Abstract
The article fabricated novel polymer/magnetic hybrid microspheres via solvothermal method by using ferroferric oxide (Fe3O4) and aggregation-induced emission (AIE) active polyarylene ether nitrile (PEN) as building components. The PEN was copolymerized through phenolphthalin and AIE-gen (4, 4′-(1, 2-di (4-hydroxyphenyl)-1, 2-diyl) diphenol), wherein AIE-gen endowed the copolymer with AIE effect so that the copolymer could aggregate correspondingly in different solvent/nonsolvent systems and produce fluorescence enhanced. In these experiments, the diameter of Fe3O4/PEN hybrid microspheres was controlled by the volume proportion of nonsolvent (ethylene glycol (EG)) and solvent (N-methyl pyrrolidone (NMP)) in preparation process based on the aggregation behavior of AIE active PEN. Moreover, the resulted hybrid microspheres also had excellent magnetic and electromagnetic absorption performance. Specifically, they all exhibited a strong reflection loss peak both in the low and high frequency range. Meanwhile, with the change of coating thickness, these hybrid microspheres had a wide wave-absorbing frequency from 5 GHz to16 GHz. The results supported that the hybrid microspheres integrated with magnetism and AIE property will have a wide application in the preparation and improvement of size-controlled inorganic organic nanocomposites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the rapid development of electronic communications, electromagnetic (EM) interference and EM pollution have become the pressing social issue. Therefore, EM absorption material has obtained more attention of researchers, for it can absorb the EM wave energy projected onto its surface and convert the EM wave energy into heat or other forms [1,2,3,4,5,6,7]. Among a variety of EM absorption materials, inorganic-organic magnetic hybrid materials have been hotspot issue and their extraordinary performances such as excellent environmental stability, low density, and facile preparation process have injected new life into the novel EM absorption material [3, 8,9,10,11,12,13,14,15].
Furthermore, it is well-known that EM absorption property of hybrid material is related to its surface morphology and structure besides the relative complex permittivity and complex permeability and the matching impedance [4, 7, 14, 16]. Therefore, varying the parameters in the preparation process to modify the morphology of the product could be a simple way to obtain high-performance EM absorption material. Previous researches have already demonstrated the feasibility of this conceived method. For example, Wei et al. [10] prepared bisphthalonitrile-benzoxine resins containing ferrocene (FPNBZ)/Fe3O4 hybrid microspheres by solvothermal method, and controlled their size and properties by altering the content of FPNBZ. Dong et al. [17] synthesized tri-substituted-bisphthalonitrile/magnetite (TPH/Fe3O4) magnetic hybrid microspheres via a one-step solvent-thermal crystallization route and changed the content of THP to vary the morphology and structure of hybrid microspheres and then to improve the absorbing property.
Recent years, the inorganic-organic magnetic hybrid materials based on Polyarylene ether nitrile (PEN) have raised concern. On the one hand, PEN possesses outstanding performance such as high chemical stability, thermal stability, high dielectric constant, low density, excellent mechanical property and good radiation resistance [18,19,20,21]. On the other hand, the combination of PEN and ferroferric oxide will lead to the increasing interfacial polarization phenomena and therefore to a frequency dependence of the effective permittivity and then get an absorber with low density and excellent absorbing performance. Our laboratory has also done a lot of research. For example, Ma et al. [8] prepared carboxylic poly (arylene ether nitrile)/Fe3O4 hybrid microspheres with hierarchical inorganic-organic microstructure successfully, and the prepared microspheres had excellent dispensability, stability, and EM absorbing properties. Tong et al. [2] prepared polyarylene ether nitriles terminated with phthalonitrile (PEN-t-Ph)/Fe3O4 hybrid microspheres via solvothermal method, and investigated the influence of the content of PEN-t-Ph [22] on the hybrid microspheres size and EM absorbing property. Nevertheless, most of the current research on inorganic-organic magnetic hybrid microspheres mainly control the morphology and properties of the microspheres by changing the content of a certain component. Here we propose a new method. It is noticed that the heterogeneous solvents (EG and NMP) were applied in the preparation process of Fe3O4/PEN microspheres. Among them, NMP is a good solvent for PEN but EG is a poor solvent, and their solvent ratio has an important influence on PEN aggregation. Therefore, we speculate whether changing the degree of PEN aggregation through varying the solvent ratio of EG to NMP will make change on the microspheres size and EM absorbing property.
About how to achieve qualitative signifying the degree and state of PEN aggregation, we noticed AIE materials whose light emission and light enhancement are induced by aggregate formation. The presence of AIE materials overturned the traditional ACQ argument (Aggregation-caused quenching), surged revolutionary changes in the field of materials. Now they are widely applied in optical resin,biological medicine,chemical sensor and other areas [23,24,25,26]. Recently, our laboratory successfully introduced AIE-gen (TPE-2OH) into the PEN main chain to synthesize PEN with AIE activity. For instance, Wang et al. [27] synthesized novel AIE-active PEN copolymer containing pendant carboxyl group, which can be further cross-linked by calcium ions to generate fluorescent polymeric nanoparticles (FPNs). They evaluated AIE properties of synthetics by testing the aggregation state and emission spectrum of the polymeric nanoparticles in the mixed dimethylformamide/water solvent-nonsolvent systems. Therefore, we were delighted to find that the degree of this AIE active PEN aggregation can be measured by fluorescent intensity. Thus in this paper, TPE and PPL were copolymerized to obtain PEN-TPE/PPL, the organic component of hybrid materials with AIE active. In addition, the involved PPL bisphenol segment introduced carboxyl groups into the side chains of PEN-TPE/PPL, which can stick to the surface of Fe3O4 by forming chemical bonding with Fe3+/Fe2+. The magnetic hybrid microspheres preparation was based on PEN-TPE/PPL via one-step solvothermal method. The surface morphology of the microspheres was controlled by altering the volume ratio of the mixture solvent and the magnetism and EM absorbing property of the resulted hybrid microspheres were investigated.
Experimental
Materials
The aromatic monomers of phenolphthalein (PPL) were synthesized in our laboratory refer to the literature [28]. 4,4′-(1,2-di(4-hydroxyphenyl)-1,2-diyl) diphenol (TPE-2OH) was obtained from Beijing HWRK Chem Co. Ltd.. BisphenolA (BPA, AR), 2, 6-dichlorobenitrile (DCBN, AR), potassium carbonate (K2CO3, AR), FeCl3.6H2O (AR), toluene (AR), N, N-Dimethylformamide (DMF, AR), N-Methy-pyrrolidone (NMP, 99%), ethylene glycol (EG, 99%), polyethylene glycol (PEG 2000, 99%) and sodium acetate (NaAc, 99%) were purchased from Kelong Reagent Co. Ltd. Chengdu, China. All the chemicals were used without further purification.
Preparation of PEN-TPE/PPL
PEN-TPE/PPL was synthesized from DCBN and bisphenol monomer (TPE-2OH and PPL) via block copolymerization method. PPL (6.08 g,19 mmol), TPE-2OH (364.4 mg, 1 mmol), K2CO3 (4.147 g), DCBN (2.78 g), toluene (5 mL) and NMP (15 mL) were added into a 100 mL three-necked round bottom flask equipped with a condenser, a Dean-Stark trap and an overhead mechanical stirrer. Then, the reactant was heated up to~130 °C for 2 h to promote the formation of phenolate, afterward, the water-toluene azeotrope was removed and the temperature was raised up to 170 °C -180 °C for 3 h to complete the polymerization. The crude products were purified with ethanol three times, and boiling with hydrochloric and water three times to remove the excessive K2CO3 and solvent. Finally, the precipitate was collected and dried at 80 °C for 48 h in a vacuum oven to obtain homopolymer of PEN-TEP/PPL. Otherwise, PEN-PPL was homopolymerized from PPL and DCBN without the using of TPE-2OH through the same process (Fig. 1).
Preparation of hybrid microsphere
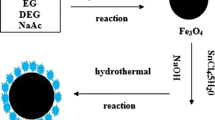
Fe3O4/PEN-TPE/PPL hybrid microspheres were prepared by solvent thermal method via one-step process as Fig. 2 shows. The volume ratio of EG to NMP was 95:5, 80:20 and 65:35 respectively. FeCl3.6H2O (2.92 g) was dissolved in EG at room temperature under the mechanical stirring, followed by the addition of PEG2000 (2.80 g). After being dissolved completely, the homogeneous solution obtained by PEN-TPE/PPL (0.5 g) dissolved in NMP was dropped. Then NaAc (10.0 g) was slowly added with vigorous mechanical stirring at 40 °C. After reaction for half an hour, the solution was sealed in a Teflon-lined stainless-steel autoclave heated at 200 °C for 15 h. After the solution was cooled to room temperature, the resulting hybrid microspheres were filtered and washed several times with ethanol and deionized water, then dried in vacuum oven at 60 °C for 12 h. The three obtained products were named as sample A (the volume ratio of EG to NMP is 95:5), sample B (the volume ratio of EG to NMP is 80:20), and sample C (the volume ratio of EG to NMP is 65:35) respectively. Fe3O4/PEN-PPL hybrid microspheres were also prepared using the above method. Fe3O4 hybrid microspheres were fabricated with same method without the addition of PEN.
Characterization
The Fourier transform infrared (FTIR) results were recorded on a Shimadzu 8400S FTIR spectrometer. Surface morphology of hybrid microspheres was studied using JMS6290LV model scanning electron microscope (SEM) and transmission electron microscope (TEM, FEI Talos F200x). X-ray diffraction (XRD, Rigaku RINT2400 with Cuka radiation). The magnetic properties were characterized by vibrating sample magnetometer at 300 k (VSM, Riken DenShi BHV-525). The EM properties were measured using a vector network analyzer (Agilent 8720ET) at 0.5 GHz–18 GHz. The samples used for EM measurements were prepared by homogeneously mixing the hybrid microspheres with wax in a mass8 ratio of 3:1 and the thickness was less than 3 mm.
Results and discussion
Figure 3 shows the FT-IR spectra of PEN-TPE/PPL. From the spectra, obviously absorption peak at 1245 cm−1 and 1720 cm−1 are corresponding to aromatic ether bond and C=O in carboxyl group respectively, and absorption peaks at 1460 cm−1, 1500 cm−1 and 1600 cm−1 are assigned to benzene. Moreover, other characteristic absorption bands, such as, symmetrical stretching vibration of nitrile band at 2231 cm−1, absorption of hydroxyl at 1020 cm−1, are all detected from the spectra. These absorption peaks prove that PEN-TPE/PPL has been successfully synthesized.
Since the backbone of PEN-TPE/PPL was introduced into AIE-gen (TPE) segment, the AIE property of PEN-TPE/PPL was investigated. The common method is to add the poor solvent into the homogeneously solution obtained from the materials dissolved in good solvent. Typically, the molecular chain of materials will curl or aggregate to form round configuration in poor solvent, and if it is an AIE material, it will exhibit fluorescence emission or fluorescence enhancement characteristics.
In this work, EG and NMP applied in the preparation process are poor solvent and good solvent, respectively, so that we can test the AIE property of PEN-TPE/PPL in mixture of EG/NMP and determine the volume ratio of the mixed solvent for further solvothermal experiments. As showed in Fig. 4a, the fluorescence intensity of PEN-TPE/PPL in mixed solutions is changed with different volume ratios of EG to NMP and enhanced by the increasing of poor solvent (EG). In addition, there is an obvious red-shift from ~400 nm to ~470 nm. These phenomena demonstrate that the PEN-TPE/PPL show as a typical AIE-active polymer in the mixture solvent of EG/NMP. In particular, when percentage of EG is less than 50 vol%, there is no obvious change of fluorescence intensity with the increase of EG content. However, when the EG content is higher than 50 vol% and below 80 vol%, the fluorescence intensity is enhanced significantly. We can interpret that when there is a low content of EG, the curl of PEN-TPE/PPL main chain is not aggregated enough to exhibit strong fluorescence. With the increasing EG content, the growing immiscibility of PEN-TPE/PPL with EG leads to the dramatic aggregation of molecular chain. Thus the blue emitting of PEN-TPE/PPL is enhanced. When EG content reaches to 80 vol%, there is the strongest luminescent intensity and when the content of EG is beyond 80 vol%, the fluorescence intensity begins to decline. This phenomenon indicates that when EG content is 80 vol%, the aggregation of PEN-TPE/PPL hits a tipping point. The decline of fluorescence intensity at EG content over 80 vol% may be due to the precipitation caused by over-aggregation of molecular chain [29, 30].
Figure 5 shows the aggregation of PEN-TPE/PPL in the solutions with different volume ratios of EG to NMP. It verifies that aggregates size becomes larger gradually, with the increase of EG content. When there is a lowestEG content, the aggregates of PEN-TPE/PPL are dispersedly smallest spheres. When the volume ratio is 80/20, there are uniformly dispersed larger spheres of aggregates. However, when EG content is reached 95 vol%, PEN-TPE/PPL appears excessive aggregation with expanding the periphery of the spheres. Thus, based on the fluorescence intensity spectra of PEN-TPE/PPL, we can learn that the degree of aggregation is represented by different intensity of fluorescence emitting. According to these phenomena, we selected three proportions of EG, tipping point content: 80 vol%, higher content: 95 vol%, and less content: 65 vol% respectively, to control the aggregation behavior of polymer in the experiment of hybrid microspheres solvothermal preparation.
After the solvothermal treating the mixture contain FeCl3.6H2O and PEN-TPE/PPL and the three selected volume EG/NMP, we obtained hybrid microspheres with different morphology characterized by SEM as shown in Fig. 6a, b and c. From Fig. 6, among the three ratios selected by the AIE effect of PEN-TPE/PPL, the size of hybrid microspheres becomes larger with increasing percentage of EG and the mean is about ~200 nm (EG: NMP = 95:5), ~120 nm (EG: NMP = 80:20) and ~55 nm (EG: NMP = 65:35) respectively, which is according to the enhancement of aggregation degree of PEN-TPE/PPL molecules with the increasing content of EG. According to the data, we suppose that the hybrid microspheres formation process may be as follows: Firstly, the aggregated PEN-TPE/PPL was dissolved in NMP to fully stretch its molecular chain which additional Fe3+ physically was adsorbed on. Next, with the presence of hydrophilic EG, hydrophobic TPE folded inward while the hydrophilic carboxyl groups on the side chain of PEN were outward to react with Fe3O4 from the Fe3+ oxidization by NaAc, so that the hydrogen of the carboxyl groups was removed while the two oxygen formed were adsorbed on theFe3O4 surface [2, 8]. Finally, under high temperature and pressure, Fe3O4 and PEN continued to shrink together to form a hybrid microsphere successfully.
Overall, the microspheres prepared at different solvent volume ratio are spherical and almost uniform in size which can also be indicated by the particle size distribution histogram of three samples below the corresponding SEM image (Fig. 6). In addition, from the histogram, we can see when the ratio of EG to NMP is 80 to 20, the tipping point ratio, the particle size distribution of the microspheres is the most uniform. For comparison, Fig. S1 (a)-(c) (Online Resource 1) indicates that without the addition of PEN-TPE/PPL, the size and aggregation of microspheres do not change, in spite of the change of solvent ratio. Furthermore, the surfaces of the microspheres are very smooth, which could be considered that PEN-TPE/PPL leads to the changes of morphology. In addition, Fig. S1(d)-(f) (Online Resource 1) suggests that when PEN-PPL without TPE segment take place of the PEN-TPE/PPL and other conditions remain unchanged, though the size of microsphere changed with solvent ratio, the morphology of the microspheres are random. Meanwhile, the phenomenon of microspheres aggregation is more serious than that of the microsphere prepared by PEN-TPE/PPL. This difference could be explained that PEN-TPE/PPL is regular block copolymer with rigid segments of TPE, while the homopolymerization of PEN-PPL is no rigid segment, the rule aggregation and rigid segments result in the difference eventually. In general, it can be concluded that interface aggregation between solvent and PEN-TPE/PPL is the main reason for adjusting the particle size of microspheres.
Figure 7 shows the TEM images of resulted hybrid microspheres. It can be seen from the Fig. 7a that the prepared hybrid microspheres have relatively uniform size and rough appearance, which are consistent with the results obtained by the SEM. The TEM images at higher magnification are shown in Fig. 7b and c, these indicate that the obtained hybrid microspheres are loose structure, which should be a result of the coalescence of small particles to grow big particles, with PEN-TPE/PPL polymer coated on the outside of dispersed Fe3O4 particles.
Figure 8 shows the XRD patterns of three resulted hybrid microspheres. The main diffraction peak at 2θ = 35.4o, 43.0o, 53.4o, 57o, 62.5o, are consist with the face-centered cubic planes of (220), (311), (400), (422), (511), (440) according to the Joint Committee on Powder Diffraction Standards (JCPDS) card No. 19–0629. There is no other diffraction peak proving good purity of prepared sample. The higher peak intensity and smaller full width of peak indicate sample B has higher crystallinity and smaller crystallite size. Due to the existence of the PEN-TPE/PPL, which may influence the crystal size and crystallinity, the peak intensity decreases compared with the pure hierarchical Fe3O4 nano-spheres reported previously [4].
Figure 9 shows the magnetization hysteresis loop of hybrid microspheres and the values of saturation magnetization (Ms), remanence magnetization (Mr) and coercivity (Hc) of samples are listed in Table 1. It can be seen that with the increasing volume ratio of EG to NMP, actually, with the increasing of hybrid microspheres size, the values of Ms and Mr are increased, as well as the value of Hc. Increasing of Ms maybe because the appearance of “Magnetic dead layer” [1, 31,32,33]. With the decreasing size of the microspheres, the surface atoms add, resulting in many dangling bonds inducing extreme asymmetry of surface atom and lattice structure. As a result, the electron cloud trajectory of the surface atoms tends to be disorderly, so that there is the increase of the disorder of the spin-on orientation of the surface atoms. However, it is difficult to make the disorder spin magnetic moment on the surface of the microsphere be ordered at the applied field, so the magnetic dead layer appears [32, 34]. Therefore, the saturation magnetization decreases as the microsphere size reduces.
About the enhancement of Hc, it is well-known that the coercive force of the particles increases with the size at room temperature when the size is below the critical size of a single domain for single domain structure, because the thermal motion has a great influence on the magnetic stability of the particles in this size range [35].
It is generally believed that the absorbing property of the absorbing material is related to its complex permittivity (εr = ε′ − jε′′) and complex permeability (μr = μ′ − jμ′′).The EM energy absorbed by the medium in the unit volume can be calculated by the formula::
Where ε0 is vacuum dielectric constant, ε″ is the imaginary part of the complex permittivity of the medium, μ″ is the imaginary part of the complex permeability of the medium, E is the electric field vector of the electromagnetic wave, and H is the magnetic field vector of the EM wave. From the Eq. (1), it can be concluded that the performance of the absorbing material depends mainly on the imaginary part of the complex permittivity and the complex permeability. Therefore, the frequency dependence of the complex permittivity and permeability of the hybrid microspheres prepared with different solvent volume ratios was investigated as showed in Fig. 10.
From Fig. 10a and b, ε ′represents energy storage, and ε″represents loss ability. It can be seen that the ε ′of three samples all decreased fluctuating as the frequency increases. The ε′value of sample B is the highest among three samples with the range of 6.12and 9.76. Sample C has similar ε ′value to sample B, whose range is between 6.47and 9.38, while ε ′value of sample A is the smallest with the range of 4.37 and 7.52. Figure 10b shows that ε″for sample B between 1.34 and 1.92 is also much higher than those of sample A between 0.8 and 1.36 and C between 0.3 and 1.2 at the range of 2.2 GHz–18 GHz, which indicates sample B possesses a good EM absorbing property. It is obvious that there are three broad peaks on ε″curves, which may be caused by the nonlinear resonant from the cooperation effect between inorganic particles and PEN-TPE/PPL, as well as wax [6]. It is also noticed that both sample A and sample C have an increase value at 8 GHz on ε″ curves, which indicates there is a dielectric loss. Similarly, the increase value on ε″ curves at 14 GHz of all samples suggests a dielectric loss as well.
The curves of μ′ and μ″ followed the frequency are shown in Fig. 10c and d. The curves of the three samples are almost identical, which may due to the fact that the dosage of FeCl3.6H2O and PEN-TPE/PPL are exactly the same in all samples. Therefore, the inter-granular magnetic interaction is almost equivalent. The main difference among the three samples is the degree of aggregation of the molecular chains in different solvent-nonsolvent systems owing to the AIE activity of PEN-TPE/PPL. It also suggests that the main reason that caused the difference of microwave absorbing property of three samples is the different dielectric loss.
Figure 11 shows the curves of dielectric loss tangent (tanδε = ε′′/ε′) and magnetic loss tangent (tanδμ = μ′′/μ′). It is generally accepted that the greater the tangent value, the greater the dielectric loss and magnetic loss, correspondingly, there is a greater reflection loss. It can be seen from Fig. 11a that sample B has a higher dielectric loss at 8.3 GHz–18 GHz, and its dielectric loss peaks can be observed at 13 GHz and 16 GHz respectively. The dielectric loss peaks of sample A and sample C can be seen at 8 GHz and 14 GHz. According to the previous studies, in the low frequency band, resonance mainly caused by vacancies or pores and high frequency resonance is attributed to atomic and electric polarization. So the resonance peak in the curves of complex permittivity could be interpreted as a result of atomic and electronic polarization [13, 14].
Magnetic loss tangent is shown in Fig. 11b. It suggests that magnetic loss decreases with the increasing of frequency at low frequency band. When the frequency is above 8 GHz, the magnetic loss of sample B and sample C begin to enhance and sample B has a larger magnetic loss tangent than others in this frequency band. The magnetic loss of sample A has almost no change in the high frequency range. In general, microwave absorption magnetic loss of the magnetic nanoparticles can be subdivided into domain-wall resonance, eddy-current loss, magnetic hysteresis loss, natural resonance and exchange resonance. However, magnetic hysteresis can be excluded in low applied fields and high frequency [1, 6, 36]. Domain-wall resonance typically occurs at the low frequency bands between in the multi-domain materials [6]. Therefore, the magnetic loss may be attributed to eddy-current loss or natural resonance and exchange resonance.
Eddy-current loss can be express by the formula:
Where f is the applied frequency, μ0is vacuum permeability; σ is electric conductivity of the particles and d is the diameter of the particles. From the above formula, it can be seen that if the magnetic loss is eddy-current loss, the value μ′′/(μ′)2f [μ′′/(μ′)2f = 2πμ0σd2/3] should be a constant with the change of frequency. As showed in Fig. 12, it can be seen that the value of μ′′/(μ′)2f changes when the frequency varies at low frequency. However, at high frequency (> 8 GHz), it is a constant exactly. Therefore, the magnetic loss may be mainly attributed by natural resonance and exchange resonance at low frequency and about high frequency band, the magnetic loss may be due to the eddy-current loss.
It is also noticed that the magnetic loss is larger than the dielectric loss at low frequency band, however, with the increasing of frequency, the dielectric loss becomes the highlight. Thence we can think that the magnetic loss plays a leading role in the process of EM absorbing and attenuation in low frequency while the dielectric loss mainly leads in low frequency band.
In general, reflection loss (RL) of the absorber is used to evaluate EM absorbing performance. According to the transmission line theory, simulation formula is as follows:
Where Zin is the input impedance of the surface of the absorber, Z0 is the impedance in free space, μr is complex permeability, εr is complex permittivity, f is the frequency of microwave,d is the thickness of sample coating, and c is the speed of light [37].
The RL of the three samples were investigated. As showed in Fig. 13a, it can be observed that the reflection loss of sample A is mostly above −10 dB with the change of frequency and thickness and the frequency band of the absorption wave moves toward the high frequency as the thickness decreases. When the thickness is 5.5 mm, 5 mm and 4.5 mm respectively, sample A has a better EM absorbing performance at low frequency. It is noticed that the RL has more or less increase near 8 GHz at all thickness, which may due to the increased dielectric loss as showed in Fig. 10b. Similarly, the decrease of RL of sample A at 14 GHz should be caused by the reduction dielectric loss showed as in Fig. 10b. From Fig. 13b, sample B has an excellent EM absorbing performance with two strong reflection loss peaks, one is −29.77 dB at 5.88 GHz with the thickness of 5.5 mm, and the other is −28.7 dB at 15.5 GHz with the thickness of 2 mm, which are also because of the enhancement dielectric loss thereat as shown in Fig. 10b. Moreover, sample B also has a broad EM absorbing range from 5.88 GHz to 15.5 GHz with the thickness ranging from 2 mm to 5.5 mm, and in this frequency range, the reflection loss peak is less than −10 dB. This indicates that sample B has a wide range of absorbing frequency and possesses more widely used in absorbing applications. In addition, the EM wave absorbing frequency also gradually moves toward the high frequency band with the thickness decreasing. The reflection loss of sample C is shown in Fig. 13c. It can be seen that sample C has a good microwave property in low frequency, with reflection loss value −29.5 dB at 5.6 GHz with the thickness of 5.5 mm. However, in the high frequency band, the EM absorbing performance is not as good as sample B, this may be caused by its lower dielectric loss and magnetic loss. Moreover, the RL variation of sample C at 8 GHz and 14 GHz is the same as that of sample A due to the dielectric loss shown in Fig. 10b.
In general, sample B prepared with the ratio of the solvent selected by aggregation phenomenon of PEN-TPE/PPL exhibits excellent microwave absorbing property that not only has strong reflection loss peak in high and low frequency but also a wide absorbing band with the change of thickness because of its dielectric loss.
Conclusion
In conclusion, Fe3O4/PEN-TPE/PPL hybrid microspheres were prepared via a simple and effective solvent-thermal route. The surface morphology of the microspheres could be fitted by the volume ratio of EG to NMP solvent, which was due to the AIE-activity of PEN-TPE/PPL in solvent-nonsolvent system. Specifically, the diameter of hybrid microspheres was increased with the increasing degree of AIE active PEN aggregation. As results, morphology of the hybrid microspheres has a great influence on the EM absorbing properties. When the volume ratio of EG to NMP was 80:20, the size is about ~120 nm, where the PEN-TPE/PPL had the strongest fluorescence emission, the obtained microspheres exhibited the best EM absorption property. The sample had a strong reflection loss peak both in the high frequency and low frequency. Meanwhile, there was a wide EM absorbing frequency among 5 GHz to 16 GHz with the change of thickness. It is believed that the prepared microsphere could be used as microwave absorbing materials or be used in the field of biopharmaceuticals because of its good absorbing performance and magnetic and stability. Thus far, the report is an approach to size control of polymer/Fe3O4 hybrid microspheres based on the aggregation degree of AIE active polymer materials, whose aggregation behavior can be adjusted by solvent/nonsolvent system and detected by fluorescence spectrometer, conventionally. The method controlling the size of hybrid microsphere will also have wide application in the preparation of inorganic-organic hybrid materials.
References
Pan H, Cheng X, Gong C, Yu L, Zhang J, Zhang Z (2013) Preparation of (FexNi1−x)4N (0.5 ≤ x ≤ 0.8) compounds and evaluation of their magnetic and microwave absorbing properties. J Appl Phys 113(11):113906
Tong L, Pu Z, Huang X, Liu X (2014) X, Ku-band microwave-absorption properties of polyarylene ether nitriles terminated with phthalonitrile/Fe3O4 hybrid submicron spheres. Mater Sci Eng B-adv 184:98–104
Wei J, Yang X, Zhan Y, Meng F, Zhao R, Zhong J, Liu XB (2012) Magnetic and electromagnetic properties of ferrocenyl organic metal magnetic resin. Mater Lett 67(1):135–138
Jia K, Zhao R, Zhong J, Liu XB (2010) Preparation and microwave absorption properties of loose nanoscale Fe3O4 spheres. J Magn Magn Mater 322(15):2167–2171
Liu L, Duan Y, Ma L, Liu S, Yu Z (2010) Microwave absorption properties of a wave-absorbing coating employing carbonyl-iron powder and carbon black. Appl Surf Sci 257(3):842–846
Zhao B, Shao G, Fan B, Guo W, Chen Y, Zhang R (2015) Preparation of SnO2-coated Ni microsphere composites with controlled microwave absorption properties. Appl Surf Sci 332:112–120
Yulan Ren CZ, Shen Z, Li C, Chen Y, Gao P, Yang P, Ouyanga Q (2013) Three-dimensional SiO2@Fe3O4 core/shell nanorod array/graphene architecture: synthesis and electromagnetic absorption properties. Nanoscale 5:12296–12303
Ma Z, Meng FB, Zhao R, Zhan YQ, Zhong JC, Liu XB (2012) Preparation and dual microwave-absorption properties of carboxylic poly (arylene ether nitrile)/Fe3O4 hybrid microspheres. J Magn Magn Mater 324(7):1365–1369
Ma Z, Zhao R, Yang X, Wei JJ, Meng FB, Liu XB (2012) Microwave absorption properties of Fe3O4/CuPc hybrid material with cooperative dual nonlinear dielectric/magnetic resonance. Mater Lett 69:30–33
Meng F, Zhao R, Zhan Y, Lei Y, Zhong J, Liu XB (2011) One-step synthesis of Fe-phthalocyanine/Fe3O4 hybrid microspheres. Mater Lett 65(2):264–267
Wei J, Zhan Y, Yang X, Meng F, Ma Z, Zhao R, Zhong J, Zhang JC, Liu XB (2011) One-step preparation of organometal/Fe3O4 hybrid microspheres and their electromagnetic properties. J Magn Magn Mater 323(24):3210–3216
Wei J, Zhao R, Liu XB (2012) Only Ku-band microwave absorption by Fe3O4/ferrocenyl-CuPc hybrid nanospheres. J Magn Magn Mater 324(20):3323–3327
Wei JJ, Zhao R, Zhan YQ, Meng FB, Yang X, Xu MZ, Liu XB (2012) One-step solvothermal syntheses and microwave electromagnetic properties of organic magnetic resin/Fe3O4 hybrid nanospheres. Appl Surf Sci 258(17):6705–6711
Xu MZ, Meng FB, Zhao R, Zhan YQ, Lei Y, Liu XB (2011) Iron phthalocyanine oligomer/Fe3O4 hybrid microspheres and their microwave absorption property. J Magn Magn Mater 323(16):2174–2178
Zhang J, Shi C, Ji T, Wu G, Kou K (2014) Preparation and Microwave Absorbing Characteristics of Multi-Walled Carbon Nanotube/Chiral-Polyaniline Composites. Open J Polym Chem 04(03):62–72
Zhang XF, Dong XL, Huang H, Liu YY, Wang WN, Zhu XG, Lv B, Lei JP, Lee CG (2006) Microwave absorption properties of the carbon-coated nickel nanocapsules. Appl Phys Lett 89(5):053115
Dong SH, Xu MZ, Wei JJ, Yang XL, Liu XB (2014) The preparation and wide frequency microwave absorbing properties of tri-substituted-bisphthalonitrile/Fe3O4 magnetic hybrid microspheres. J Magn Magn Mater 349:15–20
Feng MN, Huang X, Tang HL, Pu ZJ, Liu XB (2013) Dielectric and thermal properties of novel three-component CCTO/MWCNTs/polyarylene ether nitrile composite films. J Mater Sci Mater Electron 24(10):3652–3659
Pu ZJ, Chen L, Long Y, Tong LF, Huang X, Liu XB (2013) Influence of composition on the proton conductivity and mechanical properties of sulfonated poly (aryl ether nitrile) copolymers for proton exchange membranes. J Polym Res 20(11):281
Shen SZ, Pu ZJ, Zheng PL, Liu XB, Jia K (2015) Synthesis and properties of cross-linkable poly (arylene ether nitrile) s containing side propenyl groups. High Perform Polym 28(5):562–569
Huang X, Pu ZJ, Tong LF, Wang ZC, Liu XB (2012) Preparation and dielectric properties of surface modified TiO2/PEN composite films with high thermal stability and flexibility. J Mater Sci Mater Electron 23(12):2089–2097
Zhan Y, Yang X, Guo H, Yang J, Meng F, Liu XB (2012) Cross-linkable nitrile functionalized graphene oxide/poly (arylene ether nitrile) nanocomposite films with high mechanical strength and thermal stability. J Mater Chem 22(12):5602
Hong Y, Lam JW, Tang BZ (2009) Aggregation-induced emission: phenomenon, mechanism and applications. Chem Commun 29:4332–4353
Hu R, Lam JWY, Yu Y, Sung HHY, Williams ID, Yuen MMF, Tang BZ (2013) Facile synthesis of soluble nonlinear polymers with glycogen-like structures and functional properties from “simple” acrylic monomers. Polym Chem 4(1):95–105
Chen G, Li W, Zhou T, Peng Q, Zhai D, Li H, Yuan WZ, Zhang Y, Tang BZ (2015) Conjugation-Induced Rigidity in Twisting Molecules: Filling the Gap Between Aggregation-Caused Quenching and Aggregation-Induced Emission. Adv Mater 27:4496–4501
Hong Y, Lam JWY, Tang BZ (2011) Aggregation-induced emission. Chem Soc Rev 40:5361–5388
Wang P, Jia K, Zhou XF, Guan X, Wang L, Tian Y, Wu CH, Liu XB (2017) Ca(2+) Induced Crosslinking of AIE-Active Polyarylene Ether Nitrile into Fluorescent Polymeric Nanoparticles for Cellular Bioimaging. Macromol Rapid Commun 38(21)
Tang HL, Pu ZJ, Huang X, Wei JJ, Liu XB, Lin ZQ (2014) Novel blue-emitting carboxyl-functionalized poly (arylene ether nitrile) s with excellent thermal and mechanical properties. Polym Chem 5(11):3673
Zheng J, Huang F, Li YJ, Xu TW, Xu H, Jia JH, Ye Q, Gao JR (2015) The aggregation-induced emission enhancement properties of BF2 complex isatin-phenylhydrazone: Synthesis and fluorescence characteristics. Dyes Pigments 113:502–509
Zhang YY, Li HF, Zhang GB, Xu XY, Kong L, Tao XT, Tian YP, Yang JX (2016) Aggregation-induced emission enhancement and mechanofluorochromic properties of α-cyanostilbene functionalized tetraphenyl imidazole derivatives. J Mater Chem C 4(14):2971–2978
Peng DL, Sumiyama K, Hihara T, Yamamuro S (1999) Enhancement of magnetic coercivity and macroscopic quantum tunneling in monodispersed Co/CoO cluster assemblies. Appl Phys Lett 75(24):3856–3858
Frenkel J, Doefman J (1930) Spontaneous and Induced Magnetisation in Ferromagnetic Bodies. Nature 126(3173):274–275
Iida H, Takayanagi K, Nakanishi T, Osaka T (2007) Synthesis of Fe3O4 nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J Colloid Interface Sci 314(1):274–280
Bridger K, Watts J, Chien CL (1988) The dependence of coercivities of ultrafine Fe particles on packing fraction and microstructure. J Appl Phys 63(8):3233–3235
Mercier D, Levy JCS (2000) Magnetic resonance in spherical Co-Ni and Fe-Co-Ni particles. Phy Rev B 62:533–544
Zhang Q, Li C, Chen Y, Han Z, Wang H, Wang Z, Geng D, Liu W, Zhang Z (2010) Effect of metal grain size on multiple microwave resonances of Fe/TiO2 metal-semiconductor composite. Appl Phys Lett 97(13):133115
Qin F, Brosseau C (2012) A review and analysis of microwave absorption in polymer composites filled with carbonaceous particles. J Appl Phys 111(6):061301
Acknowledgements
The authors gratefully thank the financial support from the National Natural Science Foundation of China (Project 51403029), the Fundamental Research Funds for the Central Universities (ZYGX2016J040) and the Scientific Research Foundation for the Returned Overseas Chinese Scholars from State Education Ministry (LXHG5003).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
ESM 1
(PDF 216 kb)
Rights and permissions
About this article
Cite this article
Sang, X., Li, K., Wang, P. et al. Fabrication and microwave absorption properties of size-controlled polymer/Fe3O4hybrid microsphere based on aggregation-induced emission active polyarylene ether nitrile. J Polym Res 25, 207 (2018). https://doi.org/10.1007/s10965-018-1590-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-018-1590-7