Abstract
A series of intrinsically toughened shape memory epoxy resins (SMEPs) were prepared by curing two bisphenol-A type epoxy resins containing two and six oxyethylene units (DGEBAEO-2 and DGEBAEO-6) with hexahydrophthalic anhydride in the presence of tris-(dimethylaminomethyl) phenol. The thermal, thermomechanical, mechanical and shape-memory properties of these SMEPs were systematically investigated by DSC, DMTA, tensile test and fold-deploy shape memory test, respectively. DSC and DMTA experiments showed that as the concentration of the DGEBAEO-6 increased, the glass transition temperature and storage modulus in the rubbery plateau of the SMEPs decreased gradually. The tensile stress at yield also decreased with increase in the relative content of DGEBAEO-6 and all the SMEPs exhibit a ductile fracture feature with the appearance of yielding, according to the uniaxial tensile test. The fold-deploy shape memory test showed that all the SMEPs showed a good shape memory effect with a combination of relatively high shape fixity and shape recovery.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Shape memory polymers (SMPs) are a class of smart materials that possess the ability of storing “fixed” temporary shapes and recovering their “memorized” permanent shapes upon appropriate stimuli [1–4]. Heat, chemical, light, electrical current, etc. can be used to realize the shape memory effect of SMPs [1, 2, 5], and heat is most common among these stimuli [5, 6]. To date, several polymers, such as polyurethane [7–9], crosslinked polyethylene [10] and ethylene-vinyl acetate copolymers [11, 12], have been reported to possess shape memory properties; they can find potential applications in biomedical materials [3, 12, 13], sensors [8], actuators [14], etc.
Recently, shape memory epoxy resins (SMEPs) are of interest [15, 16] because they possess some significant properties including excellent thermal stability, superior mechanical properties, good processing abilities, and high shape fixity and shape recovery together with rapid response. More importantly, the thermal and thermomechanical properties of SMEPs can be easily tuned in a large range by varying the formulation [6, 17–22]. Up to now, several series of SMEPs have been unveiled and their properties have been studied. For example, Xie and Rousseau [6] reported the effect of crosslink density or chain flexibility of the epoxy network on the glass transition temperatures (T gs) of their SMEPs. Liu et al. [19] studied the relationships between the curing degree and the properties of their SMEPs. Wei et al. [20, 23] investigated how the content of poly(propylene glycol) diglycidyl ether affected the properties of their hydro-epoxy resin systems.
However, traditional epoxy resins are basically brittle in nature [24–26]—their strain at break (ε b) is relatively low even above their T gs [21, 27] and the durability during shape memory cycles is to be improved. Therefore, it is desirable to improve the shape-memory performances of SMEPs. In this aspect, several researches have been done so far. For example, Feldkamp and Rousseau [27] found that the ultimate strain of the formulated SMEPs could be improved three- to fivefold by simply performing the deformation of the SMEPs at the onset of the glass transition (T g E’). In addition, Feldkamp and Rousseau [21] also investigated the effect of chemical composition on the deformability of SMEPs; based on the appropriate chemical composition and thermal programming conditions, an SMEP with εb of 145 % can be obtained. Leonardi et al. [28] reported an SMEP network with chemical and physical crosslinks; this SMEP exhibited a combination of relatively large strain (75 %) and recovery stress (3 MPa). Besides, Kavitha et al. [29] prepared a series of SMEPs toughened with varying content of carboxyl-terminated butadiene-acrylonitrile (CTBN) rubber and found that the CTBN-modified SMEPs showed a significantly increase in the number of shape memory cycles compared with the unmodified SMEP. Previously, we [30] prepared a series of SMEPs based on an intrinsically toughened epoxy/amine system, and all the SMEPs showed a relative large strain at break when tested around T g E’.
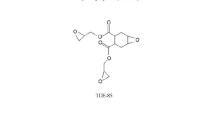
To our knowledge, amine-cured epoxy networks are common in SMEPs, while anhydride-cured epoxy resins, which are very common in electronics and electrics fields, are seldom reported in studies of SMEPs [20, 22]. In fact, some alicyclic anhydrides, such as hexahydrophthalic anhydride (HHPA) and methylhexahydrophthalic anhydride (MeHHPA), have some outstanding advantages that when they are mixed with stoichiometric amount of epoxy resins, the resulting mixtures generally exhibit a low initial viscosity and a long storage life. However, due to the relatively high crosslink density, common anhydride-cured epoxy resins often show their brittle behaviors; thus, it is necessary to enhance the toughness of epoxy/anhydride systems. In our study, two bisphenol-A type epoxy resins containing two and six oxyethylene units (DGEBAEO-2 and DGEBAEO-6, see Scheme 1) were prepared and then they were cured by HHPA in the presence of tris-(dimethylaminomethyl) phenol (DMP-30) to prepare a series of SMEPs. Note here that DGEBAEO-6 is a mixture of diglycidyl ethers of ethoxylated bisphenol-A with different numbers of oxyethylene units, and the average number of oxyethylene units in DGEBAEO-6 is almost six. It was reported that DGEBAEO-2 was tougher than DGEBA when they were cured with 4,4′-diamino diphenylsulfone (DDS) [31] or 4,4′-diamino diphenylmethane (DDM) [32], and DGEBAEO-6 can be used to toughen DGEBA/DDM system [32]. However, their use in epoxy/anhydride system is not mentioned in literature, to our knowledge. In these intrinsically toughened epoxy resins, stiff units and flexible units are connected together with covalent bonds; thus, when they were cured with HHPA, ordered intrinsically toughened epoxy resin networks can be formed. As shown in Scheme 1, DGEBAEO-6 is more flexible than DGEBAEO-2, and the molecular weight of DGEBAEO-6 is larger than that of DGEBAEO-2; hence, the flexibility and crosslink density of the SMEPs can be tuned by varying the concentration of DGEBAEO-6. In the present work, the thermal, thermomechanical, mechanical and shape-memory properties of these SMEPs with different proportions of DGEBAEO-2/DGEBAEO-6/HHPA/DMP-30 were systematically investigated by DSC, DMTA, tensile test and fold-deploy shape memory test, respectively.
Experimental
Materials
The intrinsically epoxy resins DGEBAEO-2 (epoxy equivalent weight 219 g/mol) and DGEBAEO-6 (epoxy equivalent weight 310 g/mol) were synthesized in our laboratory; the curing agent HHPA (99.4 %) was purchased from the Puyang Huicheng Electronic Materials Co., Ltd., China; the accelerator DMP-30, which was used at a level of 1.25 wt.% of HHPA, was available by Sinopharm Chemical Reagent Beijing Co., Ltd., China. The detailed formulations of the SMEPs are listed in Table 1. Note here that this series of SMEPs is labeled as EP06(x), where x indicates the relative content (weight fraction) of DGEBAEO-6 in the epoxy resin component (DGEBAEO-2 and DGEBAEO-6).
Characterization of DGEBAEO-2 and DGEBAEO-6
Fourier transform infrared (FTIR) spectroscopy
The FTIR spectra (see Fig. 1) of DGEBAEO-2 and DGEBAEO-6 were recorded on a Nicole Nexus 670 FTIR spectrometer in the range of 4,000–400 cm−1 using KBr pellet. From Fig. 1, we can observe the characteristic absorption of the epoxy group at 914 cm−1. The bands around 1,109 cm−1 and 1,122 cm−1 correspond to C-O-C stretching. Because DGEBAEO-6 contains more C-O-C bonds than DGEBAEO-2, the C-O-C bonds absorption intensity of DGEBAEO-6 is higher than that of DGEBAEO-2.
1H nuclear magnetic resonance (1H NMR) spectroscopy
1H NMR experiments were performed on a Bruker Avance 400 spectrometer (400 MHz), using CDCl3 as the solvent. Figure 2 presents the 1H NMR spectra of DGEBAEO-2 and DGEBAEO-6 with peak assignments.
Preparation of thermoset epoxy resin networks
The epoxy resin component and HHPA were pre-heated separately around 70 °C. After heating, a stoichiometric amount of HHPA and a calculated amount of DMP-30 were gradually added to the epoxy resin component with constant stirring, followed by degassing to remove air bubbles. Then the reactants were poured into a copper mold pre-coated with mold release agent and subsequently cured at 95 °C for 1.5 h, 130 °C for 2 h and 145 °C for 2 h.
Characterization of the SMEPs
Differential scanning calorimetry (DSC)
The glass transition temperatures (T gDSCs) of the SMEPs were determined using a TA Instruments Q2000 DSC with the following thermal program at the protective atmosphere of dry N2: heating from −10 °C to 260 °C at 20 °C/min, cooling to −10 °C at the maximum cooling rate, and then reheating to 115 °C at 20 °C/min. The values of T gDSCs were measured from the second heating trace.
Dynamic mechanical thermal analysis (DMTA)
Dynamic mechanical thermal analysis of the specimens measuring about 25 mm × 6 mm × 2 mm was carried out on a Rheometric Scientific DMTA V in single cantilever bending mold at a constant frequency of 1 Hz. The heating rate was fixed at 5 °C/min from 10 °C to 130 °C. The values of dynamic modulus, glass transition temperature (T gDMTA, defined by tan δ peak), and the onset temperature of the glass transition (T g E’) can be obtained from the DMTA curves. Note here that T g E’ represents the intersection temperature of the two tangents to the E’-temperature curve at the glass transition drop [21, 27].
Tensile test
Uniaxial tensile test of the SMEPs were performed on an Instron universal tester (model 1185) equipped with a thermostatic thermostatic chamber. ASTM D638 (type IV) specimens were used for testing at room temperature and around T g E’, with crosshead speeds of 5 mm/min at room temperature and 10 mm/min above room temperature. In order to prevent slippage or breaking at the grips, the initial distance (l0) between the two grips was set as 40 mm, and 2 pieces of abrasive paper were placed between the specimen and grip faces. Before testing around T g E’, the specimens were allowed to equilibrate for at least 5 min. The strain (ε) can be calculated by
where (l-l0) indicates the displacement of the crosshead.
Fold-deploy shape memory test
The shape memory behavior of this series of SMEPs was characterized by fold-deploy experiments, as mentioned in Ref.[19]. A fresh specimen with dimensions of 150 mm × 10 mm × 2 mm was heated to the deformation temperature (T d, around T gDMTA + 30 °C) by an air-blast oven (DHG-9070A, Yiheng, China) and maintained at this temperature for 20 min. The specimen was then bent onto a U-shape model with an external diameter of 20 mm by external force, followed by cooling down with air and maintaining at the setting temperature (T s, around T gDMTA − 30 °C) for 20 min with the external force; the maximum bending angle (θ max) was 180°. After that time, the external force was removed and a slight recovery can be observed. The specimen was maintained with no external force for 20 min, and then the fixing bending angle (θ fix) was then recorded. In the end, the resulting specimen was reheated to the recovery temperature (T r, in this process, T r = T d) to recover its original shape, and the final bending angle (θ end) was recorded. Thus, the shape fixity (R f) and shape recovery (R r) can be calculated as follows:
This measuring process was repeated for four times with the same specimen to obtain the average values of R f and R r. Thereafter, another fresh specimen was used to investigate the relationship between recovering ratio and recovering time at different temperatures. After the specimen was bent into U-shape and maintained at T s for 20 min with external force, the external force was removed and then the specimen was put into a pre-heated oven. The recovering process of the specimen was recorded by a digital camera.
Results and discussion
DSC and DMTA analyses
The DSC thermograms of these SMEPs are shown in Fig. 3. From this figure one can clearly observe that as the weight fraction of DGEBAEO-6 in epoxy resin component (DGEBAEO-2 and DGEBAEO-6) increases from 0 wt.% to 85 wt.%, T gDSC decreases from about 85 °C to about 33 °C. The thermomechanical properties of the SMEPs were further investigated by DMTA and the results are shown in Fig. 4. When the temperature was increased from 10 °C to 130 °C, all the samples experienced a distinctive glass transition with the storage modulus sharply decreasing from a glass state to a rubber state. As the relative content of DGEBAEO-6 increases, the crosslink density of cured epoxy resin network will decrease and the flexibility of the network will increase; therefore, the rubbery modulus and T gDMTA of the SMEPS decrease with increase in concentration of DGEBAEO-6. The values of glass transition temperature obtained from DSC and DMTA are shown in Fig. 5. In this figure, a nearly linear relationship between the T g value and the weight fraction of DGEBAEO-6 in epoxy resin component can be observed, indicating that within this temperature range, T gs of this series SMEPs can be tailored by simply varying the relative content of DGEBAEO-6. In addition, probably due to the frequency effect in dynamic mechanical thermal analysis, the T g value obtained from DMTA is higher than that obtained from DSC, as shown in Fig. 5.
Mechanical properties
Mechanical properties at room temperature
Typical stress–strain curves of these SMEPs at room temperature (23 °C) are shown in Fig. 6 and the tensile data are summarized in Table 2. Clearly, both the tensile stress at yield and the tensile stress at break of these SMEPs decrease with increasing the relative content of DGEBAEO-6. The decrease in tensile strength may be due to decrease in crosslink density and increase in flexibility of the epoxy network. With the appearance of yielding phenomenon (necking can be observed during tensile test at room temperature), all the specimens exhibit a ductile fracture feature, indicating good toughness of these SMEPs. Probably due to the relatively high glass transition temperature of the first 3 SMEPs and the necking can’t stretch steadily at room temperature, the strains at break of EP06(0), EP06(25) and EP06(50) are relatively low and numerically indistinguishable (<10 %). On the other hand, the glass transition temperatures of EP06(75) and EP06(85) are not far beyond room temperature, and the strain at break increases significantly as the relative content of DGEBAEO-6 increases. EP06(75) and EP06(85) showed relatively large strains at break of 17.0 % and 65.9 %.
Mechanical properties around Tg E’
Figure 7 shows the typical stress–strain curves of the SMEPs tested around T g E’ and Table 3 lists the results. The mechanical properties of the cured epoxy resin systems depend heavily on temperature. As the temperature increased from room temperature to the value around T g E’, the chain segment mobility of the SMEPs increased and these SMEPs then exhibit the rubber properties; thus a relatively large strain, which is desirable for shape memory applications, can be obtained. One can clearly observe from Fig. 7 that as the weight fraction of DGEBAEO-6 increases, the strain at break increases systematically; the increase in strain at break may be partly attributed to the decrease in crosslink density.
Shape-memory properties
Figure 8 shows the shape fixity of the SMEPs at setting temperatures. All the SMEPs studied here exhibit relatively high shape fixity (>95 %). The fixed specimens were then reheated to their corresponding recovery temperatures, and a nearly full recovery of the SMEPs can be observed.
The variation of recovering ratio versus recovering time of the SMEPs at different temperatures is shown in Fig. 9. Each SMEP can recover its original shape with rapid response. Moreover, we can also find that for the same sample, it takes less time to recover completely at higher temperatures. The high shape fixity and high shape recovery together with the rapid response indicate good shape memory performance of the SMEPs. As an example, a specimen of EP06(85) was rolled up at 45 °C with a external force and then cooled down to 9 °C by air. After that time, the external force was removed and a temporary shape of the specimen was fixed. In the end, the fixed specimen was put into a 40 °C water bath to recover its original shape. The recovery process of EP06(85) is shown in Fig. 10. From this figure, a relatively rapid recovery within dozens of seconds can be observed.
Conclusion
A series of SMEPs with varying proportions of DGEBAEO-2/DGEBAEO-6/HHPA/DMP-30 was prepared and thereafter the thermal properties, dynamic mechanical properties, mechanical properties and shape memory properties of these SMEPs were systematically evaluated. T g and the rubbery modulus of the SMEPs decreased gradually with increase in concentration of DGEBAEO-6. As the relative content of DGEBAEO-6 in epoxy component increased from 0 wt.% to 85 wt.%, tensile stress at yield decreased from 68.0 MPa to 38.5 MPa measured at room temperature. The strains at break of EP06(0), EP06(25) and EP06(50) were relatively low; however, EP06(75) and EP06(85) showed relatively large strains at break of 17.0 % and 65.9 %. On the other hand, tensile test around T g E’ indicated that the strain at break increased as the content of DGEBAEO-6 increased. All the SMEPs studied here showed a combination of relatively high shape fixity (> 95 %) and shape recovery (≈ 100 %), according to the fold-deploy shape memory test.
References
Lendlein A (2010) Shape-memory polymers. Springer, Heidelberg
Hu J, Institute T (2007) Shape memory polymers and textiles. Woodhead Publishing Limited, Cambridge
Liu C, Qin H, Mather PT (2007) Review of progress in shape-memory polymers. J Mater Chem 17:1543–1558
Rousseau IA (2008) Challenges of shape memory polymers: a review of the progress toward overcoming SMP’s limitations. Polym Eng Sci 48:2075–2089
Behl M, Lendlein A (2007) Shape-memory polymers. Mater Today 10:20–28
Xie T, Rousseau IA (2009) Facile tailoring of thermal transition temperatures of epoxy shape memory polymers. Polymer 50:1852–1856
Kalita H, Mandal M, Karak N (2012) Biodegradable solvent-induced shape-memory hyperbranched polyurethane. J Polym Res 19:9982
Tobushi H, Hara H, Yamada E, Hayashi S (1996) Thermomechanical properties in a thin film of shape memory polymer of polyurethane series. Smart Mater Struct 5:483–491
Hu J, Yang Z, Yeung L, Ji F, Liu Y (2005) Crosslinked polyurethanes with shape memory properties. Polym Int 54:854–859
Kumar S, Pandya MV (1997) Thermally recoverable crosslinked polyethylene. J Appl Polym Sci 64:823–829
Li F, Zhu W, Zhang X, Zhao C, Xu M (1999) Shape memory effect of ethylene-vinyl acetate copolymers. J Appl Polym Sci 71:1063–1070
Wu XL, Huang WM, Tan HX (2013) Characterization of shape recovery via creeping and shape memory effect in ether-vinyl acetate copolymer (EVA). J Polym Res 20:150
Lendlein A, Langer R (2002) Biodegradable, elastic shape-memory polymers for potential biomedical applications. Science 296:1673–1676
Metzger MF, Wilson TS, Schumann D, Matthews DL, Maitland DJ (2002) Mechanical properties of mechanical actuator for treating ischemic stroke. Biomed Microdevices 4:89–96
Santhosh Kumar KS, Biju R, Reghunadhan Nair CP (2013) Progress in shape memory epoxy resins. React Funct Polym 73:421–430
Rousseau IA, Xie T (2010) Shape memory epoxy: composition, structure, properties and shape memory performances. J Mater Chem 20:3431–3441
Song WB, Wang LY, Wang ZD (2011) Synthesis and thermomechanical research of shape memory epoxy systems. Mater Sci Eng A 529:29–34
Leng J, Wu X, Liu Y (2009) Effect of a linear monomer on the thermomechanical properties of epoxy shape-memory polymer. Smart Mater Struct 18:95031
Liu Y, Han C, Tan H, Du X (2010) Thermal, mechanical and shape memory properties of shape memory epoxy resin. Mater Sci Eng A 527:2510–2514
Wei K, Zhu G, Tang Y, Tian G, Xie J (2012) Thermomechanical properties of shape-memory hydro-epoxy resin. Smart Mater Struct 21:55022
Feldkamp DM, Rousseau IA (2011) Effect of chemical composition on the deformability of shape-memory epoxies. Macromol Mater Eng 296:1128–1141
Biju R, Nair CR (2013) Synthesis and characterization of shape memory epoxy-anhydride system. J Polym Res 20:82
Wei K, Zhu G, Tang Y, Niu L (2013) Shape-memory effects of a hydro-epoxy resin system. J Polym Res 20:123
Varley RJ (2004) Toughening of epoxy resin systems using low-viscosity additives. Polym Int 53:78–84
Thomas R, Yumei D, Yuelong H, Le Y, Moldenaers P, Weimin Y, Czigany T, Thomas S (2008) Miscibility, morphology, thermal, and mechanical properties of a DGEBA based epoxy resin toughened with a liquid rubber. Polymer 49:278–294
Yu Y, Zhang Z, Gan W, Wang M, Li S (2003) Effect of polyethersulfone on the mechanical and rheological properties of polyetherimide-modified epoxy systems. Ind Eng Chem Res 42:3250–3256
Feldkamp DM, Rousseau IA (2010) Effect of the deformation temperature on the shape-memory behavior of epoxy networks. Macromol Mater Eng 295:726–734
Leonardi AB, Fasce LA, Zucchi IA, Hoppe CE, Soulé ER, Pérez CJ, Williams RJ (2011) Shape memory epoxies based on networks with chemical and physical crosslinks. Eur Polym J 47:362–369
Kavitha RA, Rao S, Srihari S, Dayananda GN (2012) Characterization of shape memory behaviour of CTBN-epoxy resin system. J Polym Res 19:9894
Fan M, Yu H, Li X, Cheng J, Zhang J (2013) Thermomechanical and shape-memory properties of epoxy-based shape-memory polymer using diglycidyl ether of ethoxylated bisphenol-A. Smart Mater Struct 22:55034
Iijima T, Hiraoka H, Tomoi M, Kakiuchi H (1990) Synthesis and properties of new tetrafunctional epoxy resins containing oxyethylene units. J Appl Polym Sci 41:2301–2310
Yang X, Huang W, Yu Y (2012) Epoxy toughening using low viscosity liquid diglycidyl ether of ethoxylated bisphenol-A. J Appl Polym Sci 123:1913–1921
Acknowledgments
This work was supported by the National High Technology Research and Development Program of China (Grant No. 2012AA03A205).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Fan, M., Liu, J., Li, X. et al. Thermal, mechanical and shape memory properties of an intrinsically toughened epoxy/anhydride system. J Polym Res 21, 376 (2014). https://doi.org/10.1007/s10965-014-0376-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-014-0376-9