Abstract
Eco-friendly materials production from industrial wastes and renewable raw materials is being promoted for green construction applications. Therefore, considerable value can be added by combining green chemistry with microbial biotechnology to construct improved and far better, biologically stable, and compatible materials. For this purpose, herein, Schizophyllum commune IBL-06 was exploited for the production of ligninolytic enzymes (MnP, LiP, and Lac). This in-house extracted enzymatic consortium was used for the delignification of pristine rice husk. The enhanced level of delignification (40.35%) was achieved with maximum cellulose exposure (from 32 to 72%). The delignified rice husk was further reinforced with bacterial cellulose from Acetobacter xylinum. Subsequently, biocomposites were prepared from both pristine and bacterially treated rice husk using compression molding technique. Glycerol/maleic anhydride combination was used as a plasticizer/compatibilizer and chitosan (10 wt.%) as a filler for newly developed biocomposite specimens. The newly synthesized biocomposite specimens were characterized using different imaging and analytical techniques including scanning electron microscopy (SEM) and Fourier transform infrared (FT-IR) spectroscopy. The characterization studies of newly developed biocomposites revealed significant improvement in morphological and mechanical properties of biocomposites. In addition, moderate increase in water uptake capacity was also observed due to the presence of hygroscopic filler among polymer matrix. In conclusion, the improved characteristics of newly developed biocomposites based on bacterial cellulose-reinforced delignified rice husk-PVA using chitosan filler suggest high throughput of enzymatic treatment for several industrial and biotechnological sectors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
During past few years, the growing concern related to fossil-based functional materials has directed towards biobased polymer composites production for various applications [1,2,3]. Microbial polymers such as extracellular polymeric substances and agro-industrial based lignocellulosic raw-materials possess a huge potential to replace petrochemically originated materials for wide range of applications in different sectors such as pharmaceutical, food, automotive, construction, biomedical, packaging and cosmaceutical industries [2, 4,5,6]. However, the non-homogeneous nature of biofibers with a plenty of lignin remains a problem while dealing with lignocellulosic biomaterials. The improved compatibility i.e., uniform fiber-distribution and interfacial adhesion among the matrix backbone and the biofibers can be achieved by fibrous modifications [7]. Biofiber modifications can be achieved through different types of treatments including alkaline, bleaching, dewaxing, cyanoethylation, enzymatic and the grafting etc.[8]. In this study, ligninolytic enzymes were produced from Schizophyllum commune IBL-06 and the delignification of rice husk was carried out to reduce the lignin content. The delignified natural fibers were further modified using bacterial cellulosic (BC) reinforcement from Acetobacter xylinum to increase the coupling between biofiber and the polymer matrix. Several researches have reported the excellent compatibility of biopolymer composites fabricated with bacterial cellulose [9, 10]. Except compatibility, several other remarkable characteristics of BC have been reported such as water absorption, mold-ability, porosity and biodegradability for both in-vitro and in-vivo applications [11,12,13].
Polyvinyl alcohol (PVA) is a synthetic polymer commonly being applied as a matrix in composite manufacturing. It is being used in composite film formation as an adhesive agent because of its exceptional physicochemical characteristics such as emulsification, adhesion, aqueous solubility and film forming characteristics [14]. High structural stability of PVA/cellulose-based bio and nanocomposite have been reported for various applications in textile, packaging, heavy metal removal, and biomedical sectors [15,16,17]. Maleic anhydride is an organic compound often used in composites materials as a coupling agent as it enhances the compatibility among biofibers and the polymer matrix [18]. Additionally, the plasticizers have also been used in the composite development to reduce the glass transition temperature and increase the flexibility of biocomposites [19, 20]. Low molecular weight organic compounds are often used as plasticizers viz. sorbitol, glycerol and polyethylene glycol. However, glycerol due to its hygroscopic nature is considered as the favorable one for biocomposite development [21, 22].
Chitosan is a biopolymer with high compatibility and strong antimicrobial effects [23]. Highly crystalline structure and the presence of H-bonds among the polymer molecules make chitosan an excellent agent for oxygen-barrier applications [24]. So, it would be a promising way to use chitosan as a filler for PVA-based biopolymer composites for improved compatibility and oxygen barrier properties. Chitosan blends and composites have been successfully used for the development of packaging material with improved food security, ultimately leading to increased shelf-life [25, 26]. Variety of different techniques have been adapted for the development of biocomposites. Among other techniques, the compression molding poses less or no damaging effect to the fibers with potential to preserve the isotropic characteristics of biocomposites [27].
This research was designed to investigate the characteristics of biocomposites based on microbial cellulose-reinforced delignified rice husk-PVA. The effect of plasticizer/compatibilizer among polymer matrix was determined by the addition of glycerol/maleic anhydride. Finally, the filler effect of chitosan was determined in both pristine and microbial cellulose-reinforced delignified biofibers. All newly developed biocomposites were studied for their mechanical and barrier characteristic using modern techniques including SEM and FTIR. In addition, the water absorption and tensile testing was also performed using variety of different techniques to analyze the novel characteristics of biocomposites.
Materials and methods
Microbial strains, substrate and chemicals
White-rot fungus (Schizophyllum commune IBL-06) was obtained from Industrial Biotechnology Laboratory of University of Agriculture, Faisalabad, Pakistan and used for the production of ligninolytic enzymes (MnP, LiP and Lac). The bacterial spp. (Acetobacter xylinum) was obtained from Department of Mycology & Plant Pathology, University of Punjab, Pakistan and exploited for the development of microbial cellulosic fibers. Different biomaterials from agricultural-based waste (sugarcane bagasse and rice husk) were collected from students’ research farm, UAF, Pakistan. Biomaterials were dried in convection oven (LabTech LDO-150 N) at 60 °C for 48 h and pulverized to 40 mm mesh size. Dried biomaterials were preserved in polythene zipper bags to prevent moisture uptake. All the chemicals used were purchased from local distributors of Merck KGaA, USA and Sigma Aldrich, Germany.
Development of ligninolytic consortium from Schizophyllum commune IBL-06
The inoculum of Schizophyllum commune IBL-06 was prepared in Kirk’s basal medium of pH 4.5 [28]. The media was sterilized by autoclaving at 121 °C for 15 min. After autoclaving, loopful of fungal culture was added in the flasks containing basal media and incubated at 30 °C for 5–6 days.
Solid state fermentation (SSF) was carried out for the production of ligninolytic enzymes using sugarcane bagasse as a substrate. Flasks containing SSF media (15 g dried substrate moistened with 7 mL of Kirk’s basal medium) were autoclaved, pipetted with 5 mL of S. commune inoculum, and incubated at 30 °C for 5 days in still culture incubator (LabTech LDO-150 N). After five days, ligninolytic enzymes were extracted by the addition of 100 mL of 0.5 mM sodium malonate buffer (pH 4.5) and placed in incubator shaker for 30 min at 150 rpm [29]. The biomass filtrate was centrifuged at 4 °C and 3000 rpm for 5 min. The supernatant was collected and assayed for ligninolytic enzymes using standard protocol [29] and used for delignification of rice husk.
Ligninolytic enzyme assays
Enzyme assays were performed using spectrophotometric method (Dynamica, HALO DB-20S) as reported earlier [30, 31]. Briefly, the MnP activity assay was performed at 270 nm (ϵ270 = 11,570) by hydrogen peroxide-dependent oxidation of manganic-malonate. The LiP activity was assayed at 310 nm (ϵ310 = 9300) by hydrogen peroxide-dependent conversion of veratryl alcohol to veratraldehyde. The laccase activity assay was performed at 420 nm (ϵ420 = 36,000) by determining the oxidation of ABTS. Quartz cuvettes were used for ligninolytic enzyme assay having an exterior height, width and length (H × W × L) dimensions of 45 × 12.5 × 3.5 mm with 1 mm path length.
Enzymatic delignification of rice husk
The delignification of native rice husk (NRH) was performed using mixture of ligninolytic enzyme (MnP, LiP and lac). The total volume of the flasks was adjusted to 100 mL with sodium malonate buffer (0.5 mM, pH 4.5). Percentage delignification was determined from both treated and untreated substrates as reported previously [32]. Briefly, the samples were taken in glass test tubes and mixed with 25% acetyl bromide. The tubes were stoppered and kept in water bath for 30 min at 70 ± 5 °C temperature. The samples were transferred to volumetric flask of 250 mL capacity, containing CH3COOH(g) (25 mL) and 2 M-NaOH (10 mL) supplemented with 1 mL of hydroxylammonium-chloride (7.5 M). The readings were taken at 280 nm using UV/Vis spectrophotometer.
Cellulose content of the samples was determined by the method of Updegraff, [33] with modifications. Briefly, the samples were homogenized in distilled water using a waring blender with 2% (w/v) of biomass concentration. The pallets were obtained by centrifugation at 3000 rpm and added with 3 mL of acetic/nitric reagent. The mixture was incubated in boiling water bath for 30 min followed by centrifugation at 5000 rpm for 5 min. Pallets were collected and added with 10 mL of 67% H2SO4 and incubated for 1 h. The total volume of the solution was adjusted to 1L with distilled water. The hydrolysate 1 mL was added with 4 mL of distilled water and placed in ice. 4 mL of chilled anthrone reagent was gently mixed and incubated for at least 15 min. The readings were taken at 620 nm using VU/Vis spectrophotometer.
Bacterial treatment of delignified rice husk
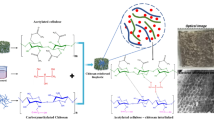
The delignified rice husk was modified by treating with bacterial cellulose from Acetobacter xylinum. For this purpose, bacterial suspension was prepared using Hestrin-Schramm (HS) medium with g/L composition of peptone (5.0), yeast extract (5.0), Na2HSO4 (1.1), citric acid (1.15) and glucose (20). Media was adjusted to pH 5.5 and autoclaved at 121 °C for 15 min. After sterilization, media was inoculated with a loopful of bacterial culture and placed in incubator shaker at 30 °C for 3 days.
The delignified rice husk was placed in 250 mL Erlenmeyer flasks in the presence of HS culture media. The media was sterilized and inoculated with 5 mL of Acetobacter xylinum culture broth. The media was placed in incubator shaker at 130 rpm and 30 °C for 7 days [34]. After 7 days, the treated biofibers were recovered by washing with NaOH (0.1 N) at 80 °C in order to remove soluble polysaccharides, media components and microorganisms [35]. The harvested biofibers were designated at bacterially treated rice husk (BTRH) which were dried in oven at 50 °C for 2–3 days and used for the development of biocomposites.
Development of biocomposites
The biocomposites were developed using pristine (NRH), bacterially treated rice husk (BTRH) and PVA with and without chitosan as a filler and glycerol/maleic anhydride as plasticizer/compatibilizer. Briefly, the PVA was gently mixed with biofibers (ratio 1:1) at 35 °C and 700 rpm for 15 min using dH2O (as required). The resulting mixture was processed for biocomposite development. In next step, the thick composite biomass was further compatibilized/ plasticized with maleic anhydride and glycerol with 25% and 50% concentration of total composite biomass respectively. To analyze the filler effect of chitosan, a 2% (w/v) solution was prepared in 1% acetic acid solution in water and the thick solution (50 mL) was blended with composite biomass at 700 rev/min for 15 min. The composite mixture was then molded using pre-heated plates (150 mm diameter; 2 mm thick) at 80 °C for 15 min. The pressure (3–4 MPa) was applied depending upon reinforcement biomass. All the newly developed biocomposites were then placed at 50 °C for 12 h [36]. The constituent composition of all newly developed biocomposites have shown in Table 1.
The resulting biocomposites were designated as PVA-NRH (synthesized using PVA and pristine rice husk), PVA-BTRH (synthesized using PVA with delignified and BC reinforced rice husk), PVA-NRH-GLY (synthesized using PVA with pristine rice husk using plasticizer/compatibilizer), PVA-BTRH-GLY (synthesized using PVA with bacterially treated rice husk using plasticizer/compatibilizer), PVA-NRH-GLY-CHT (synthesized using PVA with pristine rice husk further supplemented with plasticizers and chitosan filler), PVA-BTRH-GLY-CHT (synthesized using PVA with bacterially treated rice husk further supplemented with filler and plasticizers). Figure 1 represents the digital image of newly synthesized composites.
Characterization of biocomposites
Structural composition – FTIR
The structural components of test composites were examined by Fourier transform infrared spectrometer (Bruker Alpha FT-IR, Germany). Composites were placed directly under the infrared beam and the spectrum was recorded from 4000–500 cm−1 and given peak numbers.
Surface morphologies – SEM
All the newly developed biopolymer composites were analyzed for their surface morphologies in ultra-high vacuum model of scanning electron microscope (JSM 6409A, JEOL, Japan) at an accelerating voltage about 10 kV. The biocomposites were gold coated using sputter coater machine (Emitech-K550) and high-quality images were obtained. The deposition current of 20 mA and pressure of 7 × 102 bar was used for analysis [35].
Mechanical analysis of biocomposites
Mechanical characteristics of biocomposites, i.e., tensile strength, elongation at break and Young’s modulus were determined using Universal Testing Machine (UTM: AMETEK Lloyd Instruments Ltd.). Before mechanical measurements, the composite specimens were cut into 80 mm × 20 mm × 2 mm dimensions and were placed at 40 mm distance according to the ASTM-D790 standard. The crosshead speed of 2 mm/min and the load range of 1–6000 mN was adjusted. All measurements were taken in the form of triplicate and the results were reported as average of three repeated experiments.
Water absorption capacity
The water absorption analysis of all the newly developed biocomposites was determined. The composite specimens were weighted and immersed in distilled water. The weight gain was recorded after 30, 60, 90- and 120-min. Water absorption capacity was expressed as the percentage weight gain of biocomposite specimens. All experiments were repeated thrice, and the results were calculated using following equation:
where, Wa is weight of composite after water uptake and Wo is the initial weight of compression molded composite specimen.
Statistical analysis
All experiments were repeated thrice, and the corresponding data was presented as mean ± STD. Statistical analysis was performed using Microsoft Excel 365 ProPlus version and the mean ± STD were calculated from each experiment.
Results and discussion
Production of fungal ligninolytic enzymes
Ligninolytic enzyme consortia (LiP, MnP and Lac) was obtained from Schizophyllum commune after 5-days of incubation in SSF of sugarcane bagasse. The ligninolytic consortia with maximum enzymatic activities of 1763.1 U/mL for MnP, 1836.4 U/mL for LiP and 318.9 U/mL for laccase was used for the delignification of lignocellulosic material (rice husk). The use of microbial-based and enzymatic methodologies comprises several advantages i.e., low sugar losses, high delignification and green environmental impacts [37].
Enzymatic delignification of rice husk
Rice husk was enzymatically modified using ligninolytic enzyme mixture (MnP, LiP and Lac) and the respective results are given in Table 2. After enzymatic modification of rice husk, the lignin contents were reduced from 22.70% to 13.54% with maximum delignification of 40.35%. Moreover, the cellulose percentage was also increased from 32.06% to 72.56% after enzymatic modification as shown in Table 2. Taniguchi et al. [38] reported similar results i.e., 41% delignification of rice husk after 60 days of incubation treated with Pleurotus ostreatus. Shi et al. [39] reported 28% of delignification of cotton stalks using Phanerochaete chrysosporium. However, the presently developed in-house consortia of ligninolytic enzymes from indigenous white-rot fungus (Schizophyllum commune) showed better results in a much shorter time-period (i.e., 5-days of incubation) with maximum cellulose exposure.
Bacterial treatment of delignified rice husk
Bacterially treated delignified rice husk showed a significant change in their surface morphology. SEM micrographs showed a random deposition of bacterial cellulosic microfibrils over the delignified rice husk. The cellulosic microfibril deposition over the delignified rice husk lead towards the improvement in water uptake and mechanical characteristics of biocomposites as described in the later part of the study. Earlier, Asgher et al. [34] and Chawla et al. [40] successfully modified lignocellulosic fibers by culturing Acetobacter xylinum and showed that the fibers reinforced with BC into the interface between the fiber and the matrix. Additionally, BTRH composites exhibited a fibrous morphology because of the presence of microcellulosic content and consequently leading towards the improved mechanical characteristics of biocomposites.
Characterization of biocomposites
Structural composition – FTIR
The major structural constituents in all newly developed biocomposite specimens were investigated using FTIR spectroscopy. FTIR spectrum of PVA-NRH and PVA-NRH-GLY-CHT showed particular functional groups bands at 1595 cm−1 and 1730 cm−1 which indicate the presence of lignin components in the biocomposites (Fig. 2a). The broad stretching bands at 3337 cm−1 and 3428 cm−1 indicate the presence of -OH functional group of cellulose which are closely related to the -OH stretching bands of PVA. In pure PVA, the visible peaks around 3295 cm−1 and 1650 cm−1 indicate the presence of residual acetate functional groups [41, 42]. The characteristic broad and sharp peak at 1090 cm−1 in pure PVA and PVA-BTRH biocomposites was assigned to the stretched vibrations of C-O functional groups [3]. The broad large peak at 3437 cm−1 and 3462 cm−1 indicate the existence of -OH functional group of cellulose in BTRH and 1595 cm−1 arises from C-O functional groups of cellulose [43]. The characteristic band of BC reinforced delignified biocomposite showed a broad peak at 2867 cm−1 which indicate the vibrational stretching of aliphatic CH bonds among cellulose molecules (Fig. 2b) whereas, broad peak around 1090 cm−1 indicate the presence of ether (C–O–C) functional linkage [1]. The sharp peak around 1380 cm−1 indicate the presence of secondary alcoholic and primary aliphatic functional groups among PVA-BTRH-GLY and PVA-BTRH-GLY-CHT. The characteristic peaks around 2867 cm−1 and 1060 cm−1 in BTRH indicate the presence of asymmetric stretching of C–O–C functional groups [44].
Surface morphologies – SEM
SEM micrographs of newly developed biocomposites have been presented in Fig. 3 (left half). The SEM microstructure of pristine (NRH) exhibited few cracks and pores, and the surface was relatively rough than bacterially treated delignified rice husk. The porous/ cracked surface of PVA-NRH biocomposites was due to the deprived cellulosic dispersion in PVA matrix. The cracked surface of PVA-NRH was significantly improved by the addition of plasticizer in PVA-NRH-GLY biocomposite. The distribution of cellulose in watery environment should enhance together with the degree of cellulosic modification [45]. The SEM micrographs of 2% chitosan reinforced biocomposites (PVA-NRH-GLY-CHT and PVA-BTRH-GLY-CHT) showed compact and smoother surface as compared with other biocomposite materials (Fig. 3).
The adhesion seems quite poor in PVA-NRH and PVA-NRH-GLY biocomposites as compared with BTRH as shown in Fig. 3 (right half). Furthermore, the delignified rice husk showed smoother surface where micro-filamentous cellulosic deposition could be clearly observed. Several researches have reported the modification in chemical, physical and micro-structural properties of graft-copolymerized matrix [1, 46]. The bacterial cellulose covers the composite surface creating a fibrous network indicating the improved interfacial adhesion between BTRH and the PVA matrix [47]. The slight modification in the surface of BTRH-PVA-based biocomposites are because of the existence of PVA in BTRH matrix. Increase in bacterial cellulosic microfibril content in biocomposite sample lead towards the non-uniform integration of material indicating improved interfacial adhesion [48].
Mechanical characteristics of biocomposites
The mechanical characteristics of newly developed biocomposites have been summarized in Table 3 and Fig. 4. The mechanical profile of PVA films exhibited the Young’s modulus of 1392 ± 0.47 MPa, tensile strength of 22.34 ± 0.41 MPa and percent elongation of 118.46 ± 2.54%. The PVA-NRH biocomposites showed the percent elongation of 12.3 ± 0.31%, tensile strength of 28.30 ± 0.54 MPa and Young’s modulus of 1656 ± 0.46 MPa. The tensile strength of PVA-BTRH was improved to 37.47 ± 0.52 MPa as compared to PVA and PVA-NRH composite, while Young’s modulus reached 2824 ± 0.93 MPa. The characteristics of biocomposite, especially mechanical are highly influenced by the existence of extra spaces among cellulose molecules that weakens hydrogen bonding, ultimately leading towards reduction in mechanical strength [49]. The entanglement and interaction between composite counterparts were weak, the addition of glycerol/maleic anhydride, that hold excellent plasticization/compatibilization efficacy, led towards improved entanglement between the two components ultimately leading towards improved mechanical characteristics of newly developed biocomposites [50, 51]. The mechanical parameters of biocomposites were further improved by the addition of chitosan filler (10 wt.% of total biomass) in native as well as bacterially treated delignified rice husk. In Fig. 3, rough and crystalline structure of biocomposites could be observed with less porosity which indicate excellent filler effect of chitosan [52].
Water absorption kinetics
All newly developed biocomposites were investigated for their water absorption capacities at ambient environmental conditions using complete-dip method (Fig. 5). PVA-NRH showed a maximal of 24% water absorption after 30 min which was increased to 112% after 120 min of immersion period. Comparatively, PVA-NRH-GLY exhibited a significant decrease in water absorption capacity after the same immersion time period. This can be attributed to the fact that addition of plasticizer possesses an intense effect on water uptake capacity of biocomposites when it is utilized in optimum concentration. The chances behind decreased water absorption capacity of the composite specimens are due to the existence of microvoids in biocomposites which are responsible of water uptake [53]. Saturation point was attained after 130 min of immersion due to high water absorption kinetics [54] The water absorption was decreased after 120 min of immersion, which could be attributed to the presence of silicon-cellulosic membrane at the surface of rice husk which could impart partially hydrophobic characteristics, ultimately reducing water sorption capability of biocomposite [55].
Composite specimens from delignified rice husk (PVA-BTRH) showed much higher water absorption capacity (202%) as compared with PVA-NRH and PVA-NRH-GLY. This can be predicted to the hygroscopic nature of cellulose and the presence of -OH functional group in it. However, biocomposites with the addition of plasticizer (PVA-BTRH-GLY) exhibited 118% of water absorption capacity. When we increase the fiber mass in biocomposite matrix, this causes increased aqueous uptake potential due to higher cellulose impregnation over the substrate, ultimately leading to the swelling and increased aqueous transport through the matrix interface [56]. The addition of chitosan filler in the biocomposite specimens PVA-NRH-GLY-CHT and PVA-BTRH-GLY-CHT showed moderately increased aqueous uptake capacities i.e., 143% and 154% respectively. This could be attributed to the fact that chitosan is hygroscopic in nature having higher capability of hydrogels formation that lead to the higher water transport through the specimens [57, 58].
Conclusions
In this research, ligninolytic enzymatic consortium (MnP, LiP and Lac) produced by S. commune IBL-06 was used for the delignification of native rice husk. An enhanced level of cellulosic content was recorded (from 32 to 72%) after the treatment of ligninolytic enzymes. Further modifications were performed using cellulosic microfibrils integration from a bacterial spp. Acetobacter xylinum, and the biocomposites were developed with novel features for potential biotechnological applications. Moreover, a novel method was established for the development of microbial cellulose-reinforced delignified rice husk-PVA-based composite materials. Significantly improved morphological and mechanical characteristics were recorded in newly developed biocomposites as demonstrated by the characterization analysis. In conclusion, this technology exhibited potential to revalorize the lignocellulosic based biomaterials for functional products development.
References
Iqbal HM, Kyazze G, Tron T, Keshavarz T (2014) Laccase-assisted grafting of poly (3-hydroxybutyrate) onto the bacterial cellulose as backbone polymer: development and characterisation. Carbohydr Polym 113:131–137
Asgher M, Qamar SA, Bilal M, Iqbal HM (2020) Bio-based active food packaging materials: sustainable alternative to conventional petrochemical-based packaging materials. Food Res 109625
Asgher M, Urooj Y, Qamar SA, Khalid N (2020) Improved exopolysaccharide production from Bacillus licheniformis MS3: optimization and structural/functional characterization. Int J Biol Macromol 151:984–992
John MJ, Thomas S (2008) Biofibres and biocomposites. Carbohydr Polym 71:343–364
Swetha M, Sahithi K, Moorthi A, Srinivasan N, Ramasamy K, Selvamurugan N (2010) Biocomposites containing natural polymers and hydroxyapatite for bone tissue engineering. Int J Biol Macromol 47:1–4
Asgher M, Arshad S, Qamar SA, Khalid N (2020) Improved biosurfactant production from Aspergillus niger through chemical mutagenesis: characterization and RSM optimization. SN Applied Sciences 2:1–11
Preisner M, Kulma A, Zebrowski J, Dymińska L, Hanuza J, Arendt M, Szopa J (2014) Manipulating cinnamyl alcohol dehydrogenase (CAD) expression in flax affects fibre composition and properties. BMC Plant Biol 14:50
Sanjay MR, Siengchin S, Parameswaranpillai J, Jawaid M, Pruncu CI, Khan A (2019) A comprehensive review of techniques for natural fibers as reinforcement in composites: preparation, processing and characterization. Carbohydr Polym 207:108–121
Janpetch N, Saito N, Rujiravanit R (2016) Fabrication of bacterial cellulose-ZnO composite via solution plasma process for antibacterial applications. Carbohydr Polym 148:335–344
Chiaoprakobkij N, Seetabhawang S, Sanchavanakit N, Phisalaphong M (2019) Fabrication and characterization of novel bacterial cellulose/alginate/gelatin biocomposite film. J Biomater Sci Polym Ed 30:961–982
Helenius G, Bäckdahl H, Bodin A, Nannmark U, Gatenholm P, Risberg B (2006) In vivo biocompatibility of bacterial cellulose. J Biomed Mater Res A 76:431–438
Maneerung T, Tokura S, Rujiravanit R (2008) Impregnation of silver nanoparticles into bacterial cellulose for antimicrobial wound dressing. Carbohydr Polym 72:43–51
Picheth GF, Pirich CL, Sierakowski MR, Woehl MA, Sakakibara CN, de Souza CF, de Freitas RA (2017) Bacterial cellulose in biomedical applications: a review. Int J Biol Macromol 104:97–106
Tang X, Alavi S (2011) Recent advances in starch, polyvinyl alcohol based polymer blends, nanocomposites and their biodegradability. Carbohydr Polym 85:7–16
Tan BK, Ching YC, Poh SC, Abdullah LC, Gan SN (2015) A review of natural fiber reinforced poly (vinyl alcohol) based composites: application and opportunity. Polymers 7:2205–2222
Wang LY, Wang MJ (2016) Removal of heavy metal ions by poly (vinyl alcohol) and carboxymethyl cellulose composite hydrogels prepared by a freeze–thaw method. ACS Sustain Chem Eng 4:2830–2837
Shen D, Liu J, Gan L, Huang N, Long M (2018) Green synthesis of Fe3O4/cellulose/polyvinyl alcohol hybride aerogel and its application for dye removal. J Polym Environ 26:2234–2242
Vaisanen T, Das O, Tomppo L (2017) A review on new bio-based constituents for natural fiber-polymer composites. J Clean Prod 149:582–596
Sanyang ML, Sapuan SM, Jawaid M, Ishak MR, Sahari J (2016) Effect of plasticizer type and concentration on physical properties of biodegradable films based on sugar palm (Arenga pinnata) starch for food packaging. J Food Sci Technol 53:326–336
Sanyang M, Sapuan S, Jawaid M, Ishak M, Sahari J (2015) Effect of plasticizer type and concentration on tensile, thermal and barrier properties of biodegradable films based on sugar palm (Arenga pinnata) starch. Polymers 7:1106–1124
Avella M, Di Pace E, Immirzi B, Impallomeni G, Malinconico M, Santagata G (2007) Addition of glycerol plasticizer to seaweeds derived alginates: influence of microstructure on chemical–physical properties. Carbohydr Polym 69:503–511
Lavorgna M, Piscitelli F, Mangiacapra P, Buonocore GG (2010) Study of the combined effect of both clay and glycerol plasticizer on the properties of chitosan films. Carbohydr Polym 82:291–298
Zheng LY, Zhu JF (2003) Study on antimicrobial activity of chitosan with different molecular weights. Carbohydr Polym 54:527–530
Kjellgren H, Gällstedt M, Engström G, Järnström L (2006) Barrier and surface properties of chitosan-coated greaseproof paper. Carbohydr Polym 65:453–460
Fernandez-Saiz P, Lagaron JM, Hernandez-Muñoz P, Ocio MJ (2008) Characterization of antimicrobial properties on the growth of S. aureus of novel renewable blends of gliadins and chitosan of interest in food packaging and coating applications. Int J Food Microbiol 124:13–20
Rao MS, Kanatt SR, Chawla SP, Sharma A (2010) Chitosan and guar gum composite films: preparation, physical, mechanical and antimicrobial properties. Carbohydr Polym 82:1243–1247
Ho MP, Wang H, Lee JH, Ho CK, Lau KT, Leng J, Hui D (2012) Critical factors on manufacturing processes of natural fibre composites. Compos B Eng 43:3549–3562
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. In: Methods in enzymology. 161:238–249
Asgher M, Wahab A, Bilal M, Iqbal HMN (2016) Lignocellulose degradation and production of lignin modifying enzymes by Schizophyllum commune IBL-06 in solid-state fermentation. Biocatal Agric Biotechnol 6:195–201
Iqbal HMN, Asgher M, Bhatti HN (2011) Optimization of physical and nutritional factors for synthesis of lignin degrading enzymes by a novel strain of Trametes versicolor. BioResources 6:1273–1287
Asgher M, Ahmed N, Iqbal HMN (2011) Hyperproductivity of extracellular enzymes from indigenous white rot fungi (Phanerochaete chrysosporium) by utilizing agro-wastes. BioResources 6:4454–4467
Johnson DB (1961) The spectrophotometric determination of lignin in small wood samples. Tappi 44:793–798
Updegraff DM (1969) Semimicro determination of cellulose inbiological materials. Anal Biochem 32:420–424
Asgher M, Ahmad Z, Iqbal HM (2017) Bacterial cellulose-assisted de-lignified wheat straw-PVA based bio-composites with novel characteristics. Carbohydr Polym 161:244–252
Iqbal HM, Kyazze G, Tron T, Keshavarz T (2015) Laccase-assisted approach to graft multifunctional materials of interest: keratin-EC based novel composites and their characterisation. Macromol Mater Eng 300:712–720
Singha AS, Thakur VK (2009) Study of mechanical properties of urea-formaldehyde thermosets reinforced by pine needle powder. BioResources 4:292–308
Bhatia SK, Kim SH, Yoon JJ, Yang YH (2017) Current status and strategies for second generation biofuel production using microbial systems. Energy Convers Manage 148:1142–1156
Taniguchi M, Suzuki H, Watanabe D, Sakai K, Hoshino K, Tanaka T (2005) Evaluation of pretreatment with Pleurotus ostreatus for enzymatic hydrolysis of rice straw. J Biosci Bioeng 100:637–643
Shi J, Sharma-Shivappa RR, Chinn M, Howell N (2009) Effect of microbial pretreatment on enzymatic hydrolysis and fermentation of cotton stalks for ethanol production. Biomass Bioenerg 33:88–96
Chawla PR, Bajaj IB, Survase SA, Singhal RS (2009) Microbial cellulose: fermentative production and applications. Food Technol Biotech 47:107–124
Jayasekara R, Harding I, Bowater I, Christie GBY, Lonergan GT (2004) Preparation, surface modification and characterisation of solution cast starch PVA blended films. Polym Testing 23:17–27
Dean KM, Do MD, Petinakis E, Yu L (2008) Key interactions in biodegradable thermoplastic starch/poly (vinyl alcohol)/montmorillonite micro-and nanocomposites. Compos Sci Technol 68:1453–1462
Qiu K, Netravali AN (2012) Bacterial cellulose-based membrane-like biodegradable composites using cross-linked and noncross-linked polyvinyl alcohol. J Mater Sci 47:6066–6075
Pavaloiu RD, Stoica-Guzun A, Stroescu M, Dobre T (2014) Use of bacterial cellulose as reinforcement agent and as coating agent in drug release applications. Rev Chim 65:852–855
Suryanegara L, Nakagaito AN, Yano H (2009) The effect of crystallization of PLA on the thermal and mechanical properties of microfibrillated cellulose-reinforced PLA composites. Compos Sci Technol 69:1187–1192
Joshi JM, Sinha VK (2006) Graft copolymerization of 2-hydroxyethylmethacrylate onto carboxymethyl chitosan using CAN as an initiator. Polymer 47:2198–2204
Kirdponpattara S, Khamkeaw A, Sanchavanakit N, Pavasant P, Phisalaphong M (2015) Structural modification and characterization of bacterial cellulose–alginate composite scaffolds for tissue engineering. Carbohydr Polym 132:146–155
Ullah H, Wahid F, Santos HA, Khan T (2016) Advances in biomedical and pharmaceutical applications of functional bacterial cellulose-based nanocomposites. Carbohydr Polym 150:330–352
Eichhorn SJ, Dufresne A, Aranguren M, Marcovich NE, Capadona JR, Rowan SJ, Gindl W (2010) Current international research into cellulose nanofibres and nanocomposites. J Mater Sci 45:1–33
Clasen SH, Müller CM, Pires AT (2015) Maleic anhydride as a compatibilizer and plasticizer in TPS/PLA blends. J Brazil Chem Soc 26:1583–1590
Rouhi M, Razavi SH, Mousavi SM (2017) Optimization of crosslinked poly (vinyl alcohol) nanocomposite films for mechanical properties. Mater Sci Eng C 71:1052–1063
Abdelrazek EM, Elashmawi IS, Labeeb S (2010) Chitosan filler effects on the experimental characterization, spectroscopic investigation and thermal studies of PVA/PVP blend films. Phys B 405:2021–2027
Ishak MR, Leman Z, Sapuan SM, Rahman MZA, Anwar UMK (2013) Impregnation modification of sugar palm fibres with phenol formaldehyde and unsaturated polyester. Fibers Polym 14:250–257
Chabannes M, Bénézet JC, Clerc L, Garcia-Diaz E (2014) Use of raw rice husk as natural aggregate in a lightweight insulating concrete: an innovative application. Constr Build Mater 70:428–438
Tran TPT, Bénézet JC, Bergeret A (2014) Rice and Einkorn wheat husks reinforced poly (lactic acid)(PLA) biocomposites: effects of alkaline and silane surface treatments of husks. Ind Crops Prod 58:111–124
Dhakal HN, Zhang ZY, Richardson MOW (2007) Effect of water absorption on the mechanical properties of hemp fibre reinforced unsaturated polyester composites. Compos Sci Technol 67:1674–1683
Kumari S, Annamareddy SHK, Abanti S, Rath PK (2017) Physicochemical properties and characterization of chitosan synthesized from fish scales, crab and shrimp shells. Int J Biol Macromol 104:1697–1705
Shariatinia Z, Fazli M (2015) Mechanical properties and antibacterial activities of novel nanobiocomposite films of chitosan and starch. Food Hydrocoll 46:112–124
Acknowledgements
This study was financially supported by the Higher Education Commission, Pakistan under grant number NRPU 6417. The analytical facilities provided by Punjab Bioenergy Institute, University of Agriculture, Faisalabad, Pakistan and School of Chemical and Materials Engineering, National University of Science and Technology, Islamabad, Pakistan are thankfully acknowledged.
Author information
Authors and Affiliations
Contributions
Muhammad Asgher: Conceptualization; Supervision; Resources; Writing—review & editing; Project administration. Irum Nasir: Methodology; Investigation; Data curation; Validation. Nimrah Khalid: Methodology; Investigation; Validation. Sarmad Ahmad Qamar: Methodology, Writing – Original draft; Software; Formal analysis; Visualization; Correspondence.
Corresponding author
Ethics declarations
Conflict of interest
Authors declared that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Asgher, M., Nasir, I., Khalid, N. et al. Development of biocomposites based on bacterial cellulose reinforced delignified rice husk-PVA plasticized with glycerol. J Polym Res 27, 347 (2020). https://doi.org/10.1007/s10965-020-02314-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02314-y