Abstract
To study the effect of magnetic-responsive CNT/Fe3O4 particles on the mechanical properties and proton conductivity of sulfonated poly(arylene ether nitrile) (SPEN) proton exchange membranes, a series of composite membranes consisting of magnetic carbon nanotube/iron oxide (CNT/Fe3O4) hybrid particles and SPEN were successfully fabricated using a solution casting method, and their proton conductivity and thermal and mechanical properties were investigated. To explore the effect of the orientation of magnetic CNT/Fe3O4 hybrid particles on proton exchange membranes, a magnetic field was applied beneath the casting membrane. Thermal analysis showed good thermal stability, with 5 % weight loss at temperatures in the range of 309 to 324 °C, and the mechanical properties of the composites were enhanced with increasing CNT/Fe3O4 content. Proton conductivity increased as the content of [CNT/Fe3O4] hybrid particle content regardless of the orientation or random distribution of CNTs, while the proton conductivity of SPENH membranes decreased slightly in the presence of a magnetic field. Therefore, we believe that these membranes would find potential applications as proton exchange membranes due to their enhanced mechanical strength and thermal stability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, proton-conducting polymers have received much attention because of their significant potential for use in electrochemical applications such as electric vehicles, portable devices, cogeneration, displays, and sensors. Most importantly, they can be used as proton-exchange membranes (PEMs) in PEM fuel cells (PEMFCs) [1]. PEMs are a key component in PEMFCs, acting as reactant separator and catalyst support, and providing the required ionic pathway between the anode and cathode [2]. Hence, their properties such as proton conductivity, water maintenance, electrical resistivity, mechanical strength, and chemical stability are crucial for fuel cell performance. Commercial perfluorosulfonic acid membranes such as Nafion are well known for their superior electrochemical and chemical stability, as well as high proton conductivity [3]. However, their high cost, high methanol crossover, low mechanical strength, difficulty in preparation, and relatively low operating temperature (< 90 °C) prevent their widespread application [4]. Because membrane strength is a key factor in determining its applicability, high-performance sulfonated polymers have been extensively investigated as a promising category of alternative candidates for PEMs; these include sulfonated polyimide (SPI), sulfonated poly(aryl ether ketone) (SPAEK), sulfonated poly(arylene ether sulfone) (SPES), and sulfonated polybenzimidazole (SPBI) [5–8]. These polymers have demonstrated higher mechanical strength, improved chemical and thermal stability, lower cost, lower methanol permeability, and easier processing in comparison with Nafion.
Multi-wall carbon nanotubes (CNTs) have been an important focus of recent studies, given their outstanding electronic, mechanical, chemical, and thermal properties, and their significant potential application in nanotechnology [9]. Various inorganic particles (Pt, Au, Cu2O, Fe3O4, etc.) have been deposited onto CNTs to create new hybrids and composites for use in catalysis, fuel cells, and lithium ion batteries [10–12]. Magnetite particles have long been of scientific and technological interest due to their unique magnetic and electrical properties [13–15]. Jia and coworkers [16] and Zhan et al. [17] reported the solvothermal synthesis of the heterojunction of Fe3O4 with CNTs and graphene nanosheets, respectively, revealing that CNT/Fe3O4 and graphene nanosheets/Fe3O4 hybrid materials showed enhanced properties compared to the individual components. CNT/Fe3O4 hybrid particles used as fillers can enhance mechanical performance. In addition, the orientation of CNT/Fe3O4 hybrid particles can be easily controlled by means of an external magnetic field, which is an advantage in polymer composites applications.
Poly(arylene ether nitrile)s (PENs) have been identified as excellent matrix resins in advanced composites due to their outstanding chemical resistance, superior mechanical properties, high thermal stability, and good molding workability [18]. Studies have also reported [19] the introduction of nitrile groups into the polymer matrix with the aim of reducing the swelling of membranes via enhanced intermolecular interaction. In this work, a novel sulfonated poly(arylene ether nitrile) (SPEN), which inherited the excellent properties of PENs, was successfully prepared using a nucleophilic route to create a high-performance PEM with relatively high proton conductivity and good mechanical properties. In addition, CNT/Fe3O4 hybrid particles were dispersed into SPEN to fabricate composite membranes. The effect of CNT/Fe3O4 hybrid particles on the thermal stability, mechanical properties, proton conductivity, and water uptake of the [CNT/Fe3O4]/SPEN composite membranes was investigated in detail.
Experimental design
Materials
We purchased 2, 6-difluorobenzonitrile (DFBN) and hydroquinonesulfonic acid potassium salt (SHQ) from Sigma-Aldrich. Bisphenol A (BPA) and hydrochloric acid were purchased from Chengdu Haihong Chemical Co. N-methylpyrrolidone (NMP, AR) and potassium carbonate (K2CO3, AR) were supplied by Tianjin Bodi Chemical Co. CNTs (diameter, 10–30 nm; length, 10–50 μm; purity >95 %) were supplied by Chengdu Organic Chemistry Co. Ltd., Chinese Academy of Sciences. FeCl3 6H2O (99 %), ethylene glycol (99 %), and polyethylene glycol (99 %) were purchased from ChengDu KeLong Chemical Reagent Company, China. All the materials were used without further purification.
Synthesis of SPEN
SPEN was synthesized from bisphenol A, hydroquinonesulfonic acid potassium salt, and 2, 6-difluorobenzonitrile via nucleophilic aromatic substitution reaction in N-methylpyrrolidone (NMP) with K2CO3 as catalyst, according to a similar procedure in the literature [20]. In addition, the SPEN was purified several times before use to remove the unreacted monomers and inorganic salt.
Preparation of CNT/Fe3O4 hybrid particles
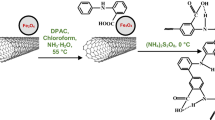
Typical synthesis of CNT/Fe3O4 hybrid particles was carried out in a hydrothermal system by reduction reactions between FeCl3 and ethylene glycol in the presence of CNTs, as previously reference reported [21]. First, 1.92 g FeCl3 6H2O was dissolved in 160 mL ethylene glycol, followed by the addition of 5.0 g polyethylene glycol and 2.0 g acid-treated CNTs to form a dispersed solution, assisted by an ultrasonic bath. Then, 18.00 g NaAc was slowly added to the solution, with vigorous stirring, at 60 °C for 2 h. The mixture was sealed in a Teflon-lined stainless steel autoclave and maintained at 200 °C for 15 h. The resulting CNT/Fe3O4 hybrid particles were isolated using bar magnets and rinsed with ethanol and deionized water several times, and dried at 80 °C for 12 h.
Preparation of [CNT/Fe3O4]/SPEN composite membranes
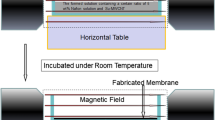
[CNT/Fe3O4]/SPEN proton-exchange membranes were fabricated on a flat glass plate by means of solution casting of a mixture of SPEN ion/exchange resin and CNT/Fe3O4 hybrid particles, combined with ultrasonic dispersion technology. The SPEN solution in NMP (wt. 10 %) was used to fabricate a polymer film with a thickness of 80 ± 10 μm. The CNT/Fe3O4 hybrid particles were dispersed into the polymer solution, with vigorous mechanical stirring for 1.5 h to avoid local aggregation, and then sonicated using ultrasonic dispersion technology for 1.5 h. The mixture was cast on a clean preheated glass plate using a sequential temperature program at 80, 100, 120, 160, and 200 °C (1 h each) to completely remove the solvent. Meanwhile, a temperature-resistance magnet was applied to the bottom of the membrane. A pure SPEN and [CNT/Fe3O4]/SPEN membranes were also prepared using the same procedure without the magnetic field. Lastly, the [CNT/Fe3O4]/SPEN proton-exchange membranes were obtained after gradually cooling to room temperature. The schematic of the casting procedure of [CNT/Fe3O4]/SPEN proton-exchange membranes is shown in Scheme 1.
Analysis and characterization
Fourier transform infrared (FTIR) spectra of the samples were recorded on a Shimadzu 8000S spectrophotometer. The cross-sectional micromorphologies of the films were observed with a scanning electron microscope (SEM, JEOL JSM-5900LV, SEMTech Solutions), and the samples were placed in liquid nitrogen to obtain brittle fracture, and then coated with a thin layer of gold before examining. The morphology of CNT/Fe3O4 hybrid particles was observed by transmission electron microscopy (TEM, H-600, Hitachi, Ltd., Tokyo, Japan) operating at 120 kV. The samples were then dispersed in anhydrous ethanol by ultrasonic treatment for 0.5 h before observation. 1H NMR spectra were recorded with a Bruker 600-MHz spectrometer, with DMSO-d 6 as a solvent. Thermogravimetric analysis (TGA) was performed on a TA Instruments TGA-Q50 module at a heating rate of 20 °C/min from room temperature to 800 °C. Magnetic study was performed using a Riken Denshi BHV-525 vibrating sample magnetometer (VSM; Riken Denshi Co., Ltd., Tokyo, Japan). The mechanical properties of the samples were tested with a SANS CMT6104 series desktop electromechanical universal testing machine (Shenzhen, China) at room temperature, and the stretching speed was 5 mm/min. The impedance of the sample film was measured using a Model 600E Series electrochemical analyzer (CH Instruments, Inc., Austin, TX, USA) in potentiostat mode, with a perturbation amplitude of 50 mV over frequencies of 0.01 Hz to 10 kHz, by means of a Nyquist plot.
Water uptake content and proton conductivity measurements
The water uptake of the membranes was determined by measuring the weight before and after the hydration. First, the membranes were dried at 100 °C overnight, and the sample membranes were then immersed in deionized water for 24 h at predetermined temperatures. Prior to obtaining measurements, the water attached to the membrane surface- was removed with filter paper. Next, the wetted membrane weight (W wet) was measured as quickly as possible. The weight of the dry membrane (W dry) was measured after thorough vacuum drying at 80 °C for 12 h. The water uptake rate was calculated using the following equation [22]:
The proton conductivity (σ) of the pure SPEN and composite membranes at ambient temperature were evaluated after equilibration of the the membrane in deionized water for 1 day. Proton conductivity [22] was calculated according to the formula
where σ, L, R, and A represent the conductivity, thickness (measured with a micrometer in each case), measured resistance, and cross-sectional area of the membrane perpendicular to current flow, respectively.
Results and discussion
Characterization of SPEN copolymer
The FTIR spectrum of the SPEN copolymer in acid form (SPENH) is shown in Fig. 1. The absorption band at 2,231 cm−1 is assigned to the -CN stretching vibration [23]. The characteristic absorption bands at 1,090 and 1,032 cm−1 are assigned to the aromatic sulfonate symmetric and asymmetric stretching vibrations [1], respectively, which confirm successful incorporation of sulfonate acid groups into the copolymers. The characteristic absorption band of the aromatic ether can be observed at 1,244 cm−1, and characteristic absorption bands of benzene rings are observed at 1,500 and 1,460 cm−1. These results suggest that SPEN copolymers were successfully synthesized, as shown in Scheme 2.
The structural properties of the synthesized polymers were also studied by liquid phase 1H NMR spectroscopy, with DMSO-d 6 as the solvent. Figure 2 shows the spectrum of the aromatic protons for the highly sulfonated homopolymer SPEN prepared from bisphenol A and sulfonated hydroquinone at a monomer ratio of 3:7. In the illustration, the spectrum can be divided into three regions: the higher frequencies (7.1–7.7 ppm), the lower frequencies (6.25–6.8 ppm), and the chemical shift of methyl proton (Hi 1.7 ppm). The three low-frequency signals were found to be the benzonitrile phenyl ring protons Hd and Hf , and the sulfonated hydroquinone phenyl ring protons Ha,b,c were found at higher frequencies due to the effect of the electron-withdrawing sulfonic acid salt group, which is consistent with reports in the literature [1]. It has also been reported [24] that the 1NMR spectra can be used to estimate the sulfonation degree (SD) by comparing the intensities of specific signals. Regarding the particularity structure of methyl proton of unsulfonated monomers, the actual SD should be the area-specific value of sulfonated hydroquinone and dihydric phenol.
where S Ha and S Hi are the spectral peak areas of the proton of sulfonated hydroquinone and dihydric phenol, respectively. According to the 1NMR spectra, the chemical shift of sulfonated hydroquinone and dihydric phenol is 7.57 and 1.70 ppm, while their area is 2.27 and 6.00, respectively. It was concluded that the experimental SD was nearly 0.69 and the original monomer ratio was 0.70, which is a slight difference. This may be caused by the different activity of the monomers resulting in an incomplete reaction.
Characterization of CNT/Fe3O4 hybrid particles
Figure 3 shows TEM images of the test sample at 30,000× magnification. The dark sphere consists of ferriferous oxide and the light gray phase represents the CNTs. It is clear that the Fe3O4 pellet is self-assembled on the surface of CNTs, caused by the electrostatic attraction. These images confirm successful formation of CNT/Fe3O4 hybrid particles. In addition, the Fe3O4 particles on the surface of the CNTs have better dispersity and no large agglomeration, which is a prerequisite for improving their compatibility with and dispersibility in the polymer matrix, further optimizing the mechanical properties of membrane composites.
Morphological properties
The morphology and structure of CNT/Fe3O4 hybrid particles were investigated using SEM. As can be seen in Fig. 4a, the distribution of magnetite nanoparticles on the CNTs surface is nearly monodisperse, consistent with the results of TEM. The cross-sectional SEM images of neat SPENH membrane and [CNT/Fe3O4]/SPENH composite membranes are shown in Fig. 4b/c. Compared with the neat membrane (Fig. 4b), the composite membrane has a rougher surface with the addition of CNT/Fe3O4 hybrid particles. The dispersion behavior of the magnetic CNT/Fe3O4 hybrid particles is clearly better in the SPENH matrix (Fig. 4c). From Fig. 4d, we can see that the roughness of one side of the membrane composite is greater than that of the other side, and the dispersion of CNTs is also different, which is the result of magnetic field interaction, indicating that the addition of a magnetic field is feasible and highly efficient. In addition, the introduction of magnetic-responsive CNT/Fe3O4 increases the roughness of the fracture surface and produces greater wire draw tension.
Thermal properties
TGA was used to quantify the Fe3O4 content in the CNT/Fe3O4 hybrid particles. The literature indicates that CNTs is oxidized at 700 °C, while Fe3O4 is thermally stable at over 750 °C [25, 26]. Figure 5a shows weight loss for pure CNTs at 700 °C and CNT/Fe3O4 hybrid particles at 600 °C of 95.47 and 68.50 %, respectively. Therefore, we can infer that the difference between these final weight loss values, 26.97 wt%, is equal to the Fe3O4 content of the CNT/Fe3O4 hybrid particles.
The thermal properties of the composite membranes and the neat SPENH membrane were determined by TGA in a nitrogen atmosphere. TGA curves of the composite membranes and neat SPENH membrane are illustrated in Fig. 6, and the detailed data are summarized in Table 1. The composite membranes and neat SPENH membrane show good thermal stability, with 5 % weight loss temperatures (T d5% ) and 10 % weight loss temperatures (T d10% ) in the ranges of 309 to 324 °C and 345 to 365 °C, respectively. As shown in Table 1, the maximum decomposition rate temperatures (T max ) for all copolymers are approximately 406 °C, with no significant change observed as hybrid particle content increases, suggesting that the incorporation of CNT/Fe3O4 hybrid particles has not damaged the thermal stability of the SPENH matrix. In general, all of the results show that the composite membranes inherit the intrinsic high thermal stability of neat SPENH, which meets thermal performance requirements for PEMs.
Mechanical properties
The tensile strength of the membranes after hydration at 25 °C is shown in Fig. 7, and it is clear that the composite membranes exhibit a marked improvement in tensile strength. Values of tensile strength for the composite membranes prepared without the magnetic field ranged from 28.40 to 42.30 MPa, while those prepared with the magnetic field ranged from 29.60 to 44.20 MPa. These values are higher than that of the neat SPENH membrane, reflecting the excellent mechanical properties of the matrix and the reinforcement of CNTs [27]. In addition, it is difficult to observe any pullout on the fracture surfaces of the composites in the SEM images of the CNT/Fe3O4 hybrid particles, confirming that the interface formed between hybrid particles and matrix is compact and continuous. The introduction of Fe3O4 particles also augments the surface roughness of CNTs, tying the molecules and interlocking them with the surrounding PEM resin over a larger region, thereby restricting the mobility of the polymer chains [28]. The addition of the magnetic field also greatly enhances the mechanical properties of composite membranes. As shown in the SEM images, the introduction of magnetic-responsive CNT/Fe3O4 increases the roughness of the fracture surface and produces greater wire draw tension. The magnetic field promotes the formation of distinct CNTs-rich regions and orientation of CNTs more likely to form a complex network structure. The compact interface and network structure all enhance the mechanical properties of composite membranes.
Figure 8 shows the break elongation of the pure SPEN and the composite membranes as a fraction of CNT/Fe3O4 hybrid particle loading. The break elongation gradually increases with the addition of CNT/Fe3O4 hybrid particles, but is lower than that for pure SPENH. This can be explained by two factors. First, the sulfonic acid groups are reduced with the addition of CNT/Fe3O4 hybrid particles, resulting in weaker hydrogen bonding and a stronger rigid network structure. Thus the break elongation of [CNT/Fe3O4]/SPEN membranes is lower than that of pure SPENH. Second, due to the special spherical structure of Fe3O4, the composite membranes may have certain water conservation qualities [29], leading to better contact and ionic interaction between the sulfonic acid groups and H2O. Consequently, the increased dose of CNT/Fe3O4 hybrid particles could enhance the elasticity and spontaneously increase the break elongation of the [CNT/Fe3O4]/SPEN composite membranes.
It should be noted that the [CNT/Fe3O4]/SPEN membranes obtained in this study exhibit much greater tensile strength than the commercial Nafion 117 membrane (10 MPa) despite the lower break elongation [30]. As such, the [CNT/Fe3O4]/SPEN membrane is suitable for application settings requiring good mechanical properties.
Water uptake content
The proton conductivity and mechanical stability of proton-exchange membranes are strongly related to the presence of water, as water uptake is the major carrier of protons. As shown in Fig. 9, water uptake content decreases slightly in the presence of the magnetic field. The addition of CNT/Fe3O4 hybrid particles in the SPENH slightly decreases the degree of water uptake compared with pure SPENH, due to a reduction in content of sulfonic acid groups within certain volume mixture. However, as the content of CNT/Fe3O4 hybrid particles increases, the water uptake of composite membranes increases gradually, possibly as a result of the retention of water by the spherical particles. Consequently, variations in the membranes reflect the synergistic reaction of reducing the sulfonic acid group content and spherical particle water retention. With the addition of CNT/Fe3O4 hybrid particles, water retention of spherical particles is the dominant factor affecting water uptake of composite membranes.
Proton conductivity
The effect of oriented CNTs on proton conductivity is shown in Fig. 10. From the illustration, we can see that the oriented [CNT/Fe3O4]/SPEN composite membranes have slightly lower proton conductivity than the pure SPENH, which may result from the fact that the tubular structure of CNTs interferes with proton transfer to a certain degree or that the addition of magnetic and Fe3O4 expends a portion of the of sulfonic acid group. Regardless of magnetic-responsive or non-magnetic field CNTs, however, proton conductivity increases as the content of CNT/Fe3O4 hybrid particles increases, indicating that filler CNT/Fe3O4 hybrid particles could effectively increase the proton conductivity of [CNT/Fe3O4]/SPENH composite membranes (not pure SPENH). Therefore, the proton conductivity and mechanical properties of [CNT/Fe3O4]/SPENH composite membranes can be easily fine-tuned using different levels of hybrid CNTs/Fe3O4.
Conclusions
In this study, a series of [CNT/Fe3O4]/SPEN composite membranes were fabricated by a solution casting method in order to enhance the mechanical properties of the sulfonated poly(arylene ether nitrile) (SPEN) proton-exchange membrane. The Fe3O4 nanoparticles self-assembled on the surface of CNTs through electrostatic attraction, and the distribution was controlled by the addition of a magnetic field during membrane fabrication. The composite membranes had good thermal stability, with 5 % weight loss at temperatures in the range of 309 to 324 °C. The addition of hybrid particles in the SPENH also provided reinforced the mechanical properties. For 2 wt% of CNT/Fe3O4-reinforced SPENH composites, the tensile strength increased by approximately 60.80 % without a magnetic field and 68.10 % with a magnetic field. Regardless of magnetic-responsive or non-magnetic field CNTs, however, the proton conductivity of [CNT/Fe3O4]/SPENH composite membranes increased with increasing content of CNT/Fe3O4 hybrid particles in all cases, while the proton conductivity of SPENH membranes decreased slightly under the interaction of a magnetic field. Nevertheless, the enhanced mechanical properties reflect the value of these membranes for application as proton-exchange membranes given their increased mechanical strength and high thermal stability.
References
Gao Y, Robertson GP, Guiver MD, Mikhailenko SD, Li X, Kaliaguine S (2007) Comparison of PEM properties of copoly(aryl ether ether nitrile)s containing sulfonic acid bonded to naphthalene in structurally different ways. Macromolecules 40:1512–1520
Miyatake K, Hirayama D, Bae B, Watanabe M (2012) Block poly(arylene ether sulfone ketone)s containing densely sulfonated linear hydrophilic segments as proton conductive membranes. Polym Chem 3:2517–2522
Mauritz KA, Moore RB (2004) State of understanding of nafion. Chem Rev 104:4535–4585
Li QF, He RH, Jensen JO, Bjerrum NJ (2003) Approaches and recent development of polymer electrolyte membranes for fuel cells operating above 100 °C. Chem Mater 15:4896–4915
Li WM, Cui ZM, Zhou XC, Zhang SB, Dai L, Xing W (2008) Sulfonated poly(arylene-co-imide)s as water stable proton exchange membrane materials for fuel cells. J Memb Sci 315:172–179
Bai H, Ho WSW (2011) Recent developments in fuel-processing and proton-exchange membranes for fuel cells. Polym Int 60:26–41
Matsumura S, Hlil AR, Lepiller C, Gaudet J, Guay D, Shi ZQ, Holdcroft S, Hay AS (2008) Ionomers for proton exchange membrane fuel cells with sulfonic acid groups on the end groups: novel bran- ched poly(ether-ketone)s. Macromolecules 41:281–284
Kim DS, Robertson GP, Guiver MD (2008) Comb-shaped poly(arylene ether sulfone)s as proton exchange membranes. Macromolecules 41:2126–2134
Jouanneau J, Mercier R, Gonon L, Gebel G (2007) Synthesis of sulfonated Polybenzimidazoles from functionalized monomers: preparation of ionic conducting membranes. Macromolecules 40:983–990
Xue LP, Shen CF, Zheng MB, Lu HL, Li NN, Ji GB et al (2011) Hydrothermal synthesis of graphene– ZnS quantum dot nanocomposites. Mater Lett 65:198–200
Zhao R, Jia K, Wei JJ, Pu JX, Liu XB (2010) Hierarchically nanostructured Fe3O4 microspheres and their novel microwave electromagnetic properties. Mater Lett 64:457–459
Pan ZW, Dai ZR, Wang ZL (2001) Nanobelts of semiconducting oxides. Science 291:1947–1949
Zhan YQ, Meng FB, Lei YJ, Zhao R, Zhong JC, Liu XB (2011) One-pot solvothermal synthesis of sandwich-like graphene nanosheets/Fe3O4 hybrid material and its microwave electromagnetic properties. Mater Lett 65:1737–1740
Jia BP, Gao L, Sun J (2007) Self-assembly of magnetite beads along multiwalled carbon nanotubes via a simple hydro-thermal process. Carbon 45:1476–1481
Zhan YQ, Zhao R, Lei YJ, Meng FB, Zhong JC, Liu XB (2011) Preparation, characterization and electromagnetic properties of carbon nanotubes/Fe3O4 inorganic hybrid material. Appl Surf Sci 257:4524–4528
Zhan YQ, Yang XL, Guo H, Yang J, Meng FB, Liu XB (2012) Cross-linkable nitrile functionalized graphene oxide/poly(arylene ethernitrile) nanocomposite films with high mechanical strength and thermal stability. J Mater Chem 22:5602–5608
Sumner MJ, Harrison WL, Weyers RM, Kim YS, McGrath JE, Riffle JS, Brink A, Brink MH (2004) Novel proton conducting sulfonated poly(arylene ether) copolymers containing aromatic nitriles. J Membr Sci 239:199–211
Tang HL, Yang J, Zhong JC, Zhao R, Liu XB (2011) Synthesis and dielectric properties of polyarylene ether nitriles with high thermal stability and high mechanical strength. Mater Lett 65:2758–2761
Zhan YQ, Zhao R, Lei YJ, Meng FB, Zhong JC, Liu XB (2011) A novel carbon nanotubes/Fe3O4 inorganic hybrid material: synthesis, characterization and microwave electromagnetic properties. J Magn Magn Mater 323:1006–1010
Lin CW, Huang YF, Kannan AM (2007) Cross-linked poly(vinyl alcohol) and poly(styrene sulfonic acid-co-maleic anhydride)-based semi-interpenetrating network as proton-conducting membranes for direct methanol fuel cells. J Power Sources 171:340–347
Li C, Tang AB, Zou YB, Liu XB (2005) Preparation and dielectric properties of polyaryleneether nitriles/TiO2 nanocomposite film. Mater Lett 59:59–63
Xing PX, Robertson GP, Guiver MD, Mikhailenko SD, Kaliaguine S (2004) Sulfonated poly(aryl ether ketone)s containing the hexafluoroisopropylidene diphenyl moiety prepared by direct copolymerization, as proton exchange membranes for fuel cell application. Macromolecules 37:7960–7967
Bom D, Andrews R, Jacques D, Anthony J, Chen BL, Meier MS, Selegue JP (2002) Thermogravimetric analysis of the oxidation of multiwalled carbon nanotubes: evidence for the role of defect sites in carbon nanotube chemistry. Nano Lett 2:616–619
Durmus Z, Kavas H, Baykal A, Sozeri H, Alpsoy L, Çelik SU, Toprak MS (2011) Synthesis and characterization of l-carnosine coated iron oxide nanoparticles. J Alloy Compd 509:2555–2561
Liu XB, Long SR, Luo DW, Chen WJ, Cao GP (2008) Preparation and properties of polyarylene ether nitrites/multi-walled carbon nanotubes composites. Mater Lett 62:19–22
Meng FB, Zhao R, Zhan YQ, Liu XB (2011) Design of thorn-like micro/nanofibers: fabrication and controlled morphology for engineered composite materials applications. J Mater Chem 21:16385–16390
Sumner JJ, Creager SE, Ma JJ, DesMarteau DD (1998) Proton conductivity in Nafion® 117 and in a novel bis[(perfluoroalkyl)sulfonyl]imide ionomer membrane. J Electrochem Soc 145:107–110
Chen L, Pu ZJ, Yang J, Yang XL, Liu XB (2013) Synthesis and properties of sulfonated polyarylene ether nitrile copolymers for PEM with high thermal stability. J Polym Res 20:45–53
Li NW, Zhang SB, Liu J, Zhang F (2008) Synthesis and properties of sulfonated poly[bis(benzimidazo-benzisoquinolinones)] as hydrolytically and thermooxidatively stable proton conducting ionomers. Macromolecules 41:4165–4172
Baughman RH, Zakhidov AA, de Heer WA (2002) Carbon nanotubes—the route toward applications. Science 297:787–792
Acknowledgments
The authors are grateful for financial support of this work from the National Natural Science Foundation (Nos. 51173021, 51373028, 51403029) and the State High-Tech Development Plan (“863” program) (2012AA03A212).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Feng, M., Meng, F., Pu, Z. et al. Introducing magnetic-responsive CNT/Fe3O4 composites to enhance the mechanical properties of sulfonated poly(arylene ether nitrile) proton-exchange membranes. J Polym Res 22, 37 (2015). https://doi.org/10.1007/s10965-015-0682-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-015-0682-x