Abstract
Synthesis of polyelectrolyte brushes on magnetic polymer particles were carried out through a two steps. Firstly, the iron oxide particles (maghemite) were embedded in a hydroxyl modified polymer phase and in the second step, polyacrylic acid (PAA) brushes were synthesized on the surface of the particles by a grafting from method. The final particles were characterized by Fourier transform infrared spectroscopy (FTIR), Fourier transform infrared attenuated total reflection spectroscopy (FTIR-ATR), powder X-ray diffraction (XRD), thermal gravimetric analysis (TGA), scanning electron microscopy (SEM), vibrating sample magnetometer (VSM) and dynamic light scattering (DLS). Experimental analysis confirmed that all the iron oxide particles were embedded in a polymer phase and the final particles have more than 82 % iron oxide content. According to magnetometry data, shape of the hysteresis loops evidence the ferromagnetic character of the particles. The DLS results show that the size of the particles is depend on ionic strengths and pH of dispersion medium.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In recent years, the synthesis of polymer brushes has received significant attention, due to their unique properties and application possibilities, such as adhesion [1], lubrication [2], wettability [3], friction [4], biocompatibility [5] and etc. Polymer brushes can be classified in planar and spherical types and neutral or charged brushes according to geometries of substrate and their charges, respectively. They are synthesized by two different methods, physisorption and chemical bonding. Typically, polymer brushes were synthesized using a physisorption approach which consist of two-component polymer chains, where one part strongly adheres to the interface by van der Waals forces or hydrogen bonding and the second part extends to generate the polymer layer [6]. In this case, brush layers are thermally and solvolytically instable due to desorption or displacement by other polymer or small molecules. A much stronger bonding between polymer chains and substrate can be achieved by chemical bonding [1, 3, 7, 8, 10–12]. The chemical bonding of polymer brushes can further be classified as “grafting to” and “grafting from” techniques. A preformed macromolecule possessing a suitable end-functionality with a reactive substrate is utilized to generate the polymer brush through the “grafting to” technique. Mir et al. prepared polyelectrolyte brushes of poly (sodium styrene sulfonate) using the “grafting to” technique for the first time [9]. Such a “grafting to” approach is generally limited because only moderate molecular weight polymers can be applied. So, this technique often leads to low grafting densities and low film thickness [9]. To overcome this problem, the “grafting from” approach can be used and has generally become the most attractive way to prepare dense, covalently tethered polymer brushes with a high grafting density [3, 10–12]. The “grafting from” technique involves the immobilization of initiators or vinyl groups onto the substrate, followed by in situ surface initiated polymerization to generate the tethered polymer brush [3, 13, 14]. As the chains are growing from the surface, the only limitation to propagation is diffusion of monomers to the chain ends, thus resulting in thick tethered polymer brushes with high grafting density. The “grafting from” technique can be also used to synthesize polyelectrolyte brushes [15]. Nowadays, polyelectrolyte brushes have attracted much attention, because they can as smart materials, respond to many external stimuli such as ionic strength, pH (in the case of weak polyelectrolyte brushes), and counter-ion valency [16]. Recently, spherical polyelectrolyte brushes (SPBs) also have been synthesized, which possess magnetic properties and are able to respond to magnetic field [17–21]. In this paper, the grafting from method was used to synthesize SPBs with magnetic properties. Firstly, we prepared magnetic polymer particles based on polystyrene by a miniemulsion polymerization. Then a layer of vinyl groups was introduced on the surface of core particles by condensation reaction. Finally PAA brushes were synthesized by in situ polymerization of AA monomers on the surface of core particles.
Experimental
Materials
All materials in this work were used without further purification, except styrene (ST, Merck) and divinyl benzene (DVB, Merck) that were washed three times with sodium hydroxide solution (5 wt%) to remove inhibitor, then dried over calcium chloride and stored at 4 °C before use. All other materials including iron oxide (γ-Fe2O3) (brown pigment Bayer 686), oleic acid (OA, Merck), ammonia (25 wt. %, Merck), hydrochloric acid (HCl, 36 wt%, Merck), hexadecane (Merck) octane (Merck), 2,2′-azobisisobutyronitrile (AIBN, Merck), 2-hydroxyethyl methacrylate (HEMA, Merck), sodium dodecyl sulfate (SDS, Merck), dimethyl formamide (DMF, Merck), methanol (Merck), acetone (Merck), potassium persulfate (KPS, Sigma-Aldrich), triethylamine (TEA, Merck), acryloyl chloride (AC, Merck), and Acrylic acid (Merck) were used as received.
Apparatus
The magnetic polymer particles were characterized using Fourier transform infrared spectroscopy (FTIR), Bruker, Equinox 55 (Germany); Fourier transform infrared attenuated total reflectance spectroscopy (FTIR-ATR) Specac, Golden gate (England); X-ray diffractometer (XRD), Siemens D5000 instrument (Germany); scanning electron microscope (SEM), VEGA, TESCAN (Czech); thermal gravimetry analysis (TGA), Mettler-Toledo TGA/DSC1 (Swiss), dynamic light scattering (DLS, VEGA, TESCAN) and vibrating sample magnetometer (VSM), Princeton Applied Research VSM 155 instrument (USA).
Methods
Synthesis of core particles (MPP)
Miniemulsion polymerization is a suitable method for synthesis of hybrid organic-inorganic spherical particles and is widely used [17]. In this work, synthesis of core particles was carried out via a miniemulsion polymerization process. Firstly, in order to increase compatibility of iron oxide particles with an organic phase, it is necessary to modify its surface. For this purpose, we used OA, which is a common reagent for modification of iron oxide particles [22]. So specifically, 20 g of iron oxide powder were dispersed in 250 ml of water. Then 46.4 g of OA was dissolved in 50 ml of acetone and added to the reaction system followed by 240 ml of ammonia solution and was stirred at room temperature. After 3 h, the sediment was neutralized by 1 N hydrochloride acid solution. Then anhydrous ethyl alcohol was introduced to get rid of the residual OA. Finally, the byproduct ammonium chloride was eluted by water from the sediment. After drying, the modified iron oxide particles were dispersed in octane with an iron oxide content of 50 wt% to form a ferro fluid. Next, 14 g of ferro fluid as oil phase was added to 24 g water containing 0.7 g SDS. The mixture was stirred for 1 h. Then octane was carefully evaporated at 80 °C to obtain stable water-based iron oxide dispersion. At the same time, the styrene monomer miniemulsion was prepared using the following recipe: 1.5 g of styrene, 0.5 g of DVB, 0.05 g of AIBN and 0.120 g of hexadecane were added to a surfactant solution containing 0.036 g of SDS dissolved in 12 g of water and was stirred for 1 h. Finally, the so as made, styrene miniemulsion and the iron oxide dispersion were mixed and stirred while the polymerization was started at 80 °C. After 2 h, 0.5 g of HEMA dissolved in 20 ml of water was added to the reaction mixture and polymerization was continued for 16 h. Then, the mixture was allowed to cool to room temperature; the sediment was washed with water and methanol respectively, and dispersed in the DMF, containing 14 wt% of magnetic polymer particles. The methanol was carefully evaporated at 80 °C to obtain DMF-based core particle dispersion.
Synthesis of magnetic spherical polyelectrolyte brushes (MSPB)
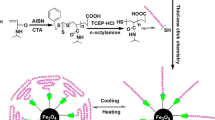
An essential step for synthesis of polymer brushes is to immobilize active groups on the substrate surface. These active groups may be initiators or also vinyl groups. By reaction of AC with surface hydroxyl groups, a layer of vinyl groups was introduced on the surface of core particles. Since AC is a very reactive compound which can easily react with any nucleophilic agent, so it is necessary to use an aprotic solvent. DMF as an aprotic polar solvent is suitable solvent for this purpose. The hydrochloric acid is byproduct of reaction between AC and surface hydroxyl groups. TEA was used as a base to neutralize HCl without reaction with AC. Since the reaction of AC with hydroxyl groups is very fast and exothermic, an ice bath was used to control temperature of the reaction. Polymerization of these vinyl groups with AA monomers led to the PAA chains grafted on maghemite-polystyrene cores and formation of spherical polyelectrolyte brushes. So briefly, 3 g of synthesized core particles were dispersed in 100 ml DMF in an ice bath and 5.5 g of TEA, then 4.5 ml of AC were added to it. After 3 h the flask content was filtered and washed with water. The obtained particles and 7 g of AA monomers were dispersed in 250 ml of water and 0.075 g potassium persulfate as initiator were added to it. Polymerization was performed at 70 °C with stirring rate of 400 rpm for 12 h. The resultant particles were washed with water and stored under distilled water in a balloon.
Results and discussion
Magnetic spherical polyelectrolyte brushes (MSPB) have been widely used at various aspects in biotechnology and biomedicine fields. To be successfully used in the above areas, they should fulfill such requirements as near nano-sized distribution, high and uniform magnetic content, no iron leaking and non-toxicity. Therefore, an indirect process based on miniemulsion polymerization was used. In other words, oleic acid coated iron oxide particles were firstly synthesized and dispersed into water using sodium dodecyl sulfate as a second emulsifier and hexadecane as an osmotic agent. The prepared water-based SDS/oleic acid bilayer coated iron oxide dispersion was mixed with monomer phase miniemulsion, and a second miniemulsification was carried out. Subsequently, the polymerization was carried out and the polymer particles with fully encapsulated iron oxide were prepared [23]. Similarly, the magnetic polymer particles were prepared with surface decorated hydroxyl groups, which are named as core particles. The hydroxyl groups on the surface of the core particle chemically transformed to vinyl groups and afterwards in the presence of acrylic acid and initiator, polyacrylic acid (PAA) brushes were synthesized on the surface of the particles by a grafting from method.
The resulting particles were studied by different methods. FTIR spectra of iron oxide and MPP, (Fig. 1a, and b, respectively) were shown in Fig. 1. The FTIR spectra of core particles (MPP) compared with iron oxide (Fig. 1b), showed seven characteristic peaks at 583, 1,382, 1,409, 3,020, 1,486, 1,717 and 3,434 cm−1, which were attributed to stretching bands of the Fe-O, symmetric CH3 and CH2, =C-H, C = C of aromatic ring, C = O and OH groups, respectively. In addition, the peak at 697 cm−1 may be related to = C-H out of aromatic ring plane bending. So, it confirms the presence of iron oxide in core particles [24]. The next stage is polymerization of acrylic acid onto the surface of MPP leading to production of MSPB. The FTIR-ATR spectra of MPP and MSPB particles are depicted in Fig. 2a, and b, respectively. In comparison FTIR-ATR spectra of MSPB particles with core particles (MPP) (Fig. 2) it is seen:
-
O-H Stretch band occurs at 3,400–2,400 cm−1, is very broad (due to strongly hydrogen-bonding), and overlaps the C-H absorptions
-
C = O Stretch, broad and occurs at 1,730–1,700 cm−1
-
C-O Stretch occurs in the range 1,320–1,210 cm−1
So, the formation of polyacrylic acid on the core particles is confirmed.
Powder XRD analysis was used to investigate the presence of iron oxide in synthesized particles. XRD spectra of bare iron oxide [25] and synthesized core particles are shown in Fig. 3a and b, respectively. The comparison of the figures shows that there are similar peaks with nearly equal relative intensities in both spectra. According to XRD results, the chemical structure of maghemite in the core particles was not changed, so the magnetic properties of the particles were kept in the core particles.
The TGA thermogram of core particles is shown in Fig. 4. As it is seen, there are 3 peaks in 260, 400 and 600 °C, which may be due to the thermal degradation of the oleic acid, polymer phase and maghemite, respectively [26]. This thermogram also shows that maghemite content of core particles is about 85 wt%.
The TGA thermogram of MSPBs (Fig. 5) shows that its organic content is about 18 wt%. So, there is an increase in organic content of particles from 15 wt% in core particles in comparison with MSPBs (18 wt %), which is due to the attachment of PAA chains onto the surface of core particles. The prepared polyelectrolyte chains are about 16 wt% of total organic content of MSPBs. The appeared peak in 450 °C (Fig. 5) is attributed to thermal degradation of PAA which usually occurs in about 500 °C [26].
The measurement of particle size can also confirms presence of PAA chains onto the core particles. As, it is schematically shown in Fig. 6, in formation step of PAA onto the core particles, a new layer is formed on the surface of core particles, thus the size of the particles must be increased due to addition of PAA chains. Figure 7 shows the size and size distribution of cores and MSPB-Na, determined by DLS. As it had been expected, the mean diameter of the particles was increased from about 1,200 nm for core particles to about 1,800 nm in MSPB-Na particles. This increase in particle size is due to attachment of fully stretched PAA chains in sodium salt form, onto the surface of core particles. It is also obvious that size distribution of MSPB-Na is wider than the core particles. This behavior is probably due to preparation of PAA with different molecular weights through radical polymerization mechanism, which leads to a wider size distribution.
For determination of molecular weight of PAA chains, it is known that the PAA chains in MSPBs structure are attached to the surface of core particles through ester bonds (Fig. 8). These ester bonds can be cleaved by applying the appropriate condition, in which the PAA chains attached to the surface can be separated and characterized by viscometry method. So, the MSPBs were heated at 110 °C in 2 M NaOH solution for 14 days, during which the PAA chains were separated from the particle surfaces. The separated PAA chains were dried and weighed. The molecular weight of PAA was determined by using Mark-Houwink-Sakurada method. The intrinsic viscosity of PAA was obtained 0.027 l/g (measured by viscometric method using an Ubbelohde viscometer in 25 °C). Constant parameters of α and k in this method (2 M NaOH, 25 °C) are 0.64 and 0.0422 ml/g, respectively [26]. The molecular weight of PAA chains calculated by this method is about 23,830 g/mol.
Morphology of the synthesized particles was studied by SEM. SEM micrographs of MPP (Fig. 9a) and MSPB (Fig. 9b) particles indicate that:
-
1.
The synthesized core particles (MPP) are spherical shaped with smooth surface. It is known that the bare iron oxide particles are no spherical and have irregular shapes. So, this confirms that the iron oxide particles are mainly located within the polymer phase.
-
2.
The size of the synthesized magnetic polymer particles (MPP) is less than 3 μm.
-
3.
After modification of MPP with PAA, the surface of particles became rough.
-
4.
After modification of MPPs with PAA, the diameter of particles was increased (nearly 20 μm).
The hydrodynamic diameter dependence of MSPBs to ionic strength and pH were studied. The hydrodynamic diameter dependence of MSPBs to ionic strength was investigated by salt concentration. A series of NaCl solutions with concentrations of 0.01, 0.1 and 1 M was prepared and size of MSPBs was determined by DLS. As depicted in Fig. 10, the results show that the size of MSPBs at higher ionic strengths is smaller in comparison with solutions with a lower ionic strength. In other words, increase in ionic strength would decrease the size of MSPBs. These counter-ions compensate the ionic sites on the polymeric chains and thus reduce the intra-/inter-molecular repulsion forces which allow the polymer chains to adopt a coiled conformation, leading to a reduction in the overall size of the particles.
The changes in particle size as a function of pH were also studied. In this case, a series of solutions with different pH values were prepared. The MSPBs were dispersed in as formed solutions and their sizes were measured by DLS. PAA chains containing weak carboxylic acids groups are partially dissociated in aqueous media. The dissociation degree of these acidic groups depends on the pH of the solution. In low pH, the PAA acid chains are almost uncharged and thus there is not any electrostatic repulsion force between adjacent polymer chains. But increasing the pH, leads to the dissociation of carboxylic acid groups, and charges will be generated along the chains. As it is shown in Fig. 11, the results show that the particle size would increase by raising pH. This effect is attributed to the dissociation degree of carboxylic acid groups and electrostatic interactions between the chains. This effect make the chains stretch away from the surface. In high pH values, the PAA chains are fully dissociated and consequently, stretched to nearly maximum length and a brush structure will be resulted.
The hysteresis loops were recorded at room temperature using vibrating sample magnetometer (VSM). The results are shown in Fig. 12. The loops are closed and symmetrical versus origin of coordinate system. Shape of the loops evidences the ferromagnetic character of the material. No evidence of superparamagnetism was seen. Coercivity and remanence of the synthesized particles in comparison to the iron oxide particles slightly decrease. But their magnetic permeability considerably decreases. These variations may be due to encapsulation of iron oxide particles within a polymer phase.
Conclusion
Magnetic spherical polyelectrolyte brushes with high content of iron oxide were synthesized. Firstly, the iron oxide particles were embedded in hydroxyl modified polymer phase via a miniemulsion polymerization process (core particles). Then, by reaction of AC with surface hydroxyl groups, a layer of vinyl groups was introduced on the surface of core particles. Finally, the polyacrylic acid brushes were synthesized onto the surface of core particles by a grafting from method. The final particles including MPP and MSPB were characterized by Fourier transform infrared spectroscopy (FTIR), Fourier transform infrared attenuated total reflection spectroscopy (FTIR-ATR), powder X-ray diffraction (XRD), thermal gravimetric analysis (TGA), scanning electron microscopy (SEM), vibrating sample magnetometer (VSM) and dynamic light scattering (DLS). In addition, their behaviors in an aqueous phase with different ionic strength and pH values were also studied. FTIR and XRD results confirmed the presence of maghemite in the final magnetic polymer particles. TGA measurements indicated that the final magnetic polymer particles have more than 82 % iron oxide content and their high thermal stability. SEM revealed that all maghemite particles were embedded in the polymer phase. According to magnetometry data, the shape of the hysteresis loops evidences the ferromagnetic character of the material and there is no any evidence for super-paramagnetism. The size and size distribution of cores and MSPB-Na were determined by DLS. As it had been observed, the particle size was increased from about 1,200 nm for core particles to about 1,800 nm in the case of MSPB-Na. The hydrodynamic diameter dependence of MSPBs to ionic strength and pH was also investigated. The results showed that the size of MSPBs at higher ionic strengths is smaller in comparison with solutions at lower ionic strengths. The data resulted from size measurements by DLS, showed the particle size would increase by raising pH.
References
Norton LJ, Smiglova V, Pralle MU, Hubenko A, Dai KH, Kramer EJ, Hahn S, Begrlund C, Dekoven B (1995) Effect of end-anchored chains on the adhesion at a thermoset-thermoplastic interface. Macromolecules 28:1999–2008
Uyama Y, Tadokoro H, Ikada Y (1990) Surface lubrication of polymer films by photoinduced graft polymerization. J Appl Polym Sci 39:489–498
Luzinov IA, Voronov SA, Minko SS, Kraus R, Wilke W, Zhuk A (1996) Encapsulation of fillers with grafted polymers for model composites. J Appl Polym Sci 61:1101–1109
Leger L, Raphael E, Hervet H (1999) Surface-anchored polymer chains: their role in adhesion and friction. Adv Polym Sci 138:185–225
Kulik E, Ikada Y (1996) In vitro platelet adhesion to nonionic and ionic hydrogels with different water contents. J Biomed Mater Res 30:295–304
Belder GF, ten Brinke G, Hadziioannou G (1997) Influence of anchor block size on the thickness of adsorbed block copolymer layers. Langmuir 13:4102–4105
Mansky P, Liu Y, Huang E, Russell TP, Hawker CJ (1997) Controlling polymer-surface interactions with random copolymer brushes. Science 275:1458–1460
Luzinov I, Julthongpiput D, Malz H, Piontech J, Tsukruk VV (2000) Polystyrene layers grafted to epoxy-modified silicon surfaces. Macromolecules 33:1043–1048
Boyes SG, Granville AM, Baum M, Akgun B, Mirous BK, Brittain WJ (2004) Polymer brushes–surface immobilized polymers Surface. Science 570:1–12
Meier LP, Shelden RA, Caseri WR, Suter UW (1994) Polymerization of styrene with initiator ionically bound to high surface area mica: grafting via an unexpected mechanism. Macromolecules 27:1637–1642
Uchida E, Ikada Y (1997) Topography of polymer chains grafted on a polymer surface underwater. Macromolecules 30:5464–5469
Zhao B, Brittain WJ (2000) Polymer brushes: surface-immobilized macromolecules. Prog Polym Sci 25:677–710
Ito Y, Ochiai Y, Park YS, Imanishi Y (1997) pH-sensitive gating by conformational change of a polypeptide brush grafted onto a porous polymer membrane. J Am Chem Soc 119:1619–1623
Prucker O, Rühe J (1998) Synthesis of poly(styrene) monolayers attached to high surface area silica gels through self-assembled monolayers of azo initiators. Macromolecules 31:592–601
Biesalski M, Rühe J, Johannsmann D (1999) Segment density profiles of polyelectrolyte brushes determined by fourier transform ellipsometry. J Chem Phys 111:7029–7037
Guo X, Ballauff M (2001) Spherical polyelectrolyte brushes: comparison between annealed and quenched brushes. Phys Rev E doi:10.1103/PhysRevE.64.051406
Chen K, Zhu Y, Zhang Y, Li L, Lu Y, Guo X (2011) Synthesis of magnetic spherical polyelectrolyte brushes. Macromolecules 44:632–639
Sharma G, Mei Y, Lu Y, Ballauff M, Irrgang T, Proch S, Kempe R (2007) Spherical polyelectrolyte brushes as carriers for platinum nanoparticles in heterogeneous hydrogenation reactions. J Catal 246:10–14
Guo X, Weiss A, Ballauff M (1999) Synthesis of spherical polyelectrolyte brushes by photoemulsion polymerization. Macromolecules 32:6043–6046
Lu Y, Wittemann A, Ballauff M, Drechsler M (2006) Preparation of polystyrene-poly(n-isopropylacrylamide) (Ps-PNIPA) core-shell particles by photoemulsion polymerization. Macromol Rapid Commun 27:1137–1141
Wang X, Xu J, Li L, Wu S, Chen Q, Lu Y, Ballauff M, Guo X (2010) Synthesis of spherical polyelectrolyte brushes by thermo-controlled emulsion polymerization. Macromol Rapid Commun 31:1272–1275
Wang J, Sun L, Mpoukouvalas K, Fassbender B, Bonaccurso E, Brunklaus G, Butt HJ, Wegner G (2009) Facile synthesis of spherical polyelectrolyte brushes as carriers for conducting polymers to be used in plastic electronics. Macromol Chem Phys 210:1504–1509
ZhengW GF, Gu H (2005) Magnetic polymer nanospheres with high and uniform magnetite content. J Magn Magn Mater 288:403–410
Poling GW (1969) Infrared reflection studies of the oxidation of copper and iron. J Electrochem Soc 116:958–963
Taylor RM, Schwertmann U (1974) Maghemite in soils and its origin. I. Properties and observations on soil maghemites. Clay Miner 10:299–31
Brandrup J, Immergut EH, Grulke EA (1999) Polymer handbook. Wiley, USA
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baharvand, H., Rabiee, A. Synthesis of polyelectrolyte brushes on spherical magnetic polymer particles. J Polym Res 21, 596 (2014). https://doi.org/10.1007/s10965-014-0596-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0596-z