Abstract
In this study, nanocomposite hydrogels of poly(methacrylic acid-co-acrylamide) (Poly(MAA-co-AAm)) containing different weight percent bentonite clay nanoparticles (0, 5, 10, and 15 wt. %) were used to remove methyl violet (MV) dye. FTIR, SEM, TGA, and XRD were used to analyze the properties of adsorbents. Results showed that bentonite nanoparticles were successfully distributed in the hydrogel system. Bentonite nanoparticles at 10 wt. % gave the maximum MV dye adsorption efficiency. The highest adsorption was obtained at a pH of 5, an adsorbent dosage of 1.5 g/L, a temperature of 25 °C, a contact time of 60 min, and a pollutant concentration of 10 mg/L. In thermodynamics studies, a negative values of Gibbs free energy (ΔG°) indicating that the adsorption process was spontaneous. In addition, Poly(MAA-co-AAm) hydrogels and Poly(MAA-co-AAm)/bentonite nanocomposite hydrogels gave the adsorption enthalpy (ΔH°) of –61.24 and –36.14 kJ/mol witch shows that the adsorption process is exothermic in the temperature range 25–45 °C using both adsorbents. Langmuir isotherm model was successfully applied in describing the equilibrium behavior of the adsorption process. The kinetic study showed that the pseudo-second-order model was more successful in describing the kinetic behavior of the adsorption process than pseudo-first-order and Elovich models. Poly(methacrylic acid-co-acrylamide) and poly(methacrylic acid-co-acrylamide)/bentonite adsorbents gave the α parameter values of 0.4276 and 22.15 mg.(g.min)−1, respectively indicating the high adsorption capacity of these adsorbents.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Water pollution from the improper discharge of industrial dyes originated from various industries such as textile, leather, paper, plastic, tanning, etc. is a very serious environmental concern, which threaten the living organisms and ecosystem [1, 2], thus, they have to be removed from wastewater streams before entering to the environment. Dyes are of major classes of polluting substances [3, 4] that are divided into cationic and anionic types [5, 6]. The annual production of industrial dyes is estimated to be 10,000 tons, of which about 1 to 10 percent of is being discharged to the environment [7]. Due to their complex structure, chemical dyes cause pollution in the environment and harm humans, animals and other living organisms, especially aquatic animals [8, 9]. Dyes are aromatic substances that have a high solubility in water, so they are easily dispersed in water and trap hydrogen molecules [10]. These pollutants are structurally divided into three categories: cationic, anionic, and non-ionic. Cationic dyes have more destructive effects than other dye pollutants. Most of them release into surface waters by the textile industry [11]. Malachite Green (MG) with the chemical formula of C23H26ClN3 is a cationic dye with a molar mass of 393.94 g/mol. MG is one of the dyeing products of aniline. In various industries such as the production of paint, paper, clothing, textiles, and printing presses, the pollution of the aquatic environments can be seen due to the release of dye pollutants. Among these, textiles play a major role in polluting the aquatic environment of the planet because about 15% of the dye used in textile factories is disposed directly to the wastewaters that are released into surface waters [12, 13]. Complete removal of dyes from the wastewater streams is a challenging issue because of their longer stability in the environment. In previous studies various techniques such as electrochemical, ion exchange, catalysis, deformation and adsorption have been suggested to remove the dyes from aqueous solutions [14, 15]. In the adsorption process, a variety of natural and synthetic adsorbents such as chitosan, alginate, activated carbon, algae, etc. can be used. Among these, natural adsorbents have a high priority due to their availability as well as their reversibility and biodegradability. The presence of carboxyl, amine, and hydroxyl functional groups on the surface of the adsorbers are important factors in increasing the adsorption of pollutants [16, 17]. Water treatment systems based on hydrogel show the potential of efficient adsorption due to the three-dimensional network of polymer chains and hydrophilic nature of hydrogels [18]. In addition, hydrogels are cheap, non-toxic, chemically and physically stable, they form a good polymer network with a good ability to be recycled [19, 20]. Hydrogels exhibit a high adsorption affinity to remove different chemicals from aqueous solutions even at lower concentrations, which makes them important for widespread use in environmental applications [21, 22]. Numerous substances including natural polymers (chitin, chitosan, carboxymethyl cellulose, etc.), agricultural wastes, silicate layers such as mineral clays and activated carbon are used as a sorbent to remove some chemicals from wastewater [23]. Most of these materials are cheap, biocompatible and abundant having good mechanical and chemical properties enabling them to be used for wastewater treatment [24]. In general, all soils have a high swelling in the aqueous medium. There is no exception for Bentonite as a mineral material to this rule and due to the presence of van der waals force in its structure, after adsorbing water, its volume reaches six times its original volume [25, 26]. Bentonite with overall formula Al2H2Na2O13Si4 possesses a large surface area and cation-exchange ability making it suitable to remove organic dyes fromwatse waters [27, 28]. Of course, it should be noted that in clays with high water adsorption, the structure of this mineral is two quadrilateral layers of silica and one octahedral layer of alumina. By replacing silica (in the quadrilateral layer) with magnesium and divalent iron (in the octahedral layer), different groups are formed [29, 30]. The aim of this study was to synthesize and describe poly nanocomposite hydrogels (MAA-co-AAm)/bentonite as a biodegradable adsorbent, inexpensive and effective in adsorbing methyl violet dye. Different techniques such as FTIR, TGA, SEM, and XRD were applied to specify the hydrogels’ structure. Adsorption parameters including process temperature, time, the concentration of dye, adsorbent dosage and pH were studied. Mathematical models of Langmuir, Freundlich, Dubinin-Radushkevich (D-R) and Temkin isotherm were used to describe the equilibrium behavior of the adsorption process. Also, to study the kinetic behavior of the process, common models such as pseudo-first-order, pseudo-second-order, and Elovich were used. In addition, the thermodynamic behavior of traditional hydrogels was evaluated. In this study, the effect of operating conditions including pH, temperature, contact time, and initial MV concentration on the adsorption performance will be studied that is a need for optimization of the operating conditions. Also, the thermodynamic adsorption parameters of synthesized hydrogel samples were investigated for this adsorption process and changes in Gibbs free energy and entropy of the adsorption will be estimated at different temperatures. These thermodynamic data for the system of this grafted hydrogel composite as an adsorbent and MV as an adsorbent were never reported similarly by any scholars.
Materials and methods
Materials
Methacrylic acid (MAA) and acrylamide (AAm) monomers and methyl violet (MV) dye (λmax = 582 nm) were purchased from Merck (Darmstadt, Germany). Potassium persulfate was obtained from Samchun (Seoul, South Korea). N,N'- Methylenebisacrylamide (MBA) and bentonite were purchased from Sigma Aldrich (Munich, Germany).
Synthesis of poly(MAA-co-AAm)/Bentonite nanocomposite hydrogels
Poly(MAA-co-AAm) and poly(MAA-co-AAm)/Bentonite nanocomposite hydrogels were prepared using free radical polymerization. For copolymerization of monomers in hydrogel synthesis, 5 ml MAA were poured in a three-necked flask equipped with nitrogen gas inlet–outlet, mechanical stirrer and a thermometer. Bentonite in a concentration of 0–15 wt. % was dispersed in 2 g of AAm and 10 mL of deionized water, and the mixture was sonicated for 20 min at 25 °C to fully disperse the nanoclay in the AAm solution. A mixture of bentonite, AAm and N,N'-Methylenebisacrylamide (MBA) as a crosslinker were added to the flask and heating was continued to 70 °C for 30 min. To produce free radicals potassium persulfate (as an initiator) was then added to the above mixture under nitrogen gas. The first signs of gel formation appeared after 20 min. The reaction was performed for a total time of 3 h to complete the polymerization process. The obtained nanocomposite hydrogels were further purified to remove the non-reactive monomers by deionized water washing for several times. The obtained adsorbents (nanocomposite hydrogels) were let to dry out gently at 55 °C for 48 h followed by pulverization to pass through a sieve with 250–500 μm mesh size. Poly(MAA-co-AAm) hydrogel without bentonite nanaoclay were synthesized according to the above-mentioned procedure for comparison purpose.
Characterization method
The chemical structure of monomers (MAA and AAm), bentonite nanoclay, poly(MAA-co-AAm) and poly(MAA-co-AAm)/bentonite nanocomposite hydrogels were examined using FTIR spectroscopy (Tensor 27, Broker, Germany) in a scanning range of 400 to 4000 cm−1. X-ray diffraction patterns were obtained using an X-ray diffractometer (Siemens S5000, Germany) according to method described previously [27]. SEM was applied to characterize the surface morphology of nanocomposite hydrogels according to the procedure described previously [24]. EDXA and dot-mapping (Microanalysis Oxford Instruments model 7718 INCA PentaFET) technique was used to determine the distribution of nanoclay at the cross-section of hydrogels. Thermogravimetric analysis (TGA) was conducted to evaluate the thermal stability of samples [31]. To study the adsorption capacity, MV-dye at different concentrations (10–100 mg.L−1 DW) was used. For this purpose, 0.05 g of dry hydrogel was added to 50 mL of MV-dye solution and magnetically stirred (800 rpm) for 60 min. After reaching equilibrium adsorption capacity (initial tests were performed at 25 °C, contact time of 60 min and weight of 1 g.L−1 of the adsorbent dosage), the solutions were filtered by centrifugation its dye content was measured by UV–Vis spectrophotometry. The adsorption capacity of the adsorbents at equilibrium (qe, mg.L−1) and adsorption efficiency (R %) were calculated using Eqs. 1 and 2, respectively.
where, C0 is the initial concentration of the contaminant, Ct is the final concentration, V is the volume of the solution used, and m (g.L−1) is amount of adsorbent used.
Swelling behavior of hydrogels
Swelling behavior of copolymer and nanocomposite hydrogel samples were investigated. For this purpose, 20 mg of adsorbent was poured into 10 mL of DW and after one day (24 h), the solution was centrifuged. The adsorbent was separated from DW and weighed. The amount of adsorbent swelling ratio was calculated using Eq. (3) [24].
where Wwet and Wdry are the wet and dry weights of the hydrogels, respectively.
Results and Discussion
Properties of hydrogels
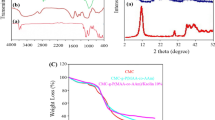
Figure 1a shows the FTIR spectra of the bentonite nanoparticles indicating characteristic peaks at wavenumber of 3616 cm−1 (corresponding to the tensile vibration of the hydrogen bonds), 1474 and 1596 cm−1 (indicating the bending of Nitrate-ions) [32]. The rest of the vibrations are in the range of 1000–1050 cm−1 due to the tension of the Si–O-C bond [33]. Figure 1b shows the FTIR spectra of poly(MAA-co-AAm) hydrogel after adsorption of the dye contaminant. The peaks at 1631 and 1699 cm−1 indicate the involvement of the carbonyl and vinyl groups of MAA monomer in the free radical polymerization. In addition, the peaks of 1728 and 1751 cm−1 are symmetrically bonded to carboxylate anions and C = O tensile vibrations in the monomers, respectively [34]. The absorption peaks at wavenumbers of 1648 and 1654 cm−1 were assigned to the C = C in MMA and AAm disappeared after polymerization. Figure 1c shows the FTIR spectra of poly(MAA-co-AAm)/bentonite nano composites after adsorption of MV dye. Compared to the shape of the copolymer hydrogel, the vibration distance was reduced, which indicates that the methyl violet (MV) dye was trapped in the pores of the nanocomposite. In addition, the increase in the transmission spectrum in 1653 cm−1, which is related to the poly(MAA-co-AAm) bonds, indicates that these bonds are filled with dye contaminants. Finally, the peaks in the nanocomposite structure between 400–500 cm−1 are related to Si–O-Si and Si–O-Al bond tension [35].
Figure 2 shows the XRD patterns of bentonite nanoparticles, poly(MAA-co-AAm), and poly(MAA-co-AAm)/bentonite nanocomposite hydrogels. As shown in the figure, bentonite nanoclay shows many diffraction peaks due to the presence of different crystalline phases. The distinct peak at 2θ = 5–10° (specified in 2θ = 5.7°) related to the basal-spacing (d001) for layered gallery structure of bentonite nanoclay. From Bragg’s equation for layered silicates such as bentonite nanoclay, the d001 is calculated as 1.55 nm. For poly(MAA-co-AAm) and poly(MAA-co-AAm)/bentonite nanocomposite hydrogels, this distinct peak hasn’t appeared which can be due to the complete disaggregation of the bentonite silicate layers during the formation of the nanocomposite structure. This means the complete dispersion of single layers of clay into polymeric matrix occurred. In this case, the exfoliated structure for the resulting nanocomposite is obtained. The XRD results showed that the formation of a layer structure (exfoliation) in the obtained nanocomposite structure, indicates the proper distribution of nanoclay particles in the polymer matrix, which is a reason to increase the resistance and thermal properties of nanocomposite hydrogel. Also, in the poly(MAA-co-AAm)/bentonite nanocomposite hydrogel XRD pattern, some peaks in 2θ = 12.5, 16, and 22° are observed which indicating the amorphous structure of copolymer samples [36].
Figure 3 shows the TGA curves of poly(MAA-co-AAm) and poly(MAA-co-AAm)/bentonite nanocomposite hydrogels and the detailed TGA analysis results are shown in Table 1. Usually, TGA analysis was performed to investigate the thermal stability behavior of poly(MAA-co-AAm) and poly(MAA-co-AAm)/bentonite nanocomposite hydrogels. The results of TGA analysis showed that the weight loss of copolymer and nanocomposite hydrogel samples was done in three stages. The first stage of weight loss occurred in the range of 50–150 °C temperature, which could be due to water loss in the sample by evaporation. It is noteworthy to mention that at this stage, the weight loss of the nanocomposite sample is more than the copolymer hydrogel sample and this is due to a large number of water molecules in the hydrogel nanocomposites structure [37]. In the 2nd degradation stage, weight loss of about 7.4 wt. % at the temperature range of 168–208 °C is related to the breaking of C–O–C bonds in the chains of methacrylic acid and the resulting hydrogels [32, 34]. The another stage of weight loss took place in the range of 200–450 °C temperature, where the hydrogels lost a significant part of their weight, which could be related to the destruction of acrylamide bonds as well as the decomposition of excellent monomer compounds. Also at this stage, the C-H bond of methacrylic acid, which is the dominant monomer of the hydrogel, is decomposed. The final weight loss can be due to the decomposition and destruction of the structure of the mentioned materials, which at this stage we see a slight weight loss. As shown in Fig. 3, the addition of bentonite nanoparticles increases the thermal stability of the nanocomposite. In addition, a comparison of the nanocomposite sample with the sample without nanoclay shows that the addition of bentonite nanoclay to the polymeric hydrogel caused a significant increase in the temperature of the nanocomposite during the heating steps. This result may be due to the exfoliated structure that creates strong hydrogen bond interactions with methacrylic acid and acrylamide monomers, evaporating water molecules, destroying the chains and opening the nanocomposite layers.
In order to study morphology and changes in samples of poly(MAA-co-AAm) and poly(MAA-co-AAm)/bentonite nanocomposite hydrogels before and after the methyl violet dye adsorption process, SEM–EDX and dot-mapping analysis were used. Figure 4 shows the SEM micrographs for poly(MAA-co-AAm) and poly(MAA-co-AAm)/bentonite nanocomposite hydrogels in the dry state. In hydrogels, the amount of swelling depends on the porosity and the average size of the pores. Due to the presence of capillary forces, water is distributed through the pores. As shown in Fig. 4b the presence of bentonite nanoparticles increases the porosity of hydrogel and thus, increases the water permeability in the structure of hydrogels. Dot mapping results (Fig. 4c) show the cross-sectional distribution of Si element (purple dots) as a marker of nanoparticles. Figure 4a also showed a uniform distribution of nanoparticles in the microstructure of nanocomposite hydrogels confirming that clay nanoparticles were not accumulated in the matrix. Also, after the adsorption process of MV-dye, the pores and surface roughness of the nanocomposite were almost saturated, which can be due to the filling of the pore by dye molecules.
The effect of different parameters on the adsorption performance
Weight concentration of nanoparticles
Figure 5a shows effect of weight percent of nanoparticles on dye adsorption efficiency. A lower dye adsorption was observed at either low (2.5 wt. %) or high (15 wt. %) concentrations of nanoparticles. The highest dye adsorption of 96% was obtained at a nanoparticle weight percent range of 7.5–12.5 (wt %). It is known that with increasing the weight percentage of bentonite nanoparticles inside the hydrogel, due to the increase of active sites and effective surface, the amount of dye adsorption increased sharply and when the amount of bentonite nanoparticles reached to 12.5 wt. %, due to the accumulation of these nanoparticles inside the hydrogel, the dye adsorption decreases because in this weight percentage, the active sites available for the adsorption process are reduced.
a) Effect of nanoparticle wt. %, b) pH, c) adsorbent dosage, d) the initial concentration of dye (MV), e) contact time, and f) temperature on adsorption efficiency of poly(MAA-co-AAm) and poly(MAA-co-AAm)/bentonite nanocomposite hydrogels. Data are mean of triplicate measurements. Error bars indicate the standard deviation
Effect of pH
The initial pH of the medium is an important factor of the adsorption process that affects adsorbent surface ionization and charge [38]. Figure 5b shows the effect of initial pH (in a range of 3–10) on the efficiency of dye adsorption for Poly(MAA-co-AAm) and Poly(MAA-co-AAm)/bentonite nanocomposite hydrogels. First, the adsorption efficiency of Poly(MAA-co-AAm)/bentonite was higher than that of Poly(MAA-co-AAm) hydrogels at all pH ranges tested. Second, either at lower (pH < 4) or higher (pH > 9) pH values, the adsorption efficiency was significantly reduced. A highest adsorption rate of 94 and 90% was obtained at pH = 7 for Poly(MAA-co-AAm)/bentonite and Poly(MAA-co-AAm) hydrogels, respectively. There are repulsive electrostatic forces between the surface of adsorbent and the dye molecules originated from H+ ions in acidic pH, and OH− ions in alkaline pH, which reduce the adsorption efficiency [39, 40].
Adsorbent dose
The adsorbent dose is another important parameter that affects the adsorption efficiency of contaminants [41]. In this study, the adsorption capacity of Poly(MAA-co-AAm) and Poly(MAA-co-AAm)/bentonite nanocomposite hydrogels was evaluated at an adsorbent dose range of 0.5–4 gL−1, an initial dye concentration of 10 mg.L−1, an initial pH of 7, and a contact time of 60 min. As can be seen from Fig. 5c, the adsorption efficiency of Poly(MAA-co-AAm)/bentonite nanocomposite hydrogel was higher than that of Poly(MAA-co-AAm) hydrogel at all adsorbent dosages tested. With increasing the adsorbent dose, a favorable increase in the adsorption efficiency for both types of hydrogels was observed. This can be explained by an increased contact surface of the adsorbents, which facilitates the adsorption process at a higher concentration. According to the results, with increasing the adsorbent dose from 0.5 to 1.5 g.L−1, the adsorption efficiency of the dye was increased. Dosage higher than 1.5 g.L−1 did not significantly change the adsorption efficiency of both adsorbent types. This could be due to the reduction of dye concentration in aqueous solution and reduction of active ingredient [42].
Effect of dye concentration
The concentration of pollutants is also an important factor in the adsorption process because it acts as a driving force of mass transfer between the adsorbent (solid phase) and the aqueous phase [43]. Figure 5d shows the effect dye concentration on the efficiency of the adsorption process. Increasing the concentration significantly decreased the adsorption efficiency for both types of adsorbents. At low dye concentrations (i.e., 10 mg.L−1), the intermolecular bonds of the dyes could be weak, while at high concentrations (i.e., 100 mg.L−1) the active sites on the adsorbent surface are completely saturated leaving extra dye molecules unabsorbed [44,45,46].
Effect of temperature
Temperature influences the kinetic energy of adsorbent molecules, and thereby, could affect the efficiency of the adsorption process [24]. The effects of temperature change in the range of 25–45 °C on the adsorption efficiency are shown in Fig. 5e. Increasing the temperature could enhance the mobility of the hydrogel side groups causing the adsorbed dye molecules to be separated from the hydrogel structure and reduce the adsorption rate. This process indicates that the adsorption reaction is exothermic. The highest adsorption rate (95%) was obtained at a temperature of 25 °C.
Contact time
The efficiency of adsorption at time intervals of 20–250 min was evaluated considering a pH of 7, and an adsorbent dose of 1.5 g.L−1. Figure 5f shows that the adsorption of dyes was faster in the biggening of the process; however, the adsorption rate reached to a plateau after 60 min. In the biggening, there are unoccupied surfaces that are active to absorb the dye. Upon increasing contact time, those active sites on the adsorbent molecules become saturated, which this leads to a reduction in the adsorption rate. According to the results, the optimal time for adsorption of dye with an initial concentration of 10 mg.L−1 was obtained at 60 min.
Adsorption isotherm models
To evaluate the adsorption behavior of dye contaminants using two types of adsorbent hydrogels the Langmuir [47], Freundlich [23], Dubinin-Radushkevich (D-R) [48], and Temkin [27] isotherm models were used according to following equations.
In these equations qm shows the maximum dye absorption capacity (mg.g−1), KF and n are Freundlich constants, ε represents Polanyi coefficient, KL is Langmuir constant, A (g−1) and bT (kJ.mol−1) are the Temkin constants, β is the activity coefficient (mol2.J−2), R is the universal gas constant and T is the absolute temperature (K). [49–51]. Table 1 shows the constants and parameters of the models. In addition, RL is a dimensionless parameter in Langmuir model, which shows the type of adsorption process. The value of RL > 1 indicates undesirable, RL = 1 shows linear, RL = 0 represents irreversible, and 0 < RL < 1 indicates desirable adsorption processes. As shown in Table 2, a value range 0–1 was obtained for RL parameter using for both adsorbent types of showing that the adsorption process was optimal [23]. The adsorbents synthesized in the present study showed a highly potential in removal of MV dyes compared to other adsorbents of researchers’’ studies (Table 3). By drawing a linear isothermal shape, a constant can be obtained [27]. The isotherm models using synthesized adsorbents showed nonlinear relationship (Fig. 6).
Using Langmuir model, the correlation coefficient (R2) was 0.9965 for Poly(MAA-co-AAm) hydrogel and 0.9986 for Poly(MAA-co-AAm)/Bentonite hydrogel adsorbents. These values were significantly higher than those of other models. In addition, the n parameters obtained using Poly(MAA-co-AAm) and Poly(MAA-co-AAm)/Bentonite hydrogels were 2.75 and 3.27, respectively, indicating that the MV dye adsorption process using these types of adsorbents are both optimal and physical. The AT and bT parameters determined by the Temkin model showed that the interaction between MV dye and adsorbent surface is weak. Also, the D-R isotherm model showed a value of 8 kJ.mol−1 for the \({\text{E = }}\frac{{1}}{{\sqrt {2 \beta } }}\) parameter which indicates the physicality of the hydrogels’ adsorption process [24].
Thermodynamic study
Thermodynamic parameters such as enthalpy (ΔH°), entropy (ΔS°) and Gibbs free energy (ΔG°) were calculated according to Eqs. 8 and 9 [52–54].
where, T is the absolute temperature (K), R is the universal constant of gases (8.314 J/mol.K) and KD (KD = qe/Ce) is the equilibrium constant.
The values ΔH° and ΔS° are determined by the slope and width of ln KD vs. 1/T in the diagram, respectively, which can be seen in Fig. 7, and the obtained parameters are summarized in Table 4. A negative value of ΔG° parameter for both types of adsorbents indicates that the MV-dye adsorption process was successful and spontaneous. Moreover, negative values of ΔH° parameter for Poly(MAA-co-AAm) (-61.24 kJ.mol−1) and Poly(MAA-co-AAm)/bentonite (-36.14 kJ.mol−1) hydrogels showed that the adsorption process was exothermic (at a temperature range 25–45 °C). The negative value of ΔSº also showed that the random collisions of dye molecules with the adsorbent surface increased during the adsorption process [24, 55].
Kinetics study
In this study, pseudo-first-order [56], pseudo-second-order [57], and Elovich [58] kinematic models were used to analyze experimental data according to Eqs. (10), (11) and (12), respectively.
where, qt and qe show the adsorption capacity at time t of dye per gram of dry adsorbent and the equilibrium adsorption capacity (mg.g−1) respectively, k1 and k2 are the constants of adsorption rates (k1 is constant adsorption rate (min−1) and k2 is the constant quasi-quadratic kinetic model rate (g.(mg.min)−1)), α and β are the initial absorbance (mg.(g.min)−1) and the desorption constant (g.mg−1) respectively, in Elovich kinetic model. The slope and cut of the log diagram (qe–qt) in front of t represent k1 and qe. In the same way, k2 and qe are calculated with graph t/qt versus t. Correlation coefficients were calculated and obtained from the analysis of experimental data using three kinetic models used in the study [59, 60]. Equation (10) for the pseudo-first-order and Eq. (11) for the pseudo-second-order were used to study the kinetics linearly. The relation between kinetic parameters using both types of adsorbents are shown in Fig. 8. The kinetic parameters of the adsorption of MV-dye using both types of adsorbents are shown in Table 5. As can be seen from Fig. 8 and Table 5, and the value of R2, the pseudo-second-order for Poly(MAA-co-AAm) and Poly(MAA-co-AAm)/bentonite nanocomposite hydrogels showed better and closer results to experimental results. In general, the quasi-quadratic kinetic model has a higher correlation and absorption coefficient compared to other kinetic models. By examining and considering the values of the qt and k2 parameters as well as matching the data with the quasi-quadratic kinetic model, the MV dye adsorption process may be followed by a combined chemical and physical mechanism. Parameter values of α for MV dye adsorption process were calculated using Poly(MAA-co-AAm) and Poly(MAA-co-AAm)/Bentonite hydrogels at 0.4276 mg.g−1.min and 22.15 mg.g−1.min. These results indicate a high adsorption value of nanocomposites.
Conclusions
Dyes and pigments are the main pollutants in wastewater streams of various industries. Poly(MAA-co-AAm) hydrogels and Poly(MAA-co-AAm)/bentonite adsorbents were used to remove MV-dye from aqueous solution. Amide and carboxylate functional groups were characterized by FTIR in the structure of adsorbents that could be important for the adsorption process. SEM confirmed the presence of numerous surface pores at in the surface of adsorbents that possibly favors the adsorption efficiency of MV-dye. The Langmuir model was found to be more capable in interpreting equilibrium behavior than other models. In addition, the values obtained from the parameters RL, n and E of isothermal models, showed that the dye adsorption process of MV dye is physical and desirable. Temkin isotherm model (AT and bT parameters) showed a weak interaction of the adsorbent surface with the MV-dye. The determined kinetic data showed a good agreement with the quasi-second-order kinetic model and the latter had a better correlation coefficient and higher adsorption capacity than those of other models. By examining and considering the values of the qt and k2 parameters as well as matching the data with the quasi-quadratic kinetic model, the MV dye adsorption process may be followed by a combined chemical and physical mechanism. The thermodynamic study showed that the adsorption process was spontaneous and exothermic.
References
Mittal H, Babu R, Alhassan SM (2020) Utilization of gum xanthan based superporous hydrogels for the effective removal of methyl violet from aqueous solution. Int J Biol Macromol 143:413–423
Wadhera P, Jindal R, Dogra R (2019) Synthesis of semi interpenetrating network hydrogel [(GrA-Psy)-cl-Poly (AA)] and its application for efficient removal of malachite green from aqueous solution. Polym Eng Sci 59:1416–1427
Eskhan A, Banat F, Selvaraj M, Abu Haija M (2019) Enhanced removal of methyl violet 6B cationic dye from aqueous solutions using calcium alginate hydrogel grafted with poly (styrene-co-maleic anhydride). Polym Bull 76:175–203. https://doi.org/10.1007/s00289-018-2378-y
Beyranvand NS, Samiey B, Tehrani AD, Soleimani K (2019) Graphene oxide–cellulose nanowhisker hydrogel nanocomposite as a novel adsorbent for methylene blue. J Chem Eng Data 64:5558–5570
Sharma G, Kumar A, Sharma S, Ala’a H, Naushad M, Ghfar AA, Ahamad T, Stadler FJ (2019) Fabrication and characterization of novel Fe0@ Guar gum-crosslinked-soya lecithin nanocomposite hydrogel for photocatalytic degradation of methyl violet dye. Sep Purif Technol 211:895–908
Gadisa BT, Appiah-Ntiamoah R, Kim H (2019) Amorphous iron sulfide nanowires as an efficient adsorbent for toxic dye effluents remediation. Environ Sci Pollut Res 26:2734–2746
Foroutan R, Mohammadi R, Ramavandi B (2019) Elimination performance of methylene blue, methyl violet, and Nile blue from aqueous media using AC/CoFe2O4 as a recyclable magnetic composite. Environ Sci Pollut Res 26:19523–19539
Foroutan R, Mohammadi R, Razeghi J, Ramavandi B (2019) Performance of algal activated carbon/Fe3O4 magnetic composite for cationic dyes removal from aqueous solutions. Algal Res 40:101509
Munagapati VS, Yarramuthi V, Kim Y, Lee KM, Kim DS (2018) Removal of anionic dyes (Reactive Black 5 and Congo Red) from aqueous solutions using banana peel powder as an adsorbent. Ecotoxicol Environ Saf 148:601–607
Cheng K, Huang C, Wei Y, Hsu S (2019) Novel chitosan – cellulose nano fi ber self- healing hydrogels to correlate self-healing properties of hydrogels with neural regeneration effects. NPG Asia Mater 11:25
Cheng-Wei T, Fang-Chang T, Jem-Kun C, Huei-Ping W, Rong-Ho L, Jiawei Z, Tao C, Chung-Chi W, Chih-Feng H (2020) Preparations of Tough and Conductive PAMPS/PAA Double Network Hydrogels Containing Cellulose Nanofibers and Polypyrroles. Polymers 12(12):2835
Chadi A, Olasupo A, Yani N, Saime W, Ngah W, Mohd B (2021) Journal of Environmental Chemical Engineering Comparative removal of malachite green dye from aqueous solution using deep eutectic solvents modified magnetic chitosan nanoparticles and modified protonated chitosan beads. J Environ Chem Eng 9(5):106281
Li Z et al (2020) Removal of malachite green dye from aqueous solution by adsorbents derived from polyurethane plastic waste. Biochem Pharmacol, no. August, p 104704
Mallakpour S, Tabesh F (2019) Tragacanth gum based hydrogel nanocomposites for the adsorption of methylene blue: Comparison of linear and non-linear forms of different adsorption isotherm and kinetics models. Int J Biol Macromol 133:754–766
Mittal H, Morajkar PP, Al Alili A, Alhassan SM (2020) In-situ synthesis of ZnO nanoparticles using gum arabic based hydrogels as a self-template for effective malachite green dye adsorption. J Polym Environ 28:1637–1653
Tsai Y, Theato P, Huang C, Hsu S (2020) A 3D-printable, glucose-sensitive and thermoresponsive hydrogel as sacrificial materials for constructs with vascular-like channels. Appl Mater Today 20:100778
Che-Hao W, Cheng-Wei T, Junko A, Jiawei Zhang TC, Chung-Chi W, Chih-Feng H (2020) Mechanochromic Double Network Hydrogels as a Compression Stress Sensor. Polym Chem 11(40):2020
Thakur S, Verma A, Sharma B, Chaudhary J, Tamulevicius S, Thakur VK (2018) Recent developments in recycling of polystyrene based plastics. Curr Opin Green Sustain Chem 13:32–38
Kaur K, Jindal R (2019) Comparative study on the behaviour of Chitosan-Gelatin based Hydrogel and nanocomposite ion exchanger synthesized under microwave conditions towards photocatalytic removal of cationic dyes. Carbohydr Polym 207:398–410
Verma A, Thakur S, Mamba G, Gupta RK, Thakur P, Thakur VK (2020) Graphite modified sodium alginate hydrogel composite for efficient removal of malachite green dye. Int J Biol Macromol 148:1130–1139
Rabipour M, Pour ZS, Sahraei R, Ghaemy M, Jazi ME, Mlsna TE (2020) pH-Sensitive Nanocomposite Hydrogels Based on Poly (Vinyl Alcohol) Macromonomer and Graphene Oxide for Removal of Cationic Dyes from Aqueous Solutions. J Polym Environ 28:584–597
Al-Aidy H, Amdeha E (2021) Green adsorbents based on polyacrylic acid-acrylamide grafted starch hydrogels: the new approach for enhanced adsorption of malachite green dye from aqueous solution. Int J Environ Anal Chem 101(15):2796–2816
Pakdel PM, Peighambardoust SJ (2018) Review on recent progress in chitosan-based hydrogels for wastewater treatment application. Carbohydr Polym 201:264–279
Sharma K, Kumar V, Kaith BS, Kumar V, Som S, Kalia S, Swart HC (2015) Synthesis, characterization and water retention study of biodegradable Gum ghatti-poly (acrylic acid–aniline) hydrogels. Polym Degrad Stab 111:20–31
Kuo S, Chang F (2011) Progress in Polymer Science POSS related polymer nanocomposites. Prog Polym Sci 36(12):1649–1696
Lu C, Kuo S, Huang C, Chang F (2009) Self-Assembled Fernlike Microstructures of Polyhedral Oligomeric Silsesquioxane / Gold Nanoparticle Hybrids. J Phys Chem C 113:3517–3524
Pakdel PM, Peighambardoust SJ (2018) A review on acrylic based hydrogels and their applications in wastewater treatment. J Environ Manage 217:123–143
Foroutan R, Peighambardoust SJ, Peighambardoust SH, Pateiro M, Lorenzo JM (2021) Adsorption of Crystal Violet Dye Using Activated Carbon of Lemon Wood and Activated Carbon/Fe3O4 Magnetic Nanocomposite from Aqueous Solutions: A Kinetic, Equilibrium and Thermodynamic Study Mol 26
Xu H, Kuo S, Huang C, Chang F (2004) Characterization of Poly (vinyl pyrrolidone- co - isobutylstyryl polyhedral oligomeric silsesquioxane) nanocomposites. J Appl Polym Sci 91:2208
Yen Y, Kuo S, Huang C, Chen J (2008) Miscibility and Hydrogen-Bonding Behavior in Organic / Inorganic Polymer Hybrids Containing Octaphenol Polyhedral Oligomeric Silsesquioxane. J Phys Chem B 112:10821–10829
Peighambardoust SJ, Aghamohammadi-Bavil O, Foroutan R, Arsalani N (2020) Removal of malachite green using carboxymethyl cellulose-g-polyacrylamide/montmorillonite nanocomposite hydrogel. Int J Biol Macromol 159:1122–1131. https://doi.org/10.1016/j.ijbiomac.2020.05.093
Moharrami P, Motamedi E (2020) Application of cellulose nanocrystals prepared from agricultural wastes for synthesis of starch-based hydrogel nanocomposites: Efficient and selective nanoadsorbent for removal of cationic dyes from water. Bioresour Technol 313:123661
Abdolhosseinzadeh M, Peighambardoust SJ, Erfan-Niya H, Pakdel PM (2018) Swelling and auramine-O adsorption of carboxymethyl cellulose grafted poly (methyl methacrylate)/Cloisite 30B nanocomposite hydrogels. Iran Polym J 27:807–818
da Silva RC, de Aguiar SB, da Cunha PLR, de Paula RCM, Feitosa JPA (2020) Effect of microwave on the synthesis of polyacrylamide-g-chitosan gel for azo dye removal. React Funct Polym 148:104491
Wang G, Shen J, Liu S, Jiang C, Qin X (2019) Three-dimensional modeling and analysis of macro-pore structure of coal using combined X-ray CT imaging and fractal theory. Int J Rock Mech Min Sci 123:104082
Nzioka AM, Kim M-G, Hwang H-U, Kim Y-J (2019) Kinetic study of the thermal decomposition process of municipal solid waste using TGA. Waste Biomass Valoriz 10:1679–1691
Ibrahim WM, Hassan AF, Azab YA (2016) Biosorption of toxic heavy metals from aqueous solution by Ulva lactuca activated carbon. Egypt J basic Appl Sci 3:241–249
Chrzanowska E, Gierszewska M, Kujawa J, Raszkowska-Kaczor A, Kujawski W (2018) Development and characterization of polyamide-supported chitosan nanocomposite membranes for hydrophilic pervaporation. Polymers (Basel) 10:868
Foroutan R, Mohammadi R, Peighambardoust SJ, Jalali S, Ramavandi B (2020) Application of nano-silica particles generated from offshore white sandstone for cadmium ions elimination from aqueous media. Environ Technol Innov 19:101031
Youssef AM, Al-Awadhi MM, Akl MA (2014) Solid phase extraction and spectrophotometric determination of methylene blue in environmental samples using bentonite and acid activated bentonite from Egypt. J Anal Bioanal Tech 5:1
Hayati-Ashtiani M (2012) Use of FTIR Spectroscopy in the Characterization of Natural and Treated Nanostructured Bentonites (Montmorillonites). Part. Sci Technol 30(6):553–564
Koosha M, Hamedi S (2019) Intelligent Chitosan/PVA nanocomposite films containing black carrot anthocyanin and bentonite nanoclays with improved mechanical, thermal and antibacterial properties. Prog Org Coatings 127:338–347
David MK, Okoro UC, Akpomie KG, Okey C, Oluwasola HO (2020) Thermal and hydrothermal alkaline modification of kaolin for the adsorptive removal of lead (II) ions from aqueous solution. SN Appl Sci 2:1–13
Forutan R, Ehsandoost E, Hadipour S, Mobaraki Z, Saleki M, Mohebbi G (2016) Kinetic and equilibrium studies on the adsorption of lead by the chitin of pink shrimp (Solenocera melantho). Entomol Appl Sci Lett 3:20–26
Rahimi K, Mirzaei R, Akbari A, Mirghaffari N (2018) Preparation of nanoparticle-modified polymeric adsorbent using wastage fuzzes of mechanized carpet and its application in dye removal from aqueous solution. J Clean Prod 178:373–383
Esvandi Z, Foroutan R, Peighambardoust SJ, Akbari A, Ramavandi B (2019) Uptake of anionic and cationic dyes from water using natural clay and clay/starch/MnFe2O4 magnetic nanocomposite. Surf Interface 21(1):100754
García FE, Plaza-Cazón J, Montesinos VN, Donati ER, Litter MI (2018) Combined strategy for removal of Reactive Black 5 by biomass sorption on Macrocystis pyrifera and zerovalent iron nanoparticles. J Environ Manage 207:70–79
Hajizadeh H, Peighambardoust SJ, Peighambardoust SH, Peressini D (2020) Physical, mechanical and antibacterial characteristics of bio-nanocomposite films loaded with Ag-modified SiO2 and TiO2 nanoparticles. J Food Sci 85:1193–1202
Agnihotri S, Singhal R (2019) Effect of sodium alginate content in acrylic acid/sodium humate/sodium alginate superabsorbent hydrogel on removal capacity of MB and CV dye by adsorption. J Polym Environ 27:372–385
Ahmadi A, Foroutan R, Esmaeili H, Tamjidi S (2020) The role of bentonite clay and bentonite clay@MnFe2O4 composite and their physico-chemical properties on the removal of Cr(III) and Cr(VI) from aqueous media. Environ Sci Pollut Res 27:14044–14057. https://doi.org/10.1007/s11356-020-07756-x
Khodaeimehr R, Peighambardoust SJ, Peighambardoust SH (2019) Preparation and characterization of corn starch/clay nanocomposite films: Effect of clay content and surface modification. Starch-Stärke 70(3–4):1700251
Ebrahimi Y, Peighambardoust SJ, Peighambardoust SH, Karkaj SZ (2019) Development of Antibacterial Carboxymethyl Cellulose-Based Nanobiocomposite Films Containing Various Metallic Nanoparticles for Food Packaging Applications. J Food Sci 84(9):2537–2548
Lee HS (1997) Biosorption of Cr, Cu and Al by Sargassum biomas. Biotechnol Bioprocess Eng 2:126–131.020–07756-x
Al-Muhtaseb SA, El-Naas MH, Abdallah S (2008) Removal of aluminum from aqueous solutions by adsorption on date-pit and BDH activated carbons. J Hazard Mater 158:300–307
Khattri S, Singh M (2009) Removal of malachite green from dye wastewater using neem sawdust by adsorption. J Hazard Mater 167:1089–1094
Chaudhary S, Sharma J, Kaith BS, Yadav S, Sharma AK, Goel A (2018) Gum xanthan-psyllium-cl-poly(acrylic acid-co-itaconic acid) based adsorbent for effective removal of cationic and anionic dyes: adsorption isotherms, kinetics and thermodynamic studies. Ecotoxicol Environ Saf 149:150–158
Kaushal S, Badru R, Kumar S, Kaur H, Singh P, (2018) Efficient removal of cationic and anionic dyes from their binary mixtures by organic–inorganic hybrid material. J Inorg Organomet 28:968–977
Sabbagh F, Khatir NM, Karim AK, Nazari Z, Jaberi R (2019) Mechanical Properties and Swelling Behav- ior of Acrylamide Hydrogels using Mont- morillonite and Kaolinite as Clays 7:211–219
Sarı A, Tuzen M (2009) Equilibrium, thermodynamic and kinetic studies on aluminum biosorption from aqueous solution by brown algae (Padina pavonica) biomass. J Hazard Mater 171:973–979
Largitte L, Pasquier R (2016) A review of the kinetics adsorption models and their application to the adsorption of lead by an activated carbon. Chem Eng Res Des 109:495–504
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest statement
This work was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Thus, the authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Safarzadeh, H., Peighambardoust, S.J., Mousavi, S.H. et al. Adsorption of methyl violet dye from wastewater using poly(methacrylic acid-co-acrylamide)/bentonite nanocomposite hydrogels. J Polym Res 29, 113 (2022). https://doi.org/10.1007/s10965-022-02956-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-022-02956-0