied to highly cross-linked thermosets. Kessler and White [25] made use of dicyclopentadiene (DCPD) as a monomer healing agent which was stored in microcapsules made up of urea-formaldehyde. These microcapsules were evenly dispersed within the polymer matrix. Upon crack propagation, these microcapsules were ruptured and as the healing agent comes in contact with dispersed Grubb’s catalyst, the polymerization reaction initiates, and the crack repairs as depicted in Fig. 1. This work was further extended by Brown et al. [26]. They studied the fracture toughness and healing efficiency of polymer matrix with respect to the size and concentration of the microcapsules and catalyst, respectively. They found that the epoxy was toughened significantly by the addition of microcapsules. The healing ability of the encapsulated polymer matrix was found to be 70% of its virgin fracture toughness. White et al. [27] also synthesized a capsule-based self-healing material wherein dicyclopentadiene (DCPD)monomer was filled in the microcapsules and these microcapsules along with Grubb’s catalyst were dispersed in the matrix system at the time of matrix formation. The healing agent comes in contact with the Grubb’s catalyst when damage occurs and the crack restores due to ring-opening metathesis polymerization ROMP (which is a chain-growth polymerization process) of the Grubbs catalyst and DCPD. The fracture toughness was recovered at room temperature and was found to be 75% in 48 h.
There were some limitations associated with the DCPD system such as a large amount of catalyst was used and the melting point was low. Lee et al. [28] proposed that these limitations were overcome by replacing DCPD with ENB (5-ethylidene-2-norbornene) or mixing ENB with DCPD [19]. The ENB was microencapsulated via in situ polymerization of urea and formaldehyde. ENB possesses some advantages as the reaction is fast, a small amount of Grubb’s catalyst is required and the product obtained with ENB has higher Tg values. It was found that ENB possesses auxiliary mechanical properties and polymerizes into a linear chain structure as compared to DCPD which possesses high toughness and strength and has the capability of forming a cross-linked structure. Thus, it was believed that the mixture of DCPD and ENB result in a more reactive repairing system that had acceptable mechanical properties, but the fracture behavior and mending efficiency of such systems were not inspected by the authors.
Schematic diagram for microencapsulation of resins and repairing agent [29]
Our group [29] recently published an article on the synthesis of high-performance self-healing coatings using a biobased epoxy resin and imidoamine curing agent. Both the epoxy resin and the curing agent were encapsulated in to the melamine formaldehyde (MF) microcapsules via in-situ polymerization technique wherein poly (ethylene-alt-maleic anhydride) (EMA) was used as an emulsifying agent, as shown in Fig. 2. The structure morphology, stability, and healing performance of these microcapsules of epoxy and the curing agents were investigated by using SEM and TGA analysis. The average particle sizes of epoxy and the curing agent filled microcapsules were found to be 83 and 95 μm, respectively. The self-healing coating specimens were prepared by mixing biobased epoxy resin and the imidoamine curing agent and adding various ratios of synthesized microcapsules. It was concluded that the coating sample containing 10 wt% of each type of microcapsules viz. epoxy as well as hardener, was found to have the highest healing efficiency of 82% and was reported to be most stable to the chemical atmosphere. Figures 3 and 4 depict SEM images of the synthesized microcapsules and force-extension curves for various coatings, respectively.
SEM images of a hollow, b epoxy filled, and c hardener filled microcapsules [29]
Force-extension curves of the self-healing coating samples containing different ratios of microcapsules before and after repairing [29]
Rodriguez et al. [30] synthesized self-contained microcapsules by suspension polymerization technique wherein both the catalyst and repairing agent were contained in same capsule and were isolated from each other. The polymeric shell wall of microcapsules was prepared from polymethyl methacrylate (PMMA) and PMMA/Sc(OTf)3. Scandium triflate (Sc(OTf)3) and diglycidyl ether of bisphenol A (DER 322) were used as a catalyst and healing agent for the polymeric matrix made up of DGEBA, Epikote 828LVEL epoxy resin and Epikure 541. The healing efficiency of PMMA-walled capsules was 46.7% at 80 °C and 55.1% at 120 °C. the healing efficiency in case of PMMA/Sc(OTf)3-walled capsules increased to 57.5% and 79.1% at same temperatures. Theis was attributed to the presence of catalyst at the walls of microcapsules which can enhance the reaction possibility among the catalyst and the encapsulated agent.
The microencapsulation concept was further used for the synthesis of fiber-reinforced self-healing composite materials. In composites also, when the catalyst was dispersed in the matrix and assimilate with the healing agent, it triggers the polymerization reaction, repairs the cracked surface, and the material’s integrity is restored. Tang and Fang [31] used organo-siloxane or organosilane (which contains Si-vinyl bonds and Si-H bonds) as healing agents. They used a polymer composite matrix consist of glass fiber and the platinum catalyst supported on these glass fibers and the microencapsulated healing agents were dispersed in the polymer matrix. the Pt catalyst initiates the hydrosilylation of the healing agent and the resulting product rebounds the cracked faces.
In contrast to the conventional self-healing materials with microcapsules, in the phase-separated capsule-based systems, one component is encapsulated within the microcapsules and the other one is phase-separated. When the two components are released and come in contact with each other, then they react and close the cracked surfaces. The phase-separated system has few advantages such as stable repair chemistry at elevated temperature and is thus applicable for healing in high-temperature thermosets. Cho et al. [32] made use of the phase-separated self-healing concept based on polycondensation reaction between the hydroxyl end-functionalized polydimethylsiloxane (HOPDMS) and polydiethoxysiloxane (PDES) in the presence of a tin catalyst DBTL (di-n-butyltindilaurate). Here the matrix used was a vinyl ester matrix where phase-separated droplets of HOPDMS and PDES were dispersed, the tin catalyst was stored in the polyurethane microcapsules, and was pre-embedded into the vinyl ester matrix. Upon mechanical damage, the microcapsules rupture, and the tin catalyst comes in contact with dispersed HOPDMS and PDES, resulted in the initiation of a polycondensation reaction between HOPDMS and PDES. This concept was further extended for the protection of self-healing coatings from corrosion. For the epoxy amine matrix system, the direct contact of siloxane-based healing agent with the matrix system results in some matrix-initiated reactions, so the authors in [33] propose a dual microcapsule system approach in which the PDMS healing agent and the catalyst both were encapsulated in the polyurea-formaldehyde microcapsules.
Some other self-healing approaches exploit epoxides as the encapsulated healing agents, planted in the matrix. Highly strained epoxide rings present in epoxy resins undergo rapid coupling reactions with the hardener or so-called curing agent such as maleimides, anhydrides, amines, alcohols, carboxylic acids, etc. At the time of fracture development, the microcapsules containing epoxy are ruptured and there is the formation of a covalent bond when the epoxide ring of the released epoxy react with the hardener embedded in the matrix due to which insoluble thermosets are generated which clog the crack and reconstruct the strength of the polymeric material. The curing of the epoxy using hardener can be done at low temperature as well as at high temperature i.e., cold curing, as well as hot curing of epoxy resins, is possible. For self-healing applications, cold curing is of great interest [34]. Since at low temperatures, the primary and secondary aliphatic amines have high reactivity, so can be usually used as hardeners or crosslinkers. The cross-linking density and the morphology of the resin depending on the curing conditions as well as the curing agents. The special advantage of epoxy is that while using epoxides as a healing agent the same kind of matrix material is used, which ensures a good adhesion among them, and the initial properties of the material are fully recovered. Wang et al. [35] Synthesized a binary self-recuperative system by using anionic polymerization of 2-methylimidazoleazine (2 MZ-AZINE) as a latent curing agent and epoxy filled microcapsules. The curing agent was dissipated in the epoxy matrix at the time of composite construction and initiate the anionic polymerization between the released epoxy and the catalytic hardener (latent curing agent). Thus, the crack was successfully repaired by curing the epoxy healing agent. The mending efficiency was highly influenced by the distribution of microcapsule and the curing agent. The ideal weight ratio of the microcapsule and the curing agent was 15 wt% and 2 wt%, resulting in a healing efficiency of 83%. Yuan et al. [36] represent an example of low-temperature curing of the bisphenol-A-diglycidyether epoxy (EPON 828) with the pentaerythritol tetrakis (3-mercaptopropionate) (PETMP) a mercaptane hardener or curing agent. Both the epoxy and the curing agent were encapsulated as two-component healing agents and were assimilated into the epoxy matrix. With a low capsule content, an attractive healing efficiency of 43.5% with 1 wt % of the capsule and about 104.5% with 5 wt % of capsules can be attained at 20 °C and after 24 h. The healing system has shown long-term stability for about 1 year. Jin et al. [37] Developed a dual capsule self-healing system based on epoxy-amine resin formulation at ambient temperature. The epoxy used was a diluted epoxy EPON 815 C and the amine hardener was a modified aliphatic polyamine EPIKURE 3274. Epoxy was encapsulated by in situ polymerization with poly urea-formaldehyde microcapsules [38] and a vacuum infiltration technique was used to prepare the amine capsules. Both these kinds of capsules were planted into the epoxy matrix. The ideal ratio of epoxy: amine microcapsules was found to be 6:4. For a specimen, containing10.5 wt% epoxy microcapsules and 7 wt% of amine capsules (an overall capsule content of 17.5wt %), cured at low temperature, the average healing efficiency of 91 ± 21% were attained.
Song et al. [39] introduced the first of its kind photo-induced extrinsic self-healing system where the mending agent (MAT-PDMS) methacryloyloxypropyl terminated poly dimethoxy silane was enclosed in microcapsules of poly urea-formaldehyde along with a photo initiator benzoin isobutyl ether. The matrix material used was a triethyl-orthosilicate based sol-gel matrix. On the appearance of the crack, the MAT-PDMS resin was photoinitiated in the presence of sunlight and the photopolymerization reaction repaired the damage.
Micro vascular approach
In this approach, the healing agent is stored in hollow channels or a network of capillaries or within brittle vessels, which are interconnected in 1D, 2D, or 3D. These vessels are filled with healing agents and are dispersed in the polymer matrix. Whenever the crack appears, the vessels rupture resulting in the release of low viscosity self-healing agents to the damaged sites and fill the micro-cracks. Unlike the microcapsule system, the main advantage of this microvascular system is that a large amount of healing agents can be stored and have the tendency to heal cracks of large dimensions. This system is capable of showing multiple healing events within a certain area, as the cracked area has multiple connectivities to the healing agent. It has been reported that though with the increase in the dimensions of these vessels, high degree of reliability and flexibility is introduced to the polymer matrix, but at higher number healing cycles, the high efficiency is still a challenge to attain [40]. The other disadvantage of the microvascular system is that during the process of healing the cross-linked healing material can block the micro pipes in the damaged area and this region gets alienated from the rest of the vascular system. Furthermore, in contrast to the microencapsulation technique, the microvascular network is complicated and its manufacturing is expensive due to multistep fabrication. The concept of repair damage by using the healing agent stored in hollow microfibers was first proposed by Dry et al. [41]. A high healing performance is obtained when 10 wt% of Grubb’s catalyst was added to the epoxy coating.
Toohey et al. [42] came up with a three-dimensional self-repairing microvascular network installed in the polymer matrix substrate. This work resembles the architecture of human skin. The direct-write assembly technique was used to insert a fully interconnected 3D microvascular network into an epoxy matrix containing Grubb’s catalyst. DCPD was used as a mending agent and stored in microvascular channels. Fracture toughness strength was used to determine the healing efficiency of the same crack for a sequence of seven successive healing cycles. The highest recovery of 70% was accomplished after the second healing cycle. Like the DCPD system, amine-epoxy chemistry was also incorporated into the microvascular system where a healing efficiency of above 60% for up to 16 healing cycles was obtained and the sample was allowed to heal for two days at 30 °C for each healing cycle [43]. In order to lower the time of healing between the cycles, the temperature was slightly raised above the room temperature. Hansen et al. [44] exhibited that autonomous mending of up to at least 30 recurring cycles has been accomplished by using epoxy-based healing chemistries from a dual microvascular design. When different microvascular networks were embedded in adjacent positions to one another, the two-part liquid repairing agents were isolated inside of the polymer matrix and react only when deterioration activates their release to the sight of damage. The interpenetrating microvascular substrate architecture was constructed through two important developments in direct-write assembly-dual ink deposition and vertical ink writing. The twin microvascular networks have been joined in many polymer matrixes, including substrates with brittle coatings and sandwich composite structures, etc. Hansen and his team [45] also reported the manufacture of self-healing material in which ternary interpenetrating microvascular networks were embedded in an epoxy coating/substrate architecture by a direct-write assembly of two fugitive inks, where one ink describe three microvascular networks and the other one functions as the spacer to separate each network. Out of three microvascular networks two were used to supply the diglycidyl ether of bisphenol-A epoxy resin (EPON 8132) and an aliphatic amidoamine (Epikure 3046) hardener mending agent. The third network was used to circulate a thermally regulated fluid. Thermal characterization affirmed that with an increase in temperature the healing kinetics were stimulated. The mechanical studies revealed that the repairing time was decreased with an increased ability of reconstruction at elevated temperatures. Postiglione et al. [46] connected successfully the 3D printing via fused deposition modeling (FDM) and the resin casting into water-soluble PVA molds. They manufactured samples with four isolated microvascular networks consisting of healing agents which were released during damage. The samples consist of microvascular network were prepared by using three polymeric materials namely PDMS (polydimethylsiloxane), and two epoxy resins formulations. The epoxy resin formulation consist of DGEBA and JD400 (O,O′-Bis(2-aminopropyl)polypropylene glycol) in volumetric proportions of 1.47:1 and was termed as EPOXY-J. In another epoxy formulation DGEBA and excess of HMDA (hexamethylenediamine) in the ratio of 1:5 was reacted together to form a liquid prepolymer. Benzyl alcohol was used as a diluent to the prepolymer. This prepolymer was used as a hardener for DGEBA. This epoxy formulation was termed as Epoxy H. The 3rd sample was a PDMS formulation synthesized by treating the PDMS and the hardener. The fractured samples regain good mechanical strength, after self-healing treatment. The healing efficiencies of 82% and 79% were determined by uniaxial tensile testing of PDMS and Epoxy-J systems, respectively. The microvascular technique repairs not only the small-scale damages at the skin core interfaces, but also the internal damages within the bulk polymeric materials. Hamilton et al. [47] embedded the vascular system within a bulk polymeric material. Two components (EPON™ 8132 epoxy resin and EPIKURE™ 3046, an aliphatic amidoamine hardener) were inserted in to the vascular system which were dispersed all over the material. These two components were inserted in alternative layers of the microchannels. The controlled cracking of the specimen was done by using the DCDC (double cleavage drilled compression) technique so that the catastrophic failure of the specimen can be obstructed. After first healing cycle, 86% of the fracture toughness was recovered when compared with the virgin sample. The polymeric material continued the healing process after 13 repeated cycles of cracking and repairing until the flow of the healing agent was blocked by the polymer that was formed at the cracked zones.
A microvascular network that possesses a dual role of sensing structural destruction before starting a triggered repairing response had been developed by Trask et al. [48] and was planted in a fiber-reinforced composite laminate. In this article, the sensing and repairing system consists of a damage sensor and a repairing agent delivery system. A single vascule was used as a sensing path, which detected the damage and microcracking in the matrix during a 10-J low-velocity impact event. The positive pressure was filled in every 2nd vascule and its release through the damaged site initiate the healing agent delivery to the impaired zone. Two different low viscosity healing resins, one commercially available epoxy healing agent (RT151) and another one an in-house healing epoxy resin system (diglycidyl ether of bisphenol-A/diethylenetriamine) were used. After damage the compression strength was recovered to 91% and 94% for RT151 and DGEBA/DETA, respectively when compared with a laminate of CFRP with no inserted healing ability. An ultrasonic C-scan TOF (time of flight) analysis pointed out that for both the systems, the overall deterioration was reduced by approximately 50% due to self-healing functionality and thus explaining the reconstruction in strength. The diameter of the vascular and the resin viscosity was important for the advancement of this kind of self-healing system as the cost to mechanical performance and the fluid delivery depend upon these parameters. Higher pressure is required to release the healing agent to the damaged site from a smaller diameter vascular network because of increased frictional loss. On the other hand, the higher infusion potential is obtained in the case of the lower viscosity fluid and the healing efficiency will be enhanced and hence the fluid delivery cost would be reduced significantly.
Hollow glass tubes/fibers approach
The introduction of the self-healing concept into the fiber-reinforced composites will help to develop the damage resistance of the materials, provide high strength, and corrosion resistance, along with self-repairing of the damaged sight. Different vessels such as hollow nano fibers, CNTs, titanium dioxide nanotubes (TNTs), hollow polymeric fibers, and halloysite nanotubes (HNTs), hollow glass fibers, etc., can act as reservoirs to the healing agent in various self-healing composite systems. The flowability of the healing agent was affected by the internal diameter and wall roughness of these vessels. For the healing of large damage zones, large-sized vessels are most convenient. The repairing agent should be liquid at healing temperature and the crack is eliminated when the healing chemical flows to the damaged area where it gets polymerized. Self-healing process for resin matrix consisting of hollow fibers filled with healing agent and the hardener is shown in Fig. 5. Epoxy-based chemicals can be used as healing agents because they can outflow when heated and then get cross-linked by epoxy hardener.
Bekas et al. [49] made use of hollow nanofibers to synthesize a polymer composite with self-mending ability. They used multi-walled CNTs to provide reinforcement to the low viscous epoxy resin. The nanocomposite of epoxy and CNTs act as a healing agent which was filled inside the vascular glass fibers and was dispersed within the polymer matrix. The viscosity of the epoxy healing agent was enhanced due to CNTs addition which was further adjusted by ethyl phenylacetate (EPA) solvent. The effect of insertion of CNTs was understood by calculating the healing efficiency and interlaminar fracture toughness of the polymeric system. The fracture toughness of 0.3 and 0.5wt% CNTs was found to increase by 33.8% and 52% relative to neat epoxy. And their healing efficiencies were 169% and 192%, respectively.
Zhu et al. [50] reported a self-pressurized healing epoxy composite system consisting of hollow polymeric fibers. They stored epoxy/mercaptan repairing agents in polypropylene (PP) tubes which were dispersed within the polymer matrix. A foaming agent was also used which had a great influence on self-healing efficiency. The foaming agent decomposes at 70 °C and produces gas within the closed PP tubes that contain a healing agent and generate pressure on repairing fluid. Under the influence of high pressure, the repairing agent outbursts from the PP tubes and spreads over a large-damaged zone which intensifies the mixing of hardener and repairing agent thus enhances the healing efficiency.
Poornima Vijayan et al. [51] reported a self-healing epoxy coating by using halloysite nanotubes (HNTs) and inorganic nanomaterials as containers for healing agents and hardener, respectively. They used TiO2 nanotubes (TNTs) for epoxy (EPON 826) healing agent and mesoporous silica for amine (Epikure 3223) hardener. The mending agent was encapsulated into the nanotubes by using a vacuum infiltration process and the amine curing agent was immobilized into mesoporous silica by shaking for 24 h. These filled containers were embedded into the matrix system and the material was coated on a carbon steel substrate. The self-healing ability of the material revealed that 57% of the anticorrosive properties of these coatings were retrieved within five days. Dry et al. [2] made use of amine hardeners for epoxy resins. Both the resin and the hardener were stored in the hollow glass fibers which were pre-embedded in the matrix system. The glass fibers containing the healing agent and the hardener were paired together in the same fiber layer of the matrix, thus assuring the mixing of both the compounds. When a fractured composite system consisting of the mending agents was allowed to heal, the repaired composite system possessed greater strength as compared to the one having no added adhesives, and have the ability to deflect the crack from the original crack path. Trask et al. [40] studied a self-healing system in which the hollow glass fiber layers were placed within both carbon fiber/epoxy and glass fiber/epoxy composites to lower the damage and rebuilt the mechanical strength. The hollow fibers were demonstrated to possess diameters between 30 and 100 μm and approximately 50% of hollowness within the fibers. The study affirmed that, when the exfoliate was exposed to quasi-static damage, a significant fraction of flexural strength can be reestablished by the self-mending effect of a healing agent stored within hollow fibers. Bleay et al. [52] prepared the epoxy composites by introducing hollow glass fibers into the matrix and vacuum filling of these hollow fibers through capillary action. To fill the viscous epoxy resin into the hollow glass fiber, a solvent such as acetone and heating of 60 °C was required. The hardener or accelerator was filled into secondary glass fibers and was also diluted with acetone for successful filling of fibers. Williams. et al. [53] described a carbon fiber reinforced composite system having self-healing functionality due to embedded hollow glass fibers into the composite system. They studied the effect of the hollow glass fibers on the healing efficiency and the mechanical properties of the carbon fiber reinforced polymer composites when subjected to damage. Pang et al. [54] extended the work on the hollow fibers approach, wherein a UV fluorescent dye was added to the healing resin stored within hollow fibers to envision the flow of mending agent in the epoxy sample. This system performed the dual function of renovating the fracture as well as highlighting the impact damage in the composites. Here two sets of hollow glass fibers were used, one set consists of a UV fluorescent dye and a mending agent, and another one consists of an epoxy hardener. A complete composite system contains solid fibers and the two sets of fibers were assimilated in plies aligned at an angle of 90° to each other. After self-healing, the recovery of strength was evaluated by conducting impact indentation followed by a four-point bending flexural test, which was found to be approx. 97% of the original strength.
Intrinsic self-healing
In the intrinsic self-healing approach, the polymeric network is able to heal cracks by its own. No stored repairing agent is assimilated into the polymer system. They consist of latent self-healing functionalities in the polymer matrix. Self-healing is established by polymer modification with functional groups that can form reversible bonds. In intrinsic self-repairing polymers, the healing can be non-autonomous, that is an external stimulus is needed for the initiation of the mending process, and either physical or chemical interactions are involved in the molecular mechanism [55]. The main utility of intrinsic self-healing materials is that numerous healing cycles are possible due to the involvement of reversible chemical and physical bonds hence materials can have long-term use [56].
In intrinsic self-healing polymers, the repair takes place via a temporary increase in movability of the polymer chains, which depends on the specific molecular structure of the polymer [57]. A certain amount of energy in the form of specific stimuli such as heat, specific load, and UV exposure, etc. is required to provide effective inter-chain mobility in the specific structures of the polymers. When the stimulus is removed, the restoration of physical/chemical bond strength takes place. The self-healing materials should possess low viscosity at the healing temperature so that the polymeric chains can move easily, i.e., can reflow and recombine chain ends easily. In general, 100% healing of the interface means that the new interface possesses the same properties as that of the bulk polymer. Intrinsic self-healing approach possess a short range of applications as it is confined to small damage zones.
Thermo-reversible Diels Alder-based self-healing polymers
The Diels Alder reaction is a [4 + 2] cycloaddition reaction of a diene and dienophile (Scheme 1). The electron-withdrawing substituents present on dienophile make them more suitable to react with diene and form DA adduct. This Diels-Alder reaction is regioselective, stereoselective, thermally reversible, and gives a high yield of the product [58]. This is a self-sufficient reaction and no by-product is formed. The Diels Alder adduct is thermally unstable and undergoes retro-Diels Alder reaction at elevated temperatures. Because of the reversibility of this reaction, the Diels Alder adduct can be decomposed to diene and dienophile at elevated temperatures. The novel materials, such as thermally reversible crosslinked polymers, can be prepared by utilizing the thermal reversibility of Diels Alder reaction.
The dienes and dienophiles are hooked up to the polymer backbone and the polymers are cross-linked with crosslinkers. At present, the most commonly used system is the DA reaction of derivatives of furan and Maleimide compounds. Due to reversible crosslinks, these polymers can elongate the lifetime of polymeric materials by increasing their mechanical properties.
To prepare a cross-linked polymer network based on DA reaction, it is convenient to functionalize the polymer chain with diene and dienophile groups such as furan and maleimide. Pratama et al. [59] synthesized a Diels Alder-based self-repairing thermosetting resin having room-temperature healing properties and suitable functionalization. The maleimide was present in the healing agent solution and furan functionalities were present in the thermoset and the reaction between these two functional groups resulted in the rebuilding of the cracked plane. The urea-formaldehyde shells were used to encapsulate the healing agent, these microcapsules were assimilated within the epoxy amine thermosets having furan functionalities. After fracturing the recovery of the self-healing thermoset was found to be 71% of its original load. The main drawback of this research was one-step repairing as the mending agent present within the microcapsules was utilized after the first cycle of repairing. Chen et al. [60] synthesized a macromolecular network by using 4 furan (4F) and 3 maleimide (3 M) groups as precursors. The synthesis of this macromolecule involves a thermally reversible Diels Alder reaction between furan and maleimide groups. This polymer network has a high density of DA bonds and high mending efficiency. A debonding of the DA adduct was demonstrated when a retro-DA reaction was performed at above 150 °C for 15 min. The furan and maleimide moieties were disconnected and had enough mobilities to reach the cracked surfaces and heal them by network reconnection at lower temperatures. Thus at 150 °C, the average mending efficiency is about 50%. The tensile strength of the macromolecular network was superior to that of epoxy resins and other properties of the system such as compression strength, flexural strength, etc. were still analogous to that of epoxy resins. It is to be noted that the self-healing behavior is repeatable in all the reported systems. Scheltjens et al. [61] synthesized a self-healing polymer by using the concept of reversible Diels Alder reaction between four furan functionalized compounds and bismaleimide. They used the Jeffamine D- series to vary the spacer length in the furan functional compounds. The reversible elastomers, with transition temperature much below the curing temperature, were formed if the spacer length is increased. The healing temperature ranges from 80 °C to 130 °C. It was determined that the complete fracture healing was obtained at 80 °C when heated for 10 minutes. Turkenburg et al. [62] studied the reversible polymer network composed of diethyl itaconate copolymerized with furfuryl methacrylate moieties which upon addition of bismaleimide undergo Diels- Alder reaction and the reversible crosslinks between the chains were established. The resulting material shows a mixed mechanical behavior. At low temperatures, there is crosslinking within the material and it behaves as a thermoset. At high temperatures the de-crosslinking takes place and the material behaves as a thermoplastic. Crack healing would occur by heating the damaged material at 160°C for one hour. Coope et al. [63] synthesized an epoxy amine system consisting of furfuryl and maleimide functional groups in two steps. In the first step, they synthesized the prepolymer by combining the furfuryl amine with DGEBA. In the second step, DA adduct is formed between the prepolymer and varied amount of 1, 1’-(methylenedi-4, 1-phenylene) BMI. Consistent multiple healing cycles (three) were achieved by heating at 150 °C for 5 min. Toncelli et al. [64] came up with thermally reversible thermosets, which were prepared by using furan functionalized polyketones and DPBM as crosslinkers. They varied the furan groups in the backbone and also varied the furan /crosslinker molar ratio. The storage and loss modulus were completely recovered. Multiple healing (seven times) would occur without any significant quality loss. Almost all the samples were fully repaired within an hour. The thermal reversibility is proved by FTIR studies and the healing efficiency was evaluated through DSC and DMTA (dynamic mechanical thermal analysis) studies. Liu and Hsieh [65] Prepared a self-healing polymer by using multifunctional furan (TF) and maleimide (TMI) compounds. The crosslinking reaction between TF and TMI was studied by using the DSC technique. Both TF and TMI are soluble in low boiling solvents and hence can be easily processed at low temperatures. The DA reaction between TF and TMI occurred at 50 °C for 12 h. Retro-DA reaction occurs at 145 °C. The self-healing property of this TF-TMI adduct was observed by using SEM (Scanning Electron Microscope). The surface of the sample was cut with a knife and the thermal treatment of the cut sample was done. Heating at 120 °C for 20 min results in debonding which provides mobility to the chain segments and heating at 50 °C for 12 h resulted in re-bonding of the chain segments. The complete recovery of the cut sample was exhibited by heating at 50 °C for 24 h. Tian et al. [66] synthesized an epoxy monomer, (DGFA) N, N-di-glycidyl-furfuryl amine. This monomer which was a liquid with lower viscosity consists of two epoxide groups and one furan group. Two types of intermonomer linkages are present in the molecular network system. The epoxide groups are cured by using conventional epoxy curing agents such as anhydride, which result in irreversible network structure and also provide good mechanical properties and thermal resistance to the system. The furan groups are cured by maleimide groups which results in thermally reversible DA bonds to introduce self-healing properties into the molecular network structure. The thermal reversibility of the network structure was monitored by using the DSC technique. The different samples were treated at different temperatures ranging from 100 to 125 °C for 20 min for disconnecting DA bonds i.e., retro-DA reaction between furan and maleimide moieties and the de-bonded groups were re-bonded together (i.e., DA reaction occurred) at 80 °C and thus the cracks were healed.
Our group has recently reported the biobased thermo-reversible self-healing coating materials derived from gum rosin, a pine tree product [67]. The self-healing is attained by using Diels-Alder chemistry. Furfuryl amine is the diene moiety that has been introduced into the gum rosin-based resin matrix. The bismaleimide units serve as a dienophile for Diels Alder reaction. The confirmation of DA bonds and thermo-reversible nature of the material has been investigated by using DSC and FT-IR spectroscopic techniques. The smoothness of the coatings was confirmed by using AFM technique. The micromechanical properties of the synthesized coatings were determined by using nano-indentation technique. Out of the synthesized coatings, the one with higher crosslink density was found to show higher thermal stability, chemical resistance to the harsh environment and adhesion strength as well. The healing ability of the synthesized resins was demonstrated by manually cracking the coatings and then heating it at respective r-DA temperatures followed by thermal annealing at DA temperatures. The optical images taken before and after healing were represented in Fig. 6. The healing efficiency of the coatings were found to be 80%. The healing process of various coatings has been investigated by using SEM technique and the SEM images are shown in Fig. 7.
Optical images of the self-healing coatings, before and after repairing [67]
SEM images of various self-healing coatings during the healing process, taken at different intervals of time [67]
Similar to the crosslinked resins, crosslinked polyamides can be prepared by modifying the polyamides by furan and maleimide groups. These furan -maleimide modified polyamides show high flexibility as compared to crosslinked epoxy resins. Liu and Chen [68] prepared a polyamide having maleimide (PA-MI) and furan (PA-F) pendent groups and synthesized their DA cross-linked polymer as shown in. Compared to PA-MI and PA-F precursors, this DA adduct shows increased toughness and mechanical properties. The self-healing ability was observed by SEM micrographs. The retro-DA reaction of the cross-linked polyamides was also studied by observing the changes in solubility. At room temperature, the crosslinked polymer was not soluble in DMAc (N, N-dimethylacetamide) but if the sample was heated at 150 °C for 2 h then it becomes soluble in DMAc. At elevated temperatures, where retro-DA would occur, the crosslinked polymer was converted into PA-MI and PA-F, which were soluble in DMAc. The cross-linked sample shows high toughness as compared to the precursors. The self-healing ability of the crosslinked sample was observed by putting a crack on the surface of the sample with the help of a knife. The thermal treatment of the cut sample was done by heating at 120 °C for 3 h (where disconnection of the DA adduct would occur) and at 50 °C for 5 days where the de-bonded polymer chains connect together to form DA adduct. Kavitha et al. [69] reported a system containing polymeric precursor prepared by using furan modified polymethacrylate (PFMA)with controlled molecular weight via atom transfer radical polymerization. Here furan modified PFMA was cross-linked by bismaleimide (BM), a dienophile. Debonding of the DA adduct would generate free BM molecules which can move freely within the polymer matrix, reach the cracked surface easily, and heal the crack. The FT-IR and DSC techniques were used to confirm the reversible nature of the Diels alder bond. The reversible materials obtained here can have potential applications in reversible coatings, self-healing materials, adhesives, and smart biomaterials, etc. The adhesive strength of PFMA-BM adducts at 25 °C was higher than the strength of the precursors. Peterson et al. [70] Synthesized a fiber-reinforced self-healing composite system in which, maleimide functionalized glass fiber was integrated within the furan functionalized epoxy amine matrix. The DA reaction between furan and maleimide functional groups would occur at room temperature and above 90 °C, the DA adduct will be de-bonded and results in original furan and maleimide moieties. The investigation of the self-healing property was done by single fiber microdroplet pull-out testing. Multiple healing is possible with intrinsic thermos-reversible self-healing systems and up to five healing cycles were successfully achieved. The average healing efficiency was found to be 41%. The DA reaction between a polyurethane prepolymer end-capped with furan groups and maleimide result in a new linear polyurethane [71]. This new linear polyurethane exhibits the property of thermal reversibility. The self-healing property was studied by using a polarized optical microscope. The healing efficiency was found to be up to 80%. Zeng et al. [72] introduced a bio-based network polymer PFS/M2 which was prepared by DA reaction between furan polymer (prepared by condensation reaction between bio-based monomers) and a bismaleimide. The mechanical properties of the network polymer were also varied depending upon the amount of bismaleimide. A wide range of polymeric materials can be prepared from bio-mass that have improved mechanical properties and self-healing ability. Bai et al. [73] prepared a new self-healing epoxy polymer based on TGAP (triglycidyl p-amino phenol). The acquisition of suitable curing conditions of 60 °C for 24 hours and the 82% degree of cure were accomplished by using near IR and DSC scans. The suitable curing condition was found to be 150 °C for 10 minutes. The swelling test and the dynamic mechanical analyzer were used to study the self-healing properties of the polymer samples. The glass transition temperature and the swelling properties of the healed sample as well as the original sample were found to be same. The TGAP epoxy polymer has higher functionality which implies more Diels Alder units and higher cross-link density as compared with the DGEBA based epoxy polymer hence extra time is required to cure and repair. Moreover, the high cross-link density of TGAP based polymer results in improved thermal stability, mechanical properties with higher Tg values as compared to DGEBA based epoxy polymer. Okhay et al. [74] manufactured a thermos-reversible polyurethane network from Diels Alder reaction between high maleimide functionalized urethane-based prepolymers (PU-M) and either of furan functionalized polyurethane (PU-F) or furan functionalized epoxy (EA-F). The solubility test, TGA (thermogravimetric analysis), DSC (differential scanning analysis) studies were used to observe the de-cross-linking and thermal behavior of the polymer network, its density was assessed from the swelling test. The crosslinking density was highly altered by average furan and maleimide functionalities. The network obtained from PU-M/EA-F was hard and dense (i.e it had high Tg value and low swelling) and that obtained from PU-M/PU-F was soft and loose (i.e it had low Tg value and high swelling). A linear polyurethane with DA bonds (PET-DA-PU) was synthesized by Xu et al. [75] using PET as the starting material, toluene-2,4-diisocyanate (TDI) as the coupling agent, and DA diol as the chain extender agent. The DA diol was synthesized by cycloaddition reaction of furfuryl alcohol and 1,1’ -(methylenedi-1,4-phenylene) bismaleimide. The glass transition temperature of the synthesized material was found to be low (-59 °C). The crack evolution was observed using polarized optical microscope which indicated the disappearance of the cracks in 9 min while heating at 100 °C. The healing efficiency of the synthesized material was found to be 89.1%. The efficiency of retro DA reaction was analyzed using NMR spectroscopy and was found to be 70%. The composite of PET-DA-PU/Al/Na2SO4 was also prepared and its healing efficiency under similar conditions was 87.8%.
Various polymeric materials with Diels Alder reactions, such as acrylate polymers, can be used in coating materials. The self-healing polymers with graphene or modified graphene oxide nanofillers improve the mechanical properties as well as the self-healing efficiencies. Based on the Diels Alder reaction, Lee et al. [76] reported a self-repairable nanocomposite consisting of graphene-based nanofillers. The maleimide-modified graphene oxide (mGO) increases the compatibility of nanofillers and the furan functionalized (FEEMA64) polymer matrix. The FEEMA64 was a copolymer in which FEEMA (furan functionalized methacrylate monomer) and PEGMMA [poly (ethylene glycol) methyl ether methacrylate Mn300] were taken in a 6:4 molar ratio. The Diels Alder reaction between FEEMA64 and bismaleimide resulted in FEEMA64 polymer film. Now graphene-based nanofiller modified with NCO-M was incorporated into the polymer film to enhance its mechanical properties. The maleimide moieties present on the surface of mGO can also participate in Diels Alder reaction and can improve the self-healing efficiency as well as the mechanical properties. The improvement in tensile strength and self-healing efficiency of the FEEMA64-mGO (0.030 wt%) was found to be 172% and 81.228% when compared to the polymer film having both furan and maleimide moieties but no nanofillers.
Disulfide bond-based self-healing polymers
This self-healing concept is mainly based on the chain exchange reactions and demands the dynamic reversibility of covalent bonds. The room temperature healing reversibility can be attained in the case of disulfide exchange reactions which are incorporated into rubbery networks and hydrogels. At the time of damage, the S-S bonds are cleaved, produce free radicals which have the potential to react immediately with other S-S bonds. The production of free radicals can be triggered by heating, photolysis, or oxidation. From earlier studies, it is found that metathesis exchange reactions are there in disulfide bonds in which two adjacent S-S linkages are obstructed and are reconstructed through ionic or free radical intermediates. The reversible behavior of the S-S bond can be attained by reduction reactions to form (S-H) thiol groups and it can be reversed by oxidation reaction. The S-S bond can be introduced into a low (Tg) glass transition temperature gel network system, can provide room temperature reversibility to the system and considerable support in self-healing. Canadell et al. [77] assimilated the disulfide links in the rubber network, which enabled the system to restore the mechanical properties at mild temperature conditions. The epoxy resin-based rubber network was obtained by cross-linking the epoxy resin (having disulfide groups) with thiols in a base-catalyzed addition reaction. Here the disulfide bonds were incorporated solely into the epoxy resin via anionic ring-opening copolymerization. The cross-linked material was a transparent thermoset rubber having a low glass transition temperature of approximately −35 °C. At a temperature above 100 °C, the cross-linked rubber showed an ability to flow which signifies that disulfide bonds detach and the chain segments attain enough flexibility to bring about the macroscopic flow. The material can show multiple repeated healing cycles. The author has also determined the effect of disulfide concentration on the self-healing properties by varying the concentration of disulfide from 0 to 20 wt%. The highest self-healing efficiency was shown by the material with the highest disulfide concentration, and no self-healing ability was shown by the material having no disulfide groups. The healable thermoset rubber recovers 100% of the original mechanical properties after mending for a short duration of one hour. Xu et al. [78] introduced shape memory effect along with the disulfide links into the self-repairing material. The shape memory effect is the potential of the material to return to its original configuration after changes such as light, heat, immersion in water, irradiation with ultraviolet light, application of electric and magnetic fields, etc. are applied to the polymeric material. This phenomenon may augment the repairing process. They synthesized a self-healing polyurethane system by using PTMEG (polytetramethylene ether glycol) and disulfide links containing chain extender, thus couple the disulfide links and shape memory effect. The mending of the damaged material would be achieved by heat-induced exchange reactions of disulfide bonds and activating the shape memory effect simultaneously. The self-repairing of the crack would occur when heated above 80 °C. The scraps on the material would disappear in just 5 min due to the shape memory effect and the mechanical properties were fully recovered after a repairing process of 4 h. Ortiz et al. [79] synthesized a self-healing epoxy resin by using thiol-disulfide oligomer. The oligomer was synthesized by partial oxidation of a multifunctional thiol and an iodide (III) compound was used as an oxidant. The DGEBA epoxy resin was photopolymerized anionically by the action of the thiol-ene system and the curing agent (tetra-allyl functionalized). The photopolymerization enhances the concentration of polythioethers and took place only in 15 min. The glass transition temperature was found to be approximately 65–75 °C. The self-repairing was attained at room temperature in 500 min or by heating the sample at 80 °C for 10 min. The mending efficiency was found to be up to 111% when compared with the unhealed sample. Li et al. [80] prepared a self-healing epoxy network (by polymerizing an aromatic amine and two epoxy resins) with exchangeable disulfide links. A poly (ethylene glycol) diglycidyl ether (DER736) and DGEBA were used as soft and rigid components respectively. When changing the amount of the soft component in the epoxy network the flexibility of the material was also varied. The materials with lower glass transition temperature were more flexible and showed superior self-healing properties but feeble mechanical strength. The tensile tests were used to measure the self-mending performance of the epoxy network with changing flexibility. The tensile strength of a fully damaged sample was reestablished to 84% of the original values at mild temperature. They also studied the variation of self-healing properties with temperature and healing time. Chang et al. [81] reported a transparent self-mending polyurethane with disulfide links. Here for the synthesis of self-healing material, the highly stretchable properties exhibited by the PEG segment, transparency, and the shape memory effect all are combined together and the polyurethane network was synthesized by a two-step synthesis route. The polyurethane network was reliable for autonomous fracture closure by using its shape memory property and exchangeable disulfide bonds for self-repairing ability. The crystal structure and the self-mending properties of the synthesized polyurethane were examined through DSC (differential scanning calorimetry), DMA (dynamic mechanical analysis), XRD (X-ray diffraction), tensile tests, and stress relaxation. The polyurethane network was transparent due to low crystallinity, demonstrated superior stretchability and self-repairing properties. At moderate mending temperature, the healing efficiency was found to be above 90% and the break elongation was 800% along with excellent healing repeatability. Yoon et al. [82] made use of the ATRP polymerization technique and synthesized a polymeric material with a branched structure having reversible cross-linking functionalities at the periphery. The cross-linked cores composed of poly(ethylene glycol diacrylate) were used for the preparation of poly(n-butyl acrylate) implanted star polymer. These polymers further acted as macroinitiators for the successive chain extension ATRP of bis(2-methacryloyloxyethyl disulfide) DSDMA where disulfide reversible cross-links (SS) were imposed at the branch periphery. Under reducing conditions, the (SS) cross-linked polymers were split to form a thiol functionalized (SH) star polymer. The solution of this SH star polymer was accumulated on silicon wafer substrates and transformed into insoluble re-cross-linked SS films through oxidation. The AFM imaging and optical microscopy were used to analyze the self-healing studies. Zhang et al. [83] recently synthesized waterborne polyurethane (WPUR) films with 2,2’disulfanediyldianiline (22DTDA) as chain extender. WPUR consist of a urea moiety and an exclusive microphase structure in WPUR was developed due to urea moiety and the ortho-substituted aromatic disulphide structure. The synthesized material showed 180 times higher mechanical strength, when compared with the material having no 22DTDA and also showed self-healing capabilities at body temperature (37 °C) in air and under ultrasound in water. Three cutting-healing cycles proved that the WPUR films could be rehabilitated at the same damaged locations and the mechanical properties of these films were found to be almost constant all through these cycles. When the repairing process was carried out at 37 °C, 50 °C or under ultrasound, the tensile strength of the healed WPUR film was found to be 13.8 MPa, 15.4 MPa, and 16 MPa, and the elongation at break was 1150%, 1215%, and 1056% respectively. Because of the re-processibility, high mechanical properties, and body temperature self-mending abilities, these films can find potential applications in the fields of smart coatings, flexible sensors, recyclable adhesives, and electronic skin, etc.
A disulphide linked self-healing silicon elastomer was developed by Huang et al. [84] based on a reaction between thiol terminated sulphur containing hetrochain polysiloxanes (P-SHs) and thiol containing crosslinkers viz.: octa(3-mercaptopropyl)silsesquioxane (POSS-SH), pentaerythritol tetrakis(β-mercaptopropionate) (PETMP), and poly[(mercaptopropyl) methylsiloxane] (PMMS). These silicon elastomers were constructed quickly at room temperature. The elastomers were cut into pieces, and either two hours of heating at 150 °C or 30 min of UV exposure were used to induce self-healing. More than 70% healing efficiency was discovered. Since the bonding was reversible and could be processed multiple times, these elastomers may be used as reversible adhesive for bonding glass sheets.
Photoinduced self-healing polymers
Photochemical reactions are generally simple, fast, environment friendly, do not require any catalyst, additive, or thermal treatment, and are a common tool for organic synthesis. Dissimilar to the thermo-reversible network, this photo reversible network when irradiated, undergo bond rearrangements. A general photo-reversible reaction is represented in Scheme 2. Some photo-initiated cycloaddition reactions, such as [2 + 2] cycloaddition reactions of some olefinic compounds, when irradiated with a certain wavelength of light, undergo cyclization to form cyclobutene; the new formed covalent bond in the cyclized adduct can be cleaved to give the starting olefinic compounds. Photo-induced reversible cross-linking can be achieved by incorporating these photosensitive groups into the polymer system. Chung et al. [85] synthesized a polymeric system incorporated with cinnamoyl groups, which results in photochemically induced healing of the polymer. Here the polymer network was cross-linked with the cinnamoyl groups to form cyclobutane dimers via photochemical cycloaddition. It was found that when a crack propagates, then the cyclobutane group of the dimer would preferentially break due to high resin strain. The cyclobutane crosslinks would be reformed when irradiated with appropriate wavelength (> 310 nm) and the mechanical strength of the material can be restored. This process was examined by using IR spectroscopy, the absorption bands of cinnamoyl C = C and C = O appear at 1637 and 1713 cm− 1 respectively. When irradiated with ultra violet radiations, the absorption band of the carbonyl group was shifted to 1734 cm− 1 and C = C absorption band almost disappeared, which confirmed the photoinduced crosslinking of the material. The measurement of flexural strength of the cracked and the healed samples successfully demonstrates the crack healing in the polymer network. But only 14% healing efficiency was obtained by photochemical healing and a combination of photochemical and thermal healing results in 26%. In both cases the healing efficiencies are very low. Ghosh and Urban [86] reported a photoinduced self-healing polyurethane network. In this network, oxetane-substituted chitosan (OXE-CHI) precursor, which was the main factor for network re-generation, was crosslinked to the polyurethane network to form oxetane-substituted-chitosan-polyurethane (OXE-CHI-PUR). The self-healing property of the network system was observed by putting a crack on the surface which opens up the oxetane ring. When exposed to the UV light of wavelength 302 nm for 30 min, the chitosan chain scission occurs and form the crosslinks with oxetane ends. The repairing time for these materials is less than an hour. It was found that with an increase in the intensity of the UV light, the scratch healing was improved. These materials can be used in coating applications, transportation, and biomedical industries. Anthracene molecule is able to undergo thermally reversible DA reactions as well as photoinduced reversible cycloaddition reactions. For the advancement of photoinduced self-repairing materials, Froimowicz et al. [87] synthesized an anthracene-modified dendritic polymer. An artificial crack made on the crosslinked dendritic polymer was exposed to irradiation at 254 nm and irradiation at 365 nm and has been recovered. It may be noted that the self-healing cycles, as well as the debonding and re-bonding of the polymer network, are repeatable many times. Coumarin is a perfume substance that is obtained from plants and possesses photo-response, capable of photodimerization via [2 + 2] cycloaddition reaction. Ling et al. [88] constructed a polyurethane network crosslinked with coumarin moieties. The coumarin dimers cross-linked to the polyurethane backbone were ruptured when the polymer network was damaged or was exposed to UV light of wavelength 254 nm and was resumed by irradiating at 350 nm. The scratches on the polymer network are healed by Ultra-violet light or even by sunlight without using any catalyst and this coumarin moiety enables the polyurethane network to heal the crack more than once on the same site in the material, and hence the multiple cycles of crack healing are possible. Dong et al. [89] synthesized a photo reversible novel class of A2-B3 type supramolecular hyperbranched polymer. The host-guest complexation between β-cyclodextrin trimer (β-CD3) and azobenzene dimer (diazo) result in a supramolecular hyperbranched polymer network which possesses excellent optical properties and has the potential to be reversed by UV/Visible light irradiation. Nishikubo et al. [90] reported the synthesis and photochemical reactions of different polymers as hyperbranched epoxy-methacrylate, hyperbranched polyurethane-methacrylate, and hyperbranched oxetane-methacrylate which when compared with corresponding linear polymers, showed superior resolution properties and greater photochemical reactivity. It was found that many radical polymerizable groups were present in these photo-functional hyperbranched polymers and UV-curing of the material was applicable. The author reported that cured HBPEMA (hyperbranched epoxy methacrylate with pendent hydroxyl groups) shows slightly lower Tg values but had greater tensile strength, cross-linking density and showed greater elongation at break when compared with the corresponding cured linear LPEMA. Thus, the cured hyperbranched HBPEMA had strong mechanical properties. Banerjee et al. [91] reported a smart coating for photovoltaic equipments which was based on a coumarin functionalized tri-arm star polyisobutylene (PIB) polymer network. The coumarin moieties present in the network were reversibly photo dimerized by UV light (λmax = 365 nm) produces crosslinked elastomeric films with self-healing properties. The photocleavage was done by irradiation with UV light at λmax = 254 nm. Attractive barrier properties against moisture and oxygen were exhibited by the crosslinked PIB films which are useful as coatings for photovoltaic appliances. The dimerization and cleavage cycles were repeated numerous times and no degradation of the healing ability was observed. The degree of photodimerization was observed by using uv-vis spectroscopy. The AFM technique was used to study the self-healing behavior of the polymer network. The healing of the cracked site can also take place under sunlight even though very slowly.
Scott et al. [92] prepared a cross-linked polymer that shows photoinduced plasticity i.e. the probability to relax the mechanical strain or stress when irradiated with light. The group of authors had synthesized a polymer network integrated with allyl sulfide groups by using photoinitiated thiol-ene polymerization. The radicals used for the construction of the polymer network were generated from the residual photo initiator by irradiation (320-500 nm). These radicals initiate the propagation reaction by associating allyl sulfide groups. The Tg value of the network was found to be low ranging from −34 to-25 °C which means that at ambient temperature the polymer is in a rubbery state and can assign quick reshuffling of the polymer network. When the stress was applied to the sample without any irradiation, the strains observed were reversible, whatsoever the density of allyl sulfide groups. The other way round, when the stress is applied under irradiation the sample did not recover to its initial length. Even though the original behavior of these polymers was not inspected as self-healing properties, but it can be a flagstone for the transposal of the concept in this field [93].
Supramolecular non-covalent self-healing polymers
Supramolecular materials, (also termed as dynamic materials) are the materials that can possess stimuli-responsiveness and reprocess ability because the components of these systems are linked through dynamic covalent connections and/or reversible noncovalent connections. The most attractive property of these supramolecular systems with reversible bonds is their potential to self-repair whenever there is crack formation. Supramolecular interactions are reversible in nature. These interactions, because of their reversible nature are very pertinent to be incorporate into mendable materials, thus granting the multiple healing cycles to the network system. Leibler et al. [94] synthesized a self-mendable rubber, by employing hydrogen bonding to the network. The self-combination of the chains within the polymer network is due to the reversible nature as well as the directionality of the hydrogen bond. The damaged surfaces were brought in contact with one another to initiate the healing process and the ruptured network would reform due to the self-association of hydrogen bonds. The groups used by “Cordier et al.” for hydrogen bonding were amido imidazolidones and urea. The elastomeric properties of the material can be maintained when these groups were mixed so that they do not form crystalline regions and result in the supramolecular network. The rubber sample was cut into two pieces and at room temperature, the sample would self-repair over time when the two pieces were brought in contact again. As the system leans on reversible hydrogen bonding, no chemical component is added to initiate self-healing and the sample is regenerated by using mechanical stimuli i.e., by bringing the damaged ends to connect with each other. Multiple healing cycles can be attained successfully due to reversible hydrogen bonding. Wang et al. [95] proposed an elastomer nanocomposite that has the potential to self-repair rapidly at room temperature. They integrated the graphene oxide cross-linkers with hydrogen-bonded polymer. Graphene oxide provided good mechanical strength and hydrogen bonding enabled it to be self-repairable. This composite system possessed a fast and spontaneous self-mending ability (without any external stimuli) of up to 50% of its original system in about 1 min. and complete mechanical healing in about an hour. Zeng et al. [96] made use of host-guest interactions and formulated a supramolecular polymer gel of triptycene-based bis (crown ether) and a copolymer having DBA (dibenzylammonium) moieties. The construction of this supramolecular polymer gel was manifested by solution viscometry and 1H NMR spectroscopy. At high concentrations, the colorless and transparent supramolecular gel was obtained which showed multi-stimuli reversible responsiveness as acid/base-, thermo-, and chemo-induced sol-gel transitions. It was concluded from rheological measurements that the thixotropic process could be replicated at least three times and the gel had intrinsic self-healing properties. It was found that the supramolecular polymer gels can behave as erasable materials when they were doped with spiropyran molecules which were predicted to be favorable for further development of smart materials. Huang et al. [97] reported a self-healing composite constructed by using (FG) a few-layer graphene and thermoplastic polyurethane (TPU). This FG-TPU polymer composite illustrates enhanced mechanical properties along with repeated healing through various methods such as IR (infrared) light, electromagnetic wave, and electricity with an attractive healing efficiency of more than 98%. Moreover, no depreciation of healing efficiencies was found after repeated healing and hence polymer composite can show a wide range of applications in construction industries, electronics, and transport industries, etc.
Uses and future scope of self-healing materials
Various polymeric and metallic materials can be used for the advancement of self-healing network structures. Since the CNTs (carbon nanotubes) have cavities in their pipeline structure along with broad interfacial area and excellent mechanical/ chemical properties, they are now given due consideration as a perfect material, like storage devices, and also can provide mechanical support to the composite system. Despite the substantial progress in the field of self-healing materials, this field is still in its initial phase. There are many issues that should be overcome before the use of these materials in an engineering discipline.
Conclusion
In this article, we have reviewed various polymer/composite systems based on two approaches of self-healing. A large number of research papers are being published every year as the research into self-repairing smart materials is an active field and an interesting aspect for drawing a contemporary self-healing system. The main focus of this review is on the self-healing concept in polymeric materials and their applications in the engineering field and our day-to-day life. There are two main types of self-healing approaches, extrinsic self-healing (micro capsule-based and microvascular-based systems) and intrinsic self-healing (Diels Alder-based, photoinduced, and supramolecular self-healing systems). The main limitation of the capsule-based system is the one-time healing and the vascular based system has the disadvantages of complexity in the design and synthesis of the polymer matrix. But the intrinsic approach can over-throw these limitations and can have the potential of multiple healing at the same site. Though the major limitation of the intrinsic approach is the poor thermal and mechanical performance. Computer simulations have catered to the important implications for guiding scientists toward the fabrication of a healing system. During the last few years, structural designs, manufacturing processes, and composite materials are improving constantly. The polymeric composite materials have finite acceptance in all engineering disciplines because of the crack initiation, propagation, and tolerance. The composite materials can be prepared by using self-mending techniques which are inspired by nature. The polymeric materials have the potential to respond to internal as well as external stimuli and serve as an interesting area of scientific research and thus find many commercial applications. Nature is the supplier of boundless inspirations, and there may be a large number of favorable circumstances in the design engineering and synthesis of stimuli-responsive polymeric materials which need to be tapped. The main challenge in this field is to design a self-healing polymeric system with high Tg value, where self-healing is induced by electric and/or magnetic fields, electromagnetic radiations, and changes in the environment or atmosphere.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
van Benthem RATM, Ming W (Marshall), de With G (eds) (2007) (Bert) Self Healing Polymer Coatings. In: Springer Series in Materials Science. pp 139–159
Dry C (1996) Procedures developed for self-repair of polymer matrix composite materials. Compos Struct 35:263–269. https://doi.org/10.1016/0263-8223(96)00033-5
Chen Y, Kushner AM, Williams GA, Guan Z (2012) Multiphase design of autonomic self-healing thermoplastic elastomers. Nat Chem 4:467–472. https://doi.org/10.1038/nchem.1314
Zhang Y, Yu Y, Zhao X et al (2021) A high strength but fast fracture-self-healing thermoplastic elastomer. Macromol Rapid Commun 42:1–6. https://doi.org/10.1002/marc.202100135
Yu K, Xin A, Feng Z et al (2020) Mechanics of self-healing thermoplastic elastomers. J Mech Phys Solids 137. https://doi.org/10.1016/j.jmps.2019.103831
Jones AR, Watkins CA, White SR, Sottos NR (2015) Self-healing thermoplastic-toughened epoxy. Polym (Guildf) 74:254–261. https://doi.org/10.1016/j.polymer.2015.07.028
Subramanian V, Varade D (2017) Self-healed materials from thermoplastic polymer composites. 153–180. https://doi.org/10.1007/978-3-319-50424-7_6
Eom Y, Kim SM, Lee M et al (2021) Mechano-responsive hydrogen-bonding array of thermoplastic polyurethane elastomer captures both strength and self-healing. Nat Commun 12:1–11. https://doi.org/10.1038/s41467-021-20931-z
Zhu DY, Wetzel B, Noll A et al (2013) Thermo-molded self-healing thermoplastics containing multilayer microreactors. J Mater Chem A 1:7191–7198. https://doi.org/10.1039/c3ta11008g
Wang HP, Yuan YC, Rong MZ, Zhang MQ (2010) Self-healing of thermoplastics via living polymerization. Macromolecules 43:595–598. https://doi.org/10.1021/ma902021v
Karami Z, Zolghadr M, Zohuriaan-Mehr MJ (2020) Self-healing diels–alder engineered thermosets. Self-Healing polymer-based Systems. INC, pp 209–233
Li G, Meng H (2015) Overview of crack self-healing. In: Recent advances in smart self-healing polymers and composites, pp 1–19
Kahar NNFNMN, Osman AF, Alosime E et al (2021) The versatility of polymeric materials as self-healing agents for various types of applications: a review. Polym (Basel) 13:1–34. https://doi.org/10.3390/polym13081194
Radl S, Kreimer M, Griesser T et al (2015) New strategies towards reversible and mendable epoxy based materials employing [4πs + 4πs] photocycloaddition and thermal cycloreversion of pendant anthracene groups. Polym (Guildf) 80:76–87. https://doi.org/10.1016/j.polymer.2015.10.043
Yuan D, Solouki Bonab V, Patel A, Yilmaz T, Gross RA, Manas-Zloczower I (2020) Design strategy for self‐healing epoxy coatings.pdf. Coatings 10
Du Y, Li D, Liu L, Gai G (2018) Recent achievements of self-healing graphene/polymer composites. Polym (Basel) 10. https://doi.org/10.3390/polym10020114
Lin C, Ge H, Wang T et al (2020) A self-healing and recyclable polyurethane/halloysite nanocomposite based on thermoreversible Diels-Alder reaction. Polym (Guildf) 206:122894. https://doi.org/10.1016/j.polymer.2020.122894
Yuan YC, Ye XJ, Rong MZ et al (2011) Self-healing epoxy composite with heat-resistant healant. ACS Appl Mater Interfaces 3:4487–4495. https://doi.org/10.1021/am201182j
Lee JY, Buxton GA, Balazs AC et al (2014) Using nanoparticles to create self-healing composites Using nanoparticles to create self-healing composites. 5531. https://doi.org/10.1063/1.1784432
Frei R, McWilliam R, Derrick B et al (2013) Self-healing and self-repairing technologies. Int J Adv Manuf Technol 69:1033–1061. https://doi.org/10.1007/s00170-013-5070-2
Gurumurthy BM, Shivaprakash YM, Hiremath A et al (2016) Self healing materials: a new era in material technology: a review. Int J Appl Eng Res 11:1373–1378
Zwaag SV, Der, Grande AM, Post W et al (2014) Review of current strategies to induce self-healing behaviour in fibre reinforced polymer based composites. 30:1633–1641. https://doi.org/10.1179/1743284714Y.0000000624
Urdl K, Kandelbauer A, Kern W et al (2017) Self-healing of densely crosslinked thermoset polymers—a critical review. Prog Org Coatings 104:232–249. https://doi.org/10.1016/j.porgcoat.2016.11.010
Hu H, Zhang L, Zhang Y et al (2020) Microencapsulation of tris(dimethylaminomethyl)phenol using polystyrene shell for self-healing materials. Sci Rep 10:1–14. https://doi.org/10.1038/s41598-020-69168-8
Kessler MR, White SR (2001) Self-activated healing of delamination damage in woven composites. Compos Part A Appl Sci Manuf 32:683–699
Brown EN, White SR, Sottos NR (2004) Microcapsule induced toughening in a self-healing polymer composite. J Mater Sci 39:1703–1710. https://doi.org/10.1023/B:JMSC.0000016173.73733.dc
White SR, Sottos NR, Geubelle PH et al (2002) Erratum: correction: autonomic healing of polymer composites. Nature 415:817–817. https://doi.org/10.1038/415817a
Lee JK, Hong SJ, Liu X (2004) Characterization of dicyclopentadiene and 5-ethylidene-2-norbornene as self-healing agents for polymer composite and its microcapsules. 12:478–483
Thakur T, Gaur B, Singha AS (2021) Bio-based epoxy/imidoamine encapsulated microcapsules and their application for high performance self-healing coatings. Prog Org Coatings 159:106436. https://doi.org/10.1016/j.porgcoat.2021.106436
Rodriguez R, Bekas DG, Flórez S et al (2020) Development of self-contained microcapsules for optimised catalyst position in self-healing materials. Polym (Guildf) 187:122084. https://doi.org/10.1016/j.polymer.2019.122084
Tang H, Fang ZP (2008) Preparation of glass fiber-supported platinum complex catalyst for hydrosilylation reactions. 9:1092–1095. https://doi.org/10.1016/j.catcom.2007.10.017
Cho SH, Andersson HM, White SR et al (2006) Polydiniethylsiloxane-based self-healing materials. Adv Mater 18:997–1000. https://doi.org/10.1002/adma.200501814
Ullah H, Azizli K, Man ZB, Ismail MBC (2016) Synthesis and characterization of urea-formaldehyde Microcapsules containing functionalized polydimethylsiloxanes. Procedia Eng 148:168–175. https://doi.org/10.1016/j.proeng.2016.06.519
Dohler D, Michael P, Binder W (2013) Part one design of self-healing materials. In: Self-healing polymers: from principles to applications, pp 5–60
Wang R, Hu H, Liu W, Guo Q (2011) Preparation and characterization of self-healing polymeric materials with microencapsulated epoxy and imidazoline derivatives curing agent. 19:279–288. https://doi.org/10.1177/0967391111019004-505
Yuan YC, Rong MZ, Zhang MQ et al (2008) Self-healing polymeric materials using epoxy / mercaptan as the healant. 5197–5202
Jin H, Mangun CL, Stradley DS et al (2012) Self-healing thermoset using encapsulated epoxy-amine healing chemistry. Polym (Guildf) 1–7. https://doi.org/10.1016/j.polymer.2011.12.005
Blaiszik BJ, Caruso MM, Mcilroy DA et al (2009) Microcapsules filled with reactive solutions for self-healing materials. Polym (Guildf) 50:990–997. https://doi.org/10.1016/j.polymer.2008.12.040
Song Y, Jo Y, Lim Y et al (2013) Sunlight-Induced Self-Healing of a Microcapsule-Type Protective Coating. ACS Appl Mater Interfaces 5:1378–1384
Trask RS, Williams GJ, Bond IP et al (2007) Bioinspired self-healing of advanced composite structures using hollow glass fibres Bioinspired self-healing of advanced composite structures using hollow glass fibres. 363–371. https://doi.org/10.1098/rsif.2006.0194
Dry CM, Sottos NR (1993) Passive smart self-repair in polymer matrix composite materials — University of Illinois Urbana-Champaign. Proc SPIE - Int Soc Opt Eng 1916:438–444
Toohey KS, Sottos NR, Lewis JA et al (2007) Self-healing materials with microvascular networks. 581–585. https://doi.org/10.1038/nmat1934
Toohey BKS, Hansen CJ, Lewis JA et al (2009) Delivery of two-part self-healing chemistry via microvascular networks.1399–1405. https://doi.org/10.1002/adfm.200801824
Hansen BCJ, Wu W, Toohey KS et al (2009) Self-healing materials with interpenetrating microvascular networks. 4143–4147. https://doi.org/10.1002/adma.200900588
Hansen CJ, White SR, Sottos NR, Lewis JA (2011) Accelerated self-healing via ternary interpenetrating microvascular networks. 4320–4326. https://doi.org/10.1002/adfm.201101553
Postiglione G, Alberini M, Leigh SJ et al (2017) Effect of 3D-printed microvascular network design on the self-healing behaviour of crosslinked polymers. https://doi.org/10.1021/acsami.7b01830
Hamilton BAR, Sottos NR, White SR (2010) Self-healing of internal damage in synthetic vascular materials. 61801:5159–5163. https://doi.org/10.1002/adma.201002561
Trask RS, Norris CJ, Bond IP (2014) Stimuli-triggered self-healing functionality in advanced fibre-reinforced composites. 25:87–97. https://doi.org/10.1177/1045389X13505006
Bekas DG, Baltzis D, Paipetis AS (2017) Nano-reinforced polymeric healing agents for vascular self-repairing composites. JMADE 116:538–544. https://doi.org/10.1016/j.matdes.2016.12.049
Zhu Y, Ji X, Zhi M, Qiu M (2016) Self-healing glass fi ber / epoxy composites with polypropylene tubes containing self-pressurized epoxy and mercaptan healing agents. Compos Sci Technol 135:146–152. https://doi.org/10.1016/j.compscitech.2016.09.020
Al-maadeed PPV MASA (2016) TiO 2 nanotubes and mesoporous silica as containers in self-healing epoxy coatings. Nat Publ Gr 1–9. https://doi.org/10.1038/srep38812
Bleay SM, Loader CB, Hawyes VJ et al (2001) A smart repair system for polymer matrix composites. Compos Part A Appl Sci Manuf 32:1767–1776. https://doi.org/10.1016/S1359-835X(01)00020-3
Williams G, Trask R, Bond I (2007) A self-healing carbon fibre reinforced polymer for aerospace applications. Compos Part A Appl Sci Manuf 38:1525–1532. https://doi.org/10.1016/j.compositesa.2007.01.013
Pang JWC, Bond IP (2005) ‘ Bleeding composites ’— damage detection and self-repair using a biomimetic approach. 36:183–188. https://doi.org/10.1016/j.compositesa.2004.06.016
Silva ACM, Moghadam AD, Singh P, Rohatgi PK (2017) Self-healing composite coatings based on in situ micro–nanoencapsulation process for corrosion protection. J Coat Technol Res 1–29. https://doi.org/10.1007/s11998-016-9879-0
Nevejans S, Ballard N, Miranda JI et al (2016) The underlying mechanisms for self-healing of poly(disulfide)s. Phys Chem Chem Phys 18:27577–27583. https://doi.org/10.1039/c6cp04028d
Garcia SJ (2014) Effect of polymer architecture on the intrinsic self-healing character of polymers. Eur Polym J 53:118–125. https://doi.org/10.1016/j.eurpolymj.2014.01.026
Zhou J, Guimard NK, Inglis AJ et al (2012) Thermally reversible Diels-Alder-based polymerization: an experimental and theoretical assessment. Polym Chem 3:628–639. https://doi.org/10.1039/c1py00356a
Pratama PA, Shari M, Peterson AM, Palmese GR (2013) Room temperature self-healing Thermoset based on the Diels – AlderAm403459E.Pdf. ACS Appl Mater Interfaces 12425–12431
Chen X, Dam MA, Ono K et al (2002) A thermally re-mendable cross-linked polymeric material. Sci (80-) 295:1698–1702. https://doi.org/10.1126/science.1065879
Scheltjens G, Diaz MM, Brancart J et al (2013) A self-healing polymer network based on reversible covalent bonding. React Funct Polym 73:413–420. https://doi.org/10.1016/j.reactfunctpolym.2012.06.017
Turkenburg DH, Durant Y, Fischer HR (2017) Bio-based self-healing coatings based on thermo-reversible Diels-Alder reaction. Prog Org Coatings 111:38–46. https://doi.org/10.1016/j.porgcoat.2017.05.006
Coope TS, Turkenburg DH, Fischer HR et al (2016) Novel Diels-Alder based self-healing epoxies for aerospace composites. Smart materials and structures. Springer, Singapore, Singapore, pp 15–39
Toncelli C, De Reus DC, Picchioni F, Broekhuis AA (2012) Properties of reversible diels-alder furan/maleimide polymer networks as function of crosslink density. Macromol Chem Phys 213:157–165. https://doi.org/10.1002/macp.201100405
Liu Y, Hsieh C (2005) Crosslinked epoxy materials exhibiting thermal remendablility and removability from multifunctional maleimide and furan compounds. 905–913. https://doi.org/10.1002/pola.21184
Tian Q, Yuan C, Rong Z, Qiu M (2009) A thermally remendable epoxy resin. 1289–1296. https://doi.org/10.1039/b811938d
Parihar S, Gaur B (2022) Thermo-reversible self-healing polymeric coatings derived from gum rosin. Prog Org Coatings 168:106889. https://doi.org/10.1016/j.porgcoat.2022.106889
Liu Y, Chen Y (2007) Thermally reversible cross-linked polyamides with high toughness and self-repairing ability from maleimide- and furan-functionalized aromatic polyamides. 224–232. https://doi.org/10.1002/macp.200600445
Kavitha AA, Singha NK (2007) A tailor-made polymethacrylate bearing a reactive diene in reversible diels – alder reaction. 4441–4449. https://doi.org/10.1002/pola
Peterson AM, Jensen RE, Palmese GR (2011) Thermoreversible and remendable glass-polymer interface for fiber-reinforced composites. Compos Sci Technol 71:586–592. https://doi.org/10.1016/j.compscitech.2010.11.022
Du P, Liu X, Zheng Z et al (2013) Synthesis and characterization of linear self-healing polyurethane based on thermally reversible Diels-Alder reaction. RSC Adv 3:15475–15482. https://doi.org/10.1039/c3ra42278j
Zeng C, Seino H, Ren J et al (2013) Bio-based furan polymers with self-healing ability. Polym (Guildf) 54:5351–5357. https://doi.org/10.1016/j.polymer.2013.07.059
Bai N, Simon GP, Saito K (2015) Characterisation of the thermal self-healing of a high crosslink density epoxy thermoset. New J Chem 39:3497–3506. https://doi.org/10.1039/c5nj00066a
Okhay N, Mignard N, Jegat C, Taha M (2013) Diels-Alder thermoresponsive networks based on high maleimide- functionalized urethane prepolymers. Des Monomers Polym 16:475–487. https://doi.org/10.1080/15685551.2012.747166
Xu M, Liu N, Mo H et al (2022) Synthesis and properties of thermally self-healing PET based Linear polyurethane containing diels–alder bonds. Polym (Basel) 14:1–13. https://doi.org/10.3390/polym14163334
Lee WJ, Cha SH (2020) Improvement of mechanical and self-healing properties for polymethacrylate derivatives containing maleimide modified graphene oxide. Polym (Basel) 12. https://doi.org/10.3390/polym12030603
Canadell J, Goossens H, Klumperman B (2011) Self-healing materials based on disulfide links. Macromolecules 44:2536–2541. https://doi.org/10.1021/ma2001492
Xu Y, Chen D (2016) A novel self-healing polyurethane based on disulfide bonds. Macromol Chem Phys 217:1191–1196. https://doi.org/10.1002/macp.201600011
Ortiz RA, Berlanga OA, Valdez AEG et al (2016) Self-healing photocurable epoxy/thiol-ene systems using an aromatic epoxy resin. Adv Mater Sci Eng. https://doi.org/10.1155/2016/8245972
Li ZJ, Zhong J, Liu MC et al (2020) Investigation on self-healing property of epoxy resins based on disulfide dynamic links. Chin J Polym Sci (English Ed) 38:932–940. https://doi.org/10.1007/s10118-020-2406-x
Chang K, Jia H, Gu S (2019) A transparent, highly stretchable, self-healing polyurethane based on disul fi de bonds. Eur Polym J 112:822–831. https://doi.org/10.1016/j.eurpolymj.2018.11.005
Yoon JA, Kamada J, Koynov K et al (2012) Self-healing polymer films based on thiol-disulfide exchange reactions and self-healing kinetics measured using atomic force microscopy. Macromolecules 45:142–149. https://doi.org/10.1021/ma2015134
Zhang L, Qiu T, Sun X et al (2020) Achievement of both mechanical properties and intrinsic self-healing under body temperature in polyurethane elastomers: a synthesis strategy from waterborne polymers. Polym (Basel) 12. https://doi.org/10.3390/POLYM12040989
Huang Y, Yan J, Wang D et al (2021) Construction of self-healing disulfide-linked silicone elastomers by thiol oxidation coupling reaction. Polym (Basel) 13. https://doi.org/10.3390/polym13213729
Chung CM, Roh YS, Cho SY, Kim JG (2004) Crack healing in polymeric materials via photochemical [2 + 2] cycloaddition. Chem Mater 16:3982–3984. https://doi.org/10.1021/cm049394+
Ghosh B, Urban MW (2009) Self-repairing oxetane-substituted chitosan polyurethane networks. Science 323(5920):1458–1460. https://doi.org/10.1126/science.1167391
Froimowicz P, Frey H, Landfester K Towards the generation of self-healing materials by means of a reversible photo-induced approach. https://doi.org/10.1002/marc.201000643
Ling J, Rong MZ, Zhang MQ (2011) Coumarin imparts repeated photochemical remendability to polyurethane. J Mater Chem 14473–14486. https://doi.org/10.1039/c1jm12321a
Dong R, Liu Y, Zhou Y, Yan D, Zhu X (2011) Photo-reversible supramolecular hyperbranched polymer based on host–guest interactions. Polym Chem 2771–2774. https://doi.org/10.1039/c1py00426c
Nishikubo T, Kudo H, Maruyama K (2009) Synthesis and properties of photo-functional hyperbranched polymers. 1–7. https://doi.org/10.1002/pat.1377
Banerjee S, Tripathy R, Cozzens D et al (2015) Photoinduced smart, self-healing polymer sealant for photovoltaics. https://doi.org/10.1021/am508096c
Scott BTF, Draughon RB, Bowman CN (2006) Actuation in crosslinked polymers via photoinduced stress relaxation . 2128–2132. https://doi.org/10.1002/adma.200600379
Ahn D, Zavada SR, Scott TF (2017) Rapid, Photomediated Healing of Hexaarylbiimidazole-Based covalently cross-linked gels. Chem Mater 29:7023–7031. https://doi.org/10.1021/acs.chemmater.7b02640
Leibler L, Cordier P, Soulie C (2008) Self-healing and thermoreversible rubber from supramolecular assembly. 451:977–980. https://doi.org/10.1038/nature06669
Wang C, Liu N, Allen R et al (2013) Communication A rapid and efficient self-healing thermo-reversible elastomer crosslinked with graphene oxide. 5785–5790. https://doi.org/10.1002/adma.201302962
Zeng F, Han Y, Yan Z et al (2013) Supramolecular polymer gel with multi stimuli responsive, self- healing and erasable properties generated by host e guest interactions. Polym (Guildf) 54:6929–6935. https://doi.org/10.1016/j.polymer.2013.10.048
Huang L, Yi N, Wu Y et al (2013) Multichannel and repeatable self-healing of mechanical enhanced graphene-thermoplastic polyurethane composites. 2224–2228. https://doi.org/10.1002/adma.201204768
Acknowledgements
The authors are grateful to Ministry of Human Resource Development (MHRD), India as well as the National Institute of Technology Hamirpur, Himachal Pradesh, India, for funding the research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Parihar, S., Gaur, B. Self healing approaches in polymeric materials-an overview. J Polym Res 30, 217 (2023). https://doi.org/10.1007/s10965-023-03590-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-023-03590-0