Abstract
Magnetic polymer particles with high and uniform magnetic content, modified with β-cyclodextrin (β-CD) were prepared. The resulting magnetic polymer particles were characterized by Fourier transform infrared spectroscopy (FTIR), powder X-ray diffractometer (XRD), thermal gravimeter analysis (TGA), scanning electron microscopy (SEM), Transmission electron microscope (TEM), and vibrating sample magnetometer (VSM) measurements. FTIR and XRD confirmed the presence of iron oxide (maghemite) and β-CD in the final magnetic polymer particles. TGA measurements indicated that the final magnetic polymer particles have more than 65 % iron oxide content and high thermal stability. SEM and TEM revealed that all maghemite particles were embedded in the polymer phase. According to magnetometry data, shape of the hysteresis loops evidence the ferromagnetic character of the material and no evidence of superparamagnetism was seen.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Magnetic polymer particles have been widely applied to various aspects in biotechnology and biomedicine fields, such as immobilized enzyme, cell separation, protein purification, immunoassay, targeting drug, NMR and hyperthermia [1–7]. Furthermore, in recent years, β-cyclodextrin (β-CD) plays important roles in many disciplines such as supramolecular chemistry, analytical chemistry, biomedicine and catalysis [8–10]. β-CD is a torus-shaped cyclic oligosaccharide consisting of seven α-1, 4-linked D-glucose units arranged in a circle with a hydrophilic exterior surface and a hydrophobic interior cavity. The special structure affords it remarkable ability to form inclusion complexes with many organic compounds and biological molecules [11–13]. Because of this unique supramolecular behavior, β-CD and its derivatives including β-cyclodextrin polymers have been extensively researched in the fields of drug delivery system, separating and absorption of material, environmental protection devices and functional catalysts, etc. [14–18]. Recently, researchers reported several successful designings and preparation methods of cyclodextrin functionalized magnetic particles or their spherical aggregates by conjugating cyclodextrin (CD) units onto the surface of magnetite particles [19–22]. On one hand, the innermost magnetite particles can sense and respond to an externally applied magnetic field. On the other hand, the outermost cyclodextrin moiety can function as inclusion sites and specific containers for drugs and biomolecules. Generally, the preparation strategies can be divided into two steps. First, magnetic particles are surface decorated by modifiers with active amino groups (−NH2). Then, the previously synthesized cyclodextrin derivatives with some active groups such as tosylate groups (−OTs) or carboxyl (− COOH) are covalently bonded with amino groups (−NH2) and thus grafted onto the surface of magnetic particles. However, the synthesis of used CD derivatives and the surface modification of magnetite particles with amino groups (−NH2) are complicated and the oxidation of magnetic particles cannot be prevented so effectively. To be successfully used in the above areas, they should fulfill such requirements as no sedimentation, near nano-sized distribution, uniform magnetic content, no iron leaking and non-toxicity. The common route for synthesizing magnetic polymer particles is monomer polymerization by dispersing magnetic particles directly in the liquid phase of a polymerizable formulation and polymerizing the monomer in the presence of the iron oxide particles to form magnetic polymer particles. Several processes have been developed including emulsion polymerization [23], dispersion polymerization [24], suspension polymerization [25], microemulsion polymerization [26] and miniemulsion polymerization [27]. To obtain a good dispersion, reaction conditions must be such that all the iron oxide particles are transferred uniformly into the resulting particles, or the iron oxide particles must provide the only site for precipitation of polymers. However, it often carries the risk of incomplete and non-uniform encapsulation, in which the resulting particles are usually of uneven sizes, lack homogeneity and the distribution of iron oxide particles in the polymer particles is not uniform. Another important challenge in the preparation of magnetic polymer particles, especially for their biological applications, is that the magnetic content of the polymer particles should be large enough for quick magnetic separation. However, it is difficult to disperse high concentrations of inorganic hydrophilic particles into droplets of hydrophobic monomers by those processes based on direct monomer polymerization. Therefore, the magnetic content in the polymer phase is usually limited [28, 29]. To avoid these problems and obtain near nano-sized magnetic polymer spheres with high and uniform magnetic content, an indirect process based on miniemulsion polymerization [30–32] was used.
In this work, an indirect process based on miniemulsion polymerization was used to preparation of magnetic polymer particles modified with β-cyclodextrin.
Experimental
Materials
All materials in this work were used without further purification, except styrene (ST, Merck) and divinyl benzene (DVB, Merck) that were washed three times with sodium hydroxide solution (5 wt. %) to remove inhibitor, dried over calcium chloride and stored at 4 °C before use. All other materials including iron oxide (γ-Fe2O3) (brown pigment Bayer 686), oleic acid (OA, Merck), ammonia (25 wt. %, Merck), hydrochloric acid (HCl, 36 wt. %, Merck), hexadecane (Merck) octane (Merck), 2,2′-azobisisobutyronitrile (AIBN, Merck), 2-hydroxyethyl methacrylate (HEMA, Merck), sodium dodecyl sulfate (SDS, Merck), dimethylformamide (DMF, Merck), methanol (Merck), acetone (Merck), maleic anhydride (MAH, Merck), acrylamide (AAm, Merck), potassium persulfate (KPS, Sigma-Aldrich) and β-cyclodextrin (β-CD, Fluka) were used as received.
Apparatus
The magnetic polymer particles were characterized using Fourier transform infrared spectroscopy (FTIR), Bruker, Equinox 55 (Germany); X-ray diffractometer (XRD), Siemens D5000 instrument (Germany); scanning electron microscope (SEM), VEGA, TESCAN (Czech); transmition electron microscope (TEM), Philips CM120 (Poland); thermal gravimetry analysis (TGA), Mettler-Toledo TGA/DSC1 (Swiss) and vibrating sample magnetometer (VSM), Princeton Applied Research VSM 155 instrument (USA).
Methods
Synthesize of core particles (MPP)
Ten grams of iron oxide powder dispersed in 250 mL of water. Then 23.2 g of OA was dissolved in 25 mL of acetone and added to the reaction system followed by 120 mL of ammonia solution and was stirred at room temperature. After 3 h, the sediment was neutralized by 1 N hydrochloride acid solution. Then anhydrous ethyl alcohol was introduced to get rid of the residual OA. Finally, the by product ammonium chloride was eluted by water from the sediment. After drying, the coated iron oxide particles were dispersed in the octane with a iron oxide content of 14 wt. % to form to a ferrofluid. Next, 14 g ferrofluid with 0.3 g hexadecane constituted the oil phase was added to 24 g water containing 0.7 g SDS. The mixture was stirred for 1 h. Then octane was carefully evaporated at 80 °C to obtain stable water-based iron oxide dispersion. At the same time, the styrene monomer miniemulsion was prepared using the following recipe: 3.0 g of styrene,1.0 g of DVB, 0.1 g of AIBN and 0.120 g of hexadecane were added to a surfactant solution containing 0.036 g of SDS dissolved in 12 g of water and was stirred for 1 h. Finally, the styrene miniemulsion and the iron oxide dispersion as obtained above were mixed and stirred. After 3 h, 1 g of HEMA dissolved in 20 mL of water and was added to the reaction mixture and polymerization was carried out at 80 °C for 16 h with continual stirring at 600 rpm. After the polymerization reaction was completed, the mixture was allowed to cooling to room temperature; the sediment was washed with water and methanol respectively, and dispersed in the DMF with iron oxide content of 14 wt. %. Then methanol was carefully evaporated at 80 °C to obtain DMF-based core particles dispersion.
Synthesis of β-CD based reactive monomer (β-CD-MAH)
In order to obtain a β-CD based reactive monomer which can be copolymerized and cross-linked with acrylamide component, a modified β-CD carrying five vinyl carboxylic acid groups was designed and synthesized [33]. Specifically, 5.68 g of β-CD (0.005 mol) was dissolved in 30 mL of DMF, and 4.90 g of MAH (0.05 mol) was added afterwards. The mixture solution was heated at 80 °C for 10 h under the vigorously stirring. After the reaction was completed, the mixture was allowed to cooling to room temperature, and then, 30 mL of trichloromethane was added. A white precipitate obtained was filtrated, and washed at least three times using large amount of acetone, finally, dried in a vacuum oven at room temperature for 1 day, and 80 °C for 3 days.
Vinyl modification of core particles (MPP-MAH)
In order that to have reactive groups on the core particles which can be participate in copolymerization of β-CD-MAH and AAm to form a hydrogel, a modified core particles carrying vinyl groups were designed and synthesized. Particularly, in 30 g of DMF-based core particles dispersion was dissolved 4.90 g of MAH (0.05 mol). Afterwards, the mixture solution was heated at 80 °C for 10 h under the vigorously stirring. After completion of the reaction, the mixture was allowed to cooling to room temperature. Then, the sediment was filtrated, and washed at least three times using large amount of distilled water, and dispersed in the distilled water with iron oxide content of 5 wt. %.
Modification of core particles by β-cyclodextrin (MPP-β-CD)
The AAm/β-CD-MAH hydrogels on the core particles were synthesized by the copolymerization of AAm with β-CD-MAH in the presence of vinyl modified core particles water-based dispersion. Specifically, 0.5 g of β-CD-MAH was dissolved in 30 mL core particles water-based dispersion, and 0.5 g of AAm and 0.01 g of KPS were added afterwards. The mixture solution was heated at 80 °C for 24 h under the vigorously stirring. After finishing the reaction, the mixture was allowed to cooling to room temperature. Then, the sediment was filtrated, and washed at least three times using large amount of distilled water, and dispersed in the distilled water with iron oxide content of 5 wt. %.
Results and discussion
As it is known, magnetic polymer particles modified with β-CD have been widely applied to various aspects in biotechnology and biomedicine fields. To be successfully used in the above areas, they should fulfill such requirements as no sedimentation, near nano-sized distribution, high and uniform magnetic content, no iron leaking and non-toxicity. Therefore, an indirect process based on miniemulsion polymerization was used. In other words, oleic-acid-coated iron oxide particles were firstly synthesized and dispersed into water using sodium dodecyl sulfate as a second emulsifier and hexadecane as an osmotic agent. The prepared water-based SDS/oleic acid bilayer coated iron oxide dispersion was mixed with monomer phase miniemulsion, and a second miniemulsification was carried out. Subsequently, the polymerization generated polymer particles with iron oxide fully encapsulated [34]. In this work, similarly the magnetic polymer particles were prepared with surface decorated by hydroxyl groups, and named as core particles. The hydroxyl groups on the surface of the core particle chemically transformed to vinyl groups and at the same time, aside β-cyclodextrin derivatives with the vinyl groups were synthesized. Finally, the vinyl β-CD monomer in the presence of acrylamide is polymerized onto the surface of core particles.
The resulting magnetic polymer particles were examined and characterized using Fourier transform infrared spectroscopy (FTIR), X-ray diffractometer (XRD), thermal gravimetry analysis (TGA), scanning electron microscope (SEM), transmition electron microscope (TEM) and vibrating sample magnetometer (VSM).
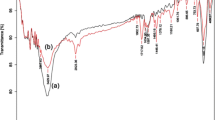
FTIR spectra of MPP, MPP-MAH, β-CD, β-CD-MAH and MPP-β-CD were shown in Fig. 1. In FTIR spectra of core particles (MPP) (Fig. 1a), seven signals were distinguished at 581, 1382, 1409, 1713 and 3432 cm−1, which were attributed to the Fe-O, symmetric CH3 and CH2, C=O and OH groups stretching bands of iron oxide and polymer backbone respectively. By modification of MPP with MAH, a sharp peak was observed at 1,704 cm−1 due to conjugated carbonyl group in MAH (Fig. 1b) that approved the successful functionalization of MPP by MAH. Comparing the spectrum of modified β-CD with β-CD (Fig. 1c, d), there is an additional signal at 1,724 cm−1 was assigned to the stretching of C=O in both carboxylate and carboxylic parts of the molecule. So, it is verified, functionalization of β-CD by MAH. At the final step, the modified β-CD was copolymerized with AAm on the surface of MPP-MAH particles and immobilization of β-CD onto the surface of magnetic microspheres was confirmed by FTIR spectra as shown in Fig. 1e. In resulted MPP-β-CD, there are three signals at 555, 1041 and 1739 cm−1 attributed to Fe-O vibration band, C-O and carbonyl stretching bands. The sharp signal at 1,739 cm−1 is an evidence for elimination of conjugated state of C=O groups in MAH modified parts, and indicates the ester groups of the prepared copolymer on the surface of core particles.
One of the confident methods to find out the presence of a chemical in a compound is XRD. In this method, crystal compounds give sharp peaks at various angles; however, this is not the case in amorphous compounds. Powder XRD pattern of bare iron oxide, β-CD, MPP-β-CD were shown in the Fig. 2. Presence of intensive sharp peaks at 33.15, 33.55, and 62.72˚ in the powder XRD pattern (Fig. 2a) indicate a maghemite structure of bare iron oxide particles. Reiteration characteristic peaks of maghemite in XRD pattern of synthesized powder (Fig. 2c) were shown the existence of a maghemite in the final synthesized particles and the fact that the structure of iron oxide after encapsulation was not changed. Besides, as it is observed in XRD of pure β-CD (Fig. 2b), presence of sharp peaks indicates the crystal structure of β-CD. β-CD exhibited characteristic peaks at 9.1, 10.8, 12.5, 14.6, 15.4, 17.0, 17.7, 18.8, 19.0, 19.5, 20.9, 21.4, and 22.88° because of its crystalline nature. Reiteration some of these peaks and presence of other sharp peaks in the powder XRD pattern of the synthesized particles (Fig. 2c), which may be show the presence of β-CD in the final product.
Thermal behavior of the synthesized particles was studied by TGA. The TGA results can quantify iron oxide content and the thermal stability of polymer/iron oxide composite particles. Figure 3 shows the TGA results of synthesized particles (core particles and final particles). As it is seen, the initial weight loss of 1.96 % (up to 250 ° C) is due to the evaporation of physically adsorbed water. The subsequent weight loss in the range of 300–500 ° C is consequence of thermal degradation of polymer phase. In addition, the TGA measurements were indicated the iron oxide content of core and final particles was 84.42 % and 67.04 % by weight, respectively and the synthesized particles have high thermal stability (about 350 °C). So, the results confirmed the encapsulation of iron oxide particles and also show the successful modification of core particles with β-CD.
Morphology of the synthesized particles was studied by SEM and TEM (Figs. 4 and 5). SEM micrographs of MPP and MPP-β-CD (Fig. 4a, b) indicate that:
-
1.
The synthesized core particles (MPP) are spherical and the surface of them is smooth. This hints that the iron oxide particles mainly located inside of the polymer phase.
-
2.
The size of the synthesized magnetic polymeric particles (MPP) is less than 3 μm.
-
3.
After modification of MPP with β-CD (MPP-β-CD), the surface of particles was found rough, due to polymerization on the surface of MPPs.
-
4.
The MPPs after modification with β-CD have larger size (nearly 20 μm).
Transmission electron microscope (TEM) was used to obtain direct information about the internal structure and morphology of the prepared composite particles. The sample for TEM was prepared by dropping dispersions of composite particles in aqueous solution onto carbon coated copper grids after sonication and drying under vacuum. The iron oxide particles absorb electron beam and appear as dark spots within the MPP-β-CDs particles and the bright layer surrounded them exhibited the polymer phase. TEM images of MPP-β-CD (Fig. 5a, b) show the particles are spherical in shape and were qualitatively suggested high content of iron oxide in the composite particles. In addition, the TEM images show no evidence for the presence of polymer sphere without incorporated iron oxide particles; also there is no iron leaking outside the polymer sphere. Therefore, the results confirmed the successful encapsulation of iron oxide particles.
The magnetic properties of the particles were determined by vibrating sample magnetometer (VSM). The hysteresis loops were recorded at room temperature are shown in Fig. 6. The loops are closed and symmetrical versus origin of coordinate system. Shape of the loops evidences the ferromagnetic character of the material. No evidence of superparamagnetism was seen. Coercivity and remanence of the synthesized particles in comparison to the iron oxide particles slightly decrease. But their magnetic permeability considerably decreases. These variations may be caused by encapsulation of iron oxide particles with a polymer phase.
Conclusion
Magnetic polymer particles with high and uniform magnetic content and surface decorated by hydroxyl groups based on miniemulsion polymerization were prepared. The hydroxyl groups on the surface of these particles chemically modified to vinyl groups and in the same manner the β-cyclodextrin derivatives synthesized with the vinyl groups. Finally, the vinyl β-CD monomer in the presence of acrylamide is polymerized onto the surface of modified magnetic polymer particles. The resulting magnetic polymer particles were characterized by FTIR, XRD, TGA, SEM, TEM, and VSM measurements. FTIR and XRD confirmed the presence of iron oxide and β-CD in the final magnetic polymer particles. The TGA measurements indicated that the final magnetic polymer particles have more than 65 % iron oxide content and high thermal stability. The high maghemite content of the final particles indicates that they have a strong magnetic sensitivity under an outer magnetic field and move toward the outer field very quickly and obviously can be separated completely from water in very short time. SEM and TEM revealed that all maghemite particles were embedded in the polymer phase. According to magnetometry data, shape of the loops evidences the ferromagnetic character of the material and no evidence of superparamagnetism was seen.
References
Kronick P, Gilpin RW (1986) Use of superparamagnetic particles for isolation of cells. J Biochem Biophys Methods 12:73–80
Hallier-Soulier S, Guillot E (1999) An immunomagnetic separation polymerase chain reaction assay for rapid and ultra-sensitive detection of Cryptosporidium parvum in drinking water. FEMS Microbiol Lett 176:285–289
Abudiab T, Beitle RR (1998) Preparation of magnetic immobilized metal affinity separation media and its use in the isolation of proteins. J Chromatogr A 795:211–217
Khng HP, Cunliffe D, Davies S et al (1998) The synthesis of sub-micron magnetic particles and their use for preparative purification of proteins. Biotechnol Bioeng 60:419–424
Yu H, Raymonda JW, McMahon TM et al (2000) Detection of biological threat agents by immunomagnetic microsphere-based solid phase fluorogenic- and electro-chemiluminescence. Biosens Bioelectron 14:829–840
Kim DK, Zhang Y, Voit W et al (2001) Superparamagnetic iron oxide nanoparticles for bio-medical applications. Scripta Mater 44:1713–1717
Mitsumori M, Nagae H, Hasegawa M, Abe M, Nishimura Y et al (1996) Targeted hyperthermia using dextran magnetite complex: a new treatment modality for liver tumors. Hepatogastroenterology 43:1431–1437
Szejitli J (1998) Introduction and general overview of cyclodextrin chemistry. Chem Rev 98:1743–1753
Dodziuk H (2006) Cyclodextrins and their complexes: chemistry, analytical methods applications. Wiley-VCH, New York
Davis ME, Brewster ME (2004) Cyclodextrin-based pharmaceutics: past, present and future. Nat Rev Drug Discov 3:1023–1035
Szejitli J (1998) Cyclodextrin technology, 1st edn. Kluwer Academic Press, Dordrecht, pp 79–81
Harada A, Furue M, Nozakura SI (1976) Cyclodextrin-containing polymers: 1. Preparation of polymers. Macromolecules 9:701–704
Harada A, Furue M, Nozakura SI (1977) Interaction of cyclodextrin-containing polymers with fluorescence compounds. Macromolecules 10:676–681
Sreenivasan K (1997) On the restriction of the release of water-soluble component from polyvinyl alcohol film by blending b-cyclodextrin. J Appl Polym Sci 65:1829–1832
Harada A, Furue M, Nozakura SJ (1978) Optical resolution of mandelic acid derivatives by column chromatography on crosslinkedcyclodextrin gels. Polym Sci Polym Chem Ed 16:189–196
He BL, Zhao X (1992) Synthesis of novel beta-cyclodextrin polymer. Sci China (Ser B) 12:1240–1247
Crini G, Janus L, Morcellet M, Torri G, Naggi A, Bertini S, Vecchi C (1998) Macroporous polyamines containing cyclodextrin: synthesis, characterization, and sorption properties. J Appl Polym Sci 68:1973–1978
Hanessian S, Benalil A, Laferriers C (1995) The synthesis of functionalized cyclodextrins as scaffolds and templates for molecular diversity, catalysis, and inclusion phenomena. J Org Chem 60:4786–4797
Cao HN, He J, Deng L, Gao XQ (2009) Fabrication of cyclodextrin-functionalized super-paramagnetic Fe3O4/amino-silane core–shell nanoparticles via layer-by-layer method. J Appl Surf Sci 255:7974–7980
Banerjee SS, Chen DH (2009) Cyclodextrin-conjugated nanocarrier for magnetically guided delivery of hydrophobic drugs. J Nanoparticle Res 11:2071–2078
Banerjee SS, Chen DH (2007) Magnetic nanoparticles grafted with cyclodextrin for hydrophobic drug delivery. Chem Mater 19:6345–6349
Xia HB, Yi JB, Foo PS, Liu BH (2007) Facile fabrication of water-soluble magnetic nanoparticles and their spherical aggregates. Chem Mater 19:4087–4091
Richard J, Vaslin S (1999) Latex of calibrated monodispersemagnetizable microspheres, process of preparation and use of the said latex in chemistry or in biology. US Patent 5:976,426
Horák D (2001) Magnetic polyglycidylmethacrylate microspheres by dispersion polymerization. J Polym Sci A Polym Chem 39:3707–3715
Cocker TM, Fee CJ, Evans RA (1997) Preparation of magnetically susceptible polyacrylamide/magnetite beads for use in magnetically stabilized fluidized bed chromatography. Biotechnol Bioeng 53:79–87
Zaitsev VS, Filimonov DS, Presnyakov IA, Gambino RJ, Chu BJ (1999) Physical and chemical properties of magnetite and magnetite-polymer nanoparticles and their colloidal dispersions. Colloid Interface Sci 212:49
Hoffmann D, Landfester K, Antonietti M (2001) Magnetohydrodynamics 37:217–221
Liu ZL, Ding ZH, Yao KL et al (2003) Preparation and characterization of polymer-coated core–shell structured magnetic microbeads. J Magn Magn Mater 265:98–105
Dresco PA, Zaitsev VS, Gambino RJ, Chu B (1999) Preparation and properties of magnetite and polymer magnetite nanoparticles. Langmuir 15:1945–1951
Antonoett M, Landfester K (2002) Polyreactions in miniemulsions. Prog Polym Sci 27:689–757
Asua JM (2002) Miniemulsion polymerization. Prog Polym Sci 27:1283–1346
Tiarks F, Landfester K, Antonietti M (2001) Encapsulation of carbon black by miniemulsion polymerization. Macromol Chem Phys 202:51–60
Liu YY, Fan XD (2002) Synthesis and characterization of pH- and temperature-sensitive hydrogel of N-isopropylacrylamide/cyclodextrin based copolymer. Polymer 43:4997–5003
ZhengW GF, Gu H (2005) Magnetic polymer nanospheres with high and uniformmagnetite content. J Magn Magn Mater 288:403–410
Acknowledgments
Financially support from the department of chemistry, Islamic Azad University Central Tehran Branch, Tehran, Iran is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghorbani, Z., Baharvand, H., Nezhati, M.N. et al. Magnetic polymer particles modified with β-cyclodextrin. J Polym Res 20, 199 (2013). https://doi.org/10.1007/s10965-013-0199-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-013-0199-0