Abstract
A series of six photo-crosslinkable thermotropic liquid crystalline copolyesters were prepared by polycondensation method at room temperature using tetra-n-butylammonium bromide as the phase transfer catalyst. The diacid chloride involved in this process was adipoyl chloride, the common diol (diol-1), also referred to as bischalcone diol, used was 3,3′-benzene-1,4-diylbis[1-(4-hydroxyphenyl)prop-2-en-1-one] and six different arylidene diols were involved as varying diols (diol-2), in the ratio 2:1:1. The common diol and the six arylidene diols were synthesized by the acid-catalyzed Claisen-Schmidt synthesis. The inherent viscosity ηinh of these six copolyesters was found between 0.35 and 0.45. The microstructure of the repeating unit present in the copolyester chain was confirmed by FT-IR, 1H–NMR and 13C–NMR spectroscopic techniques. Thermal transition temperatures, resolved from the DSC thermograms, were found to be well above room temperature. Optical polarizing microscopy was employed to establish the liquid crystalline property and all the polymers were found to have nematic phase. The photo-crosslinking behaviour of these copolyesters was studied in DMF solution at different time intervals. The copolyesters having methoxy group in them absorb UV-A rays (315–400 nm) for a longer duration, which promotes them to be good candidates for UV filters and sunscreens.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Photo-crosslinkable polymeric materials have attracted the attention of the scientific community for more than half a century [1]. They are employed in photocurable paints [2], photoresists [3], printing plates [4], and so on. A systematic survey of literature indicates that certain moieties such as arylidene-keto group [5], chalcone mesogen [6], cinnamate group [7], stilbene group [8], Schiff base [9], azobenzothiazole [10] and propargyl group [11] are found to be responsible for photo-crosslinking behaviour. Chalcone moiety is a versatile chromophore, and this is incorporated in the polymeric main chain [12] and also as a pendant moiety or side chain in the polymeric system [13]. Photo-crosslinkable chalcone moiety is involved in the synthesis of many types of polymeric materials such as polyimides [14], polyphosphazenes [15], poly(arylene-ethers) [16], copolyphosphoramide esters [17], poly(arylene ether sulfone) [18], poly(amide-imide)s [19], poly(ester-amides) [20], and acryamide polymers [21]. Photocrosslinkable diarylidene part is roped in the main chain or as pendant units in flame-retardant poly(benzylidene phosphoramide ester)s [22] and hyperbranched benzylidene polyesters [23]. Non-linear optically active polymeric systems are generated from bis(hydroxy-arylidene)alkanones [24], azo-bisbenzylidene polymers, and [25] polyethers [26] are only a few of rich literature available in this area. Recently we have reported the synthesis and characterization of thermotrophic liquid crystalline copolyesters containing arylidene-keto and monochalcone moieties [27].
However, there are no reports available to indicate the synthesis and characterization of copolyesters containing bischalcone and arylidene-keto moiety in the main chain. Hence, synthesis and characterization of a few liquid crystalline copolyesters containing photo-crosslinkable arylidene-keto and chalcone moiety in the main chain are studied and the results are presented here. The idea of incorporation of rigid rods [28] and flexible spacers [29] is carefully executed in the synthesis of copolyesters to achieve thermotropic liquid crystalline property.
Methods and materials
Materials
Adipoyl chloride, terephthalaldehyde (Sigma Aldrich), cyclohexanone, cyclopentanone, acetone, 4-hydroxyacetophenone (Spectrochem Puriss grade), 3-methoxy-4-hydroxy benzaldehyde (vanillin), 4-hydroxybenzaldehyde (Loba Chemie AR), tetra-n-butylammonium bromide (EMerck), sulphuric acid and sodium hydroxide (Rankem) were used as received. All other materials were used after purification [30].
Methods
Synthesis of arylidene diols
The six arylidene-keto diols utilized in this work were synthesized by the standard procedure [31] available in the literature to ensure good yield. A typical synthesis of an arylidene diol by acid catalyzed Claisen-Schmidt condensation is mentioned here. The same method was adopted to synthesise other monomers as well.
Synthesis of 2,6-bis(4-hydroxybenzylidene)cyclohexanone (BHCH)
Cyclohexanone 0.98 g (0.01 mol) and 4-hydroxybenzaldehyde 2.44 g (0.02 mol) were dissolved in 20 mL of methanol. To the methanolic solution kept on an ice bath, concentrated sulphuric acid (2 mL) was added in drops in 2 min. During the addition an exothermic reaction was set in, the mixture was shaken and the temperature was kept under 20 °C. The reaction mixture was kept in ambient temperature for 12 h. The solid separated out was washed with water and recrystallized from methanol. Yield: 92%; m.p. >250 °C; IR (KBr) 3383, (b, O-H), 1651(s, C = O) cm−1; 1H NMR (400 MHz, DMSO-d6) δ 3.11 (s, 4H), 6.88–7.65 (m, 10H) and 9.61 (s, 2H).
The same method was adopted to synthesise other monomers as well by varying the ketone and aldehyde.
2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BVCH)
Cyclohexanone 1.0 mL (0.01 mol) and vanillin 3.04 g (0.02 mol). Yield: 86%, m.p. 182 °C; IR (KBr) 3383 (b, OH), 1652 (s, C = O) cm−1; 1H NMR (400 MHz, DMSO-d6) 2.93 (s, 4H), 3.91 (s, 6H), 6.92–7.79 (m, 10H) and 9.61 (s, 2H).
2,5-bis(4-hydroxybenzylidene)cyclopentanone (BHCP)
Cyclopentanone 0.9 mL (0.01 mol) and 4-hydroxybenzaldehyde 2.44 g (0.02 mol). Yield: 92%, m.p. >250 °C; IR (KBr) 3300 (b, O-H), 1667(s, C = O) cm−1; 1H NMR (400 MHz, DMSO-d6) 3.11 (s, 4H), 6.85–7.67 (m, 10H) and 9.61 (s, 2H).
2,5-bis(4-hydroxy-3-methoxybenzylidene)cyclopentanone (BVCP)
Cyclopentanone 0.9 mL (0.01 mol) and vanillin 3.04 g (0.02 mol). Yield: 89% m.p. 212 °C; IR (KBr) 3448 (b, OH), 1667 (s, C = O) cm−1; 1H NMR (400 MHz, CDCl3) 3.10 (s, 4H), 3.94 (s, 6H), 6.63–7.94 (m, 10H) and 9.61 (s, 2H).
1,5-bis(4-hydroxyphenyl)penta-1,4-dien-3-one (BHAC)
Acetone 0.75 mL (0.01 mol) and 4-hydroxybenzaldehyde 2.44 g (0.02 mol). Yield: 93% m.p. 241 °C; IR (KBr) 3452 (b, O-H), 1658 (s, C = O) cm−1; 1H NMR (400 MHz, DMSO-d6) 6.82–6.96 (dd, 4H, −CH = CH-), 7.49–8.33 (m, 8H, aromatic) and 9.61 (s, 2H).
1,5-bis(4-hydroxy-3-methoxyphenyl)penta-1,4-dien-3-one (BVAC)
Acetone 0.75 mL (0.01 mol) and vanillin 3.44 g (0.02 mol). Yield: 92% m.p. 161 °C; IR (KBr) 3446 (b, O-H) 1658 (s, C = O) cm−1; 1H NMR (400 MHz, DMSO-d6) 3.94 (s, 6H), 6.72–7.14 (dd, 4H, −CH = CH-), 7.52–8.89 (m, 6H, aromatic) and 9.60 (s, 2H).
A general scheme for the synthesis of bisbenzylidene diols is presented here.
General structure of copolyesters is represented below.
Synthesis of bischalcone diol
xThe bischalcone diol namely 3,3′-benzene-1,4-diylbis[1-(4-hydroxyphenyl)prop-2-en-1-one] (BHAP) associated with this work was synthesized by the usage of appropriate procedure [32].
Synthesis of BHAP
Dry HCl gas was passed through a well-cooled and stirred solution of 4-hydroxyacetophenone 16.32 g (0.12 mol) and terephthalaldehyde 8.04 g (0.06 mol) in 50 mL of dry methanol for 10 min. The BHAP was separated out as solid by the addition of ice-cold water. It was washed with double-distilled water and then re-crystallized from hot methanol. Yield: 92%, m.p.: 262–264 °C; IR (KBr) 3450 (b, O–H), 1655 (s, C = O) cm−1; 1H NMR (DMSO-d6) δ 6.93–6.96 (dd, 2H, −CH = CH–), δ 7.45–7.99 (m, 12H, aromatic) and δ 10.03 (s, 2H, −OH).
Synthesis of copolyesters
The copolyesters were all prepared by phase transfer catalyzed polycondensation method reported in literature [33]. The typical procedure for the synthesis of copolyester PABHH is presented here. The common diol, BHAP, namely diol-I (0.002 mol) and the varying diol, BHCH, namely diol II (0.002 mol) were dissolved in double distilled water (25 mL) containing dissolved sodium hydroxide (0.004 mol) taken in a three necked 100 mL round bottomed flask. The mixture was stirred continuously at room temperature for 30 min in nitrogen atmosphere. A solution of 2 mL of 2% tetra-n-butylammonium bromide was added and stirred. About 25 mL solution containing adipoyl chloride (0.004 mol) in dichloromethane was added using a pressure equalizer with constant stirring. The mixture was maintained at room temperature with continuous stirring for 3 h and it was cooled and poured into 300 mL of methanol. The copolyester got precipitated; it was filtered, washed with methanol and then dried in vacuum pump at ambient temperature.
The remaining five copolyesters were all prepared by employing the above method. Monomers used in the preparation of copolyesters along with polymer code are presented in Table 1.
Characterisation techniques
Shimadzu 8400 and JASCO Model P-4600 FT-IR instrument were utilized to record the FT-IR spectra of all the monomers and the six copolyesters in KBr pellets. Bruker Avance instrument was involved to record the 1H–NMR at 400 MHz and 13C–NMR at 75 MHz. To record 1H–NMR and 13C–NMR spectra, all the six copolyesters were dissolved in DMSO-d6. DSC thermograms were recorded at the rate of heating 20 °C/min in nitrogen atmosphere using Dupont 2000 model Perkin Elmer Thermal Analyser. Olympus BX 51 Optical Polarizing Microscope attached with Linkem hot stage was employed to record the optical polarizing micrograph of the copolyesters. The UV light from a 125 W - 365 nm mercury lamp at a distance of 9 cm in Heber Annular Photochemical reactor model HVAR 123 was irradiated on copolyesters in DMF solution of concentration 0.02 gdL−1 at different time intervals to study their photocrosslinking behaviour. Photocrosslinking kinetics was studied by on JASCO V650 UV-Visible spectrophotometer.
Results and discussion
The amorphous copolyesters when tested for their solubility (0.1 g/10 mL) disclosed that they were insoluble in non-polar solvents, sparingly soluble in moderately polar solvents and completely soluble in highly polar solvents (Table 2).
Inherent viscosity (ηinh) values for the copolyesters were determined at 30 °C in DMF of concentration 0.1 gdL−1 using Ubbelohde viscometer, which are found between 0.35 and 0.45. The (ηinh) values are related to the molecular weights and rigidity of the polymers [34]. The copolyesters derived from monomers having methoxy groups such as PABHV, PABPV and PABCV show higher (ηinh) values. This trend is attributed to interlocking effect offered by methoxy group [35]. Similar observation is made by Murugavel and coworkers [22] in a series of poly(benzylidene phosphoramide ester)s containing a benzylidene chromophore in the main chain.
FT-IR spectra of all the six monomers and copolyesters in KBr pellets are recorded between 400 and 4000 cm−1 and the results are produced here. The ester linkage resulting from the polycondenzation of diols and adipoyl chloride appears between 1752 and 1762 cm−1 due to strong stretching vibration of C = O in the FT-IR spectra. FT-IR spectra of all the copolyesters are given in Fig. 1.
The carbonyl stretching frequency of ester linkage in copolyester PABHH is observed at 1755 cm−1
, whereas that of PABPV is observed at 1760 cm−1. This may be accounted for as follows: the difference in stretching frequency is due to the presence of methoxy group in the monomeric unit of 2,6-bis(4-hydroxy-3-methoxybenzylidene)cyclohexanone (BVCH) as shown below. The 3-methoxy group in the benzylidene moiety withdraws electron density by – I effect from the ring, which in turn withdraws electron density from the phenoxy oxygen atom of ester linkage and decreases electron density at acyl group. As a consequence, the carbonyl group of the ester group absorbs at higher frequency when compared to copolyester derived from unsubstituted monomer of 2,6-bis(4-hydroxybenzylidene)cyclohexanone (BHCH). It should be noted that the methoxy oxygen is not in a position to exert the mesomeric effect as it goes out of plane due to steric interaction it encounters with adipate chain.
FT-IR spectra of typical two monomers, BHAC and BHAP are given in Fig. 2 for comparison. (FTIR spectra of remaining four monomers are given in ESM). BHAC and BHAP have their keto carbonyl stretching frequencies at 1700 cm−1 and 1655 cm−1 respectively. The copolyesters have both the two carbonyl groups; one in benzylidene part which is observed at 1700 cm−1 and the other in bischalcone part, observed at 1655 cm−1, which clearly shows that both these monomers are incorporated in the copolyesters. The C-H bending of aromatic rings has a signal at 818–819 cm−1. The signal around 1600 cm−1 is due to olefinic group. The absorption at ῡ = 1193–1219 cm−1 is due to ester C-O stretching vibration. The broad peaks at 3250–3370 cm−1 are due to O-H stretching of end OH groups.
In the 1H–NMR spectra, the methylene protons of the diacid part are observed at δ 1.2–2.2 ppm. The methylene protons of cyclopentanone and cyclohexanone rings of diarylidene moieties absorb at δ 2.2–3.2 ppm [36]. The deshielded vinylic protons of chalcone moiety due to the presence of electron-withdrawing keto groups and phenyl rings at adjacent positions absorb at δ 6.7–7.3 ppm [37, 38]. The arylidene protons of diarylidene keto moieties resonate at δ 7.5–7.7 [39]. The methoxy protons of diarylidenealkanone system derived from vanillin absorb at δ 3.7–3.9 ppm. The aromatic protons of diarylidene ketones and chalcone, absorb at δ 7.1–8.4 ppm. A typical 1H–NMR spectrum of the copolyester PABPV is presented in Fig. 3. (1H–NMR spectra of all other polymers are given in ESM). In all the spectra a very intense signal at δ 3.45 ppm is due to methyl protons of deuterated DMSO-d6, which is used as solvent and a signal at δ 2.56 ppm is due to protons of water, which may be an impurity in DMSO [26].
The 13C–NMR spectra of the copolyesters show a signal at δ 170–175, which is due to the carbonyl carbon of the ester group conspicuously indicating that the arylidene-keto and chalcone moieties are incorporated in the polymeric backbone. The signal at δ 185–190 ppm is due to the carbonyl carbon of the arylidene keto and chalcone moieties. Aromatic carbons of diarylidene and chalcone parts absorb at δ 115–140 ppm [39]. 13C–NMR spectrum of the copolyesters PABHV is represented in Fig. 4. (13C–NMR spectra of all other polymers are given in ESM).
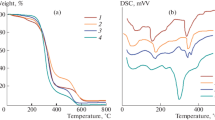
Figure 5 represents the thermograms of these copolyesters, which give adequate information on thermal transition temperatures, namely the glass transition temperature (Tg), mesophase formation temperature (Tm) and isotropic temperature (Tiso). The glass transition temperature (Tg) of all the copolyesters is above room temperature. The copolyesters synthesized from divanillidene alkanones show higher Tg than copolyesters of simple diarylidene alkanones. This is due the interlocking effect offered by the methoxy group in vanillin moiety [35]. Once Tg is crossed, the unsubstituted copolyesters show higher phase transition temperatures when compared to methoxy substituted ones, as the former are more rigid than the latter due to the easier stacking of aromatic rings over the other [40]. After the interlocking is released the methoxy substituents tend to force the chains out of phase and reduce the intermolecular forces of attraction [41, 42], thereby reducing the melting temperature (Table 3).
Figure 6a-d represent the optical polarizing micrographs of the copolysters, (also refer ESM) which throws light on the nematic formation in the tiny blobs and flowing fluidic appearance that is generated above Tm to unveil that it is typical of thermotropic liquid crystalline material.
Photo-crosslinking studies
Figure 7a-d represent the overlaid UV-vis spectra of the copolyesters at different time of exposure to light. The absorbance decreased with increase in time of irradiation, convincing that there is photo-crosslinking by 2π + 2π cycloaddition between the olefinic bonds of the arylideneketo and chalcone units [43, 44]. The plots of [(Ao – At)/Ao] against the time duration of irradiation impresses on the extent of crosslinking, where Ao is initial absorbance and At values are absorbance values for different times of irradiation. Fig. 8 represents rate of crosslinking due to the irradiation of ultraviolet light on the copolyesters.
The plots show that the crosslinking is faster in the beginning and it gradually slows down after 5–8 min. This may be due to the freedom in the form of flexibility available in the beginning than after getting crosslinked here and there. The copolyesters derived from vanillin-based diarylidenealkanonediols show gradual decrease in absorption, whereas the other copolyesters show steeper decrease. This is attributed to the hindrance by methoxy group’s bulkiness during photo-crosslinking [45]. When the unsubtituted copolyesters are compared, the crosslinking is in the order PABCH > PABHH > PABPH. Other workers [46, 47] have reported that cyclopentanone-bearing polymers crosslink faster than the ones that have cyclohexanone rings. They have correlated the sizes of the rings with the rates (smaller the ring faster the rate). But in the present work, the cyclohexanone ring systems show faster crosslinking ability. This may be attributed to the consequence of copolymerization by the incorporation of bischalcone system in to the copolyester [47].
A closer look at the UV spectra of copolyester PABPH (Fig. 9) shows that there are two peaks merge to give a humped peak. The peak at 377 nm is due to diarylidene (BHCP) system in the copolyester and the one at 361 nm is due to bischalcone part. During photolysis the peak at longer wavelength decreases faster than the peak at shorter wavelength, that suggests the BHCP part (which contains the cyclopentanone ring) of the polymer undergoes faster crosslinking, which is in conformity with others’ report [46, 47]. Whereas the peak due to bischalcone system takes longer time to decrease in intensity. In other copolyesters such distinction is not noticed. This explains the deviation from the trend observed by other researchers.
The copolyesters PABHV, PABPV and PABCV having methoxy groups in their bisbenzylidenealkanone parts, absorb at UV-A (315–400 nm) region for longer duration. Due to the nondistruction of chromophore (Fig. 10) by cycloaddition due to the mismatch offered by copolymerization [47], makes them good candidates for UV filters and sunscreens.
It is to be noted that PABCV is derived from BVAC, which is prepared from acetone a chemical cheaper when compared to cyclopentanone or cyclohexanone. The combination of bichalcone unit and methoxy-substituted bisbenzylidenealkanone parts in the copolymer yields materials that absorb at UV-A region. This property can be exploited in the commercial production of UV filtering materials.
Conclusions
A series of six copolyesters were synthesized using a diacid chloride, diol-1 and diol-2. The dicarboxylic acid chloride used was adipoyl chloride, the diol-1 used was BHPP. The diol-2 used were BHAC, BVAC, BHCP, BVCP, BHCH and BVCH. The copolyesters were characterized by viscosity and spectral data. The DSC thermograms identify phase transition temperatures and were helpful in OPM analysis. The OPM studies indicate nematic liquid crystalline phase formed in copolyesters. In the photo-crosslinking studies it was found that the copolyesters ABHV, ABPV and ABCV having methoxy group absorb UV-A rays longer, which promotes them to be good candidates for UV filters, sunscreens and photoresists.
References
Unruh CC, Smith Jr AC (1960) Condensation of poly(4-vinylacetophenone) with various araldehydes. J Appl Polym Sci 3:310–315. doi:10.1002/app.1960.070030908
Bibaut RC, Burget D, Fouassier JP, Varelas CG, Thomatos J, Tsagaropoulos G, Ryrfors LO, Karlsson OJ (2002) Use of α-diketones as visible photoinitiators for the photocrosslinking of waterborne latex paints. J Polym Sci Part A Polym Chem 40:3171–3181. doi:10.1002/pola.10407
Havard JM, Yoshida M, Pasini D, Vladimirov N, Frechet JMJ, Medeiros DR, Patterson K, Yamada S, Willson CG, Byers JD (1999) Design of photoresists with reduced environmental impact. II. Water-soluble resists based on photocrosslinking of poly(2-isopropenyl-2-oxazoline). J Polym Sci Part A Polym Chem 37:1225–1236. doi:10.1002/(sici)1099-0518(19990501)37:9<1225::aid-pola2>3.0.co;2-5
Gaston M, Colette R, Lablache CA, Loucheux C (1984) Photochemistry of polymeric systems. VI Photocrosslinking of Basic Polymers and Copolymers dyeing J Appl Polym Sci 29:651–660. doi:10.1002/app.1984.070290221
Roop SD, Vasanthi S, Arul MJ (2012) Synthesis of certain poly[bis(benzylidene)]esters and investigation on their photocrosslinkability. E-J Chem 9:145–148
Sung HK, Chi HA, Sam RK, Kwangnak K (2005) Synthesis and properties of spiroxazine polymer having photocrosslinkable chalcone moiety. Dyes Pigments 65:179–182. doi:10.1016/j.dyepig.2004.07.013
Kaniappan K, Murugavel SC, Daniel TT (2014) A review on photopolymers for polymer nanocomposite applications. J Environ Nanotechnol 3:1–15. doi:10.13074/jent.2014.09.143104
Ravikrishnan A, Sudhakara P, Kannan P (2010) Stilbene-based liquid crystalline and photocrosslinkable polynaphthylphosphate esters. J Mater Sci 45:435–447. doi:10.1007/s10853-009-3959-9
Noordini MS, Sheikh MRK, Rosiyah Y, Karim MR, Ahmad DA, Aziz H (2013) Effect of the lateral substituent on the mesomorphic behavior of side-chain liquid-crystalline polymers containing a Schiff base ester. J Polym Res 20:296. doi:10.1007/s10965-013-0296-0
Karim MR, Yahya R, Sheikh MRK, Noordini MS, Aziz H, Mahmud HNME (2014) Synthesis, thermal stability, optical and electrochemical properties of halogen terminated azo-benzothiazole mesogen containing smectic side chain liquid crystalline polymers. J Polym Res 21:487. doi:10.1007/s10965-014-0487-3
Quan L, Jian PG, Zhi YW (2003) Metal catalyzed photocrosslinking of polymers containing pendant propargyl groups. Polymer 44:5527–5531. doi:10.1016/s0032-3861(03)00607-4
Hossein NI, Khalil F, Zohreh M (2009) New photosensitive poly(amid-imide)s containing chalcone moiety and hydantoin derivatives in the main chain: synthesis and characterization. J Appl Polym Sci 112:1097–1103. doi:10.1002/app.29548
Jae HK, Si YB, Shen K, Dong HC (2003) Photochromic behavior of new bifunctional copolymer containing spiropyran and chalcone moiety in the side chain. Dyes Pigments 58:105–112. doi:10.1016/S0143-7208(03)00052-4
Feng K, Tsushima M, Matsumoto T, Kurosaki T (1998) Synthesis and properties of novel photosensitive polyimides containing chalcone moiety in the main chain. J Polym Sci A Polym Chem 36:685–693. doi:10.1002/(sici)1099-0518(19980415)36:5<685::aid-pola2>3.0.co;2-l
Harry RA, Charles GC (1994) Synthesis of photo-cross-linkable chalcone-bearing polyphosphazenes. Macromolecules 27:3131–3135. doi:10.1021/ma00090a003
Weiwei T, Zhenxin Z, Pushan W, Myong-Hoon L, Xiang-Dan L (2009) Synthesis and characterization of a novel photocrosslinkable fluorinated poly(arylene ether) for optical waveguide. Mater Lett 63:1381–1383. doi:10.1007/%252Fbf00576275
Kaniappan K, Murugavel SC (2009) Photocrosslinkable phosphorus containing homo- and copolyesters: synthesis, characterization, and photosensitive properties. J Appl Polym Sci 111:1606–1614. doi:10.1002/app.29190
Pushan W, Lei W, Aiqing Z, Xiang-Dan L, Myong-Hoon L (2011) Synthesis and properties of novel photosensitive poly(arylene ether sulfone) containing chalcone moiety in the main chain. Mater Chem Phys 126:832–835. doi:10.1016/j.matchemphys.2010.12.033
Khalil F, Zohreh M (2008) Synthesis and properties of novel photosensitive poly(amide-imide)s containing chalcone moiety and aromatic Diamines in the main chain. Turk J Chem 32:673–683
Abd-Alla MA (1992) Synthesis, characterization and thermal studies of polyamide esters containing aryl-azo, azomethine and thianthrene units. J Mater Sci 27:6299–6302. doi:10.1007/%252Fbf00576275
Selvam P, Nanjundan S (2005) Synthesis and characterization of new photoresponsive acrylamide polymers having pendant chalcone moieties. React Funct Polym 62:179–193. doi:10.1016/j.reactfunctpolym.2004.10.003
Murugavel SC, Kaliappan T, Swaminathan CS, Kannan P (1997) Synthesis and spectral, thermal, and photocrosslinking studies of poly(benzylidene phosphoramide ester)s. J Appl Polym Sci 65:2151–2157. doi:10.1002/(sici)1097-4628(19970912)65:11<2151::aid-app11>3.0.co;2-6
Murali M, Samui AB (2006) Photoactive, liquid-crystalline, hyperbranched benzylidene polyesters: synthesis and characterization. J Polym Sci Part A Polym Chem 44:53–61. doi:10.1002/pola.21118
Yakimansky AV, Tenkovtsev AV, Dudkina MM, Voigt-Martin IG, Kolb U, Lukoshkin VA, Bohme F (2002) Studies of structures and properties of polymeric systems containing bis-(hydroxy-arylidene)alkanones as NLO-active chromophores. J Non-Cryst Solids 303:237–245. doi:10.1016/s0022-3093(01)01215-7
Balakrishna K, Someshwarnath P, Sarada PM, Tapan K, Mukesh PJ, Raj SM, Tham SD, Lalit MK, Asit BS (2013) Synthesis and characterization of azo-bisbenzylidene-based polymers for second order nonlinear optics. J Polym Sci Part A Polym Chem 51:4317–4324. doi:10.1002/pola.26842
Srinivasa RV, Samui AB (2009a) Structure–property relationship of photoactive liquid crystalline polyethers containing benzylidene moiety. J Polym Sci Part A Polym Chem 47:2143–2155. doi:10.1002/pola.23303
Sidharthan J, Reuben JD, Peter Amaladhas T (2012) Synthesis and characterization of certain thermotrophic liquid crystalline copolyesters containing α, β- unsaturated ketone in the main chain. Int J Chem Appl 4:241–250
Finkelmann H, Ringsdorf H, Wendorf JH (1978) Model considerations and examples of enantiotropic liquid crystalline polymers. Polyreactions in ordered systems. Makromol Chem 179:273–276. doi:10.1002/macp.1978.021790129
Percec V, Yourd R (1988) Liquid crystalline polyethers based on conformational isomerism. 1. Quasi-rigid polyethers containing methyleneoxy units. Macromolecules 21:3379–3386. doi:10.1021/ma00190a006
Armarego WLF, Perrin DD (2000) Purification of laboratory chemicals. Butterworth-Heinemann, Oxford
Kannappan V, Arumugasamy E, Ravichandran E, Baskar B (2000) Synthesis and characterization of certain thermotrophic liquid crystalline random copolyesters. J Polym Mater 17:4–9
Chitra M, Rajendran TV, Duraipandiyan V, Rajan YC, Reuben JD (2010) A study on the synthesis and bactericidal activity of certain copolyesters containing bischalcone moiety in the main chain. Indian J Sci Technol 3:890–893
Sugaraj SR, Reuben JD, Christurajan Y, Jayakumar S, Pichai R (2010) Synthesis, characterization and ultrasonic determination of certain copolyesters containing bis(arylidene)acetone moiety in the main chain. Indian J Sci Technol 3:696–701
Berry GC, Fox TG (1968) The viscosity of polymers and their concentrated solutions. Adv Polym Sci 5:261–357. doi:10.1007/BFb0050985
Lenz RW (1985) Characterization of thermotropic liquid crystalline polymers. J Pure and Appl Chem 57:977–984. doi:10.1351/pac198557070977
Gangadhara and Kishore K (1995) Synthesis and characterization of photo-crosslinkable main-chain liquid-crystalline polymers containing bis(benzylidene)cycloalkanone units. Polymer 36: 1903–1910. doi:10.1016/0032-3861(95)90938
Dong HC, Sang JO (2002) Photochemical reactions of a dimethacrylate compound containing a chalcone moiety in the main chain. Eur Polym J 38:1559–1564. doi:10.1016/s0014-3057(02)00038-1
Jeyasheela S, Subramanian K (2012) Synthesis and characterization of novel liquid crystalline photoactive polymers with pendant chalcone moiety. Mol Cryst Liq Cryst 552:53–70. doi:10.1080/15421406.2011.599218
Jayalatha D, Balamurugan R, Kannan P (2008) Novel photo-crosslinkable liquid crystalline polymers containing vanillylidene cycloalkanones and azobenzene units. Liq Cryst 35:275–285. doi:10.1080/02678290701862256
Griffin AC, Havens SJ (1981) Mesogenic polymers. III. Thermal properties and synthesis of three homologous series of thermotropic liquid crystalline “backbone” polyesters. J Polym Sci Polym Phys Ed 19:951–969. doi:10.1002/pol.1981.180190605
Lenz RW, Jin J-L (1986) Liquid crystal polymers: a new state of matter. Polymer News 11:200
Hartmann J (1991) Polymers: chemistry and physics of modern materials. Von J. MG Cowie, 2nd edn. Blakkie, Glasgow ISBN 0-216-92980-6
Gangadhara, Kishore K (1995) A new class of photo-cross-linkable side chain liquid crystalline polymers containing bis(benzylidene)cyclohexanone units. Macromolecules 28:806–815. doi:10.1021/ma00108a002
Sudhakara P, Vijaya PG, Balamurugan S, Kannan P, Song JI (2013) Photocrosslinkable liquid crystalline polymers based on cyclohexanone and fluorescent heterocyclic ring system. J Polym Res 20:55. doi:10.1007/s10965-012-0055-7
Srinivasa RV, Samui AB (2009) Structure–property relationship of photoactive liquid crystalline polyethers containing benzylidene moiety. J Polym Sci Part A Polym Chem 47:2143–2155. doi:10.1002/pola.23303
Balamurugan R, Kannan P (2008) Photoreactive main chain liquid crystalline polyesters containing oxadiazole and bis(benzylidene) cycloalkanone units. J Polym Sci Part A Polym Chem 46:5760–5775. doi:10.1002/pola.22891
Kaniappan K, Murugavel SC (2005) Synthesis and characterization of photosensitive phosphorus based polymers containing α, β-unsaturated ketones in the main chain. J Macromol Sci Part A Pure Appl Chem 42:1589–1602. doi:10.1080/10601320500246636
Acknowledgement
One of the authors (Sidharthan) wishes to thank University Grants Commission (UGC), New Delhi for granting Minor Research Project (MRP-5334/14 (SERO/UGC) to carry out this research. The authors are grateful to Indian Institute of Science (IISc), Bangalore for OPM analysis.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
ESM 1
(PDF 7461 kb)
Rights and permissions
About this article
Cite this article
Sidharthan, J., Peter Amaladhas, T. Synthesis and characterization of photo-crosslinkable liquid crystalline copolyesters containing arylidene-keto and chalcone moieties. J Polym Res 24, 53 (2017). https://doi.org/10.1007/s10965-017-1206-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-017-1206-7