Abstract
The copolymerization of sodium-2-acrylamido-2-methylpropane sulfonate with acrylonitrile and acrylamide in water has been studied at different concentrations of monomers, the initiator and the external electrolyte (NaCl). It was shown that an increase in the total concentration of the monomers leads to enriching the copolymers with units of the ionic monomer. A decrease in the initiator concentration causes the increase of the nitrile content in the product. Using of reactivity ratios r1 and r2 was shown to be invalid for this system as it does not allow one to appropriately predict the copolymer composition or its microstructure. The observed effects of significant dependence of copolymers composition on these factors have been explained by the influence of prepolymerization processes of formation of monomer assemblies and aggregates of monomers with growing macroradicals.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The problem of controlling the compositional heterogeneity is topical for many copolymers widely used in practice. Particularly, copolymers of sodium-2-acrylamido-2-methylpropane sulfonate (Na-AMPSA) with acrylamide (AAm) and acrylonitrile (AN) have a high performance in processes of petroleum production and water treatment [1, 2]. In addition, they have a potential application in controlled drug delivery [3], as superabsorbents [4]. The copolymerization of above monomers in aqueous solutions was studied in a number of works. For example, the reactivity ratios were determined for pairs Na-AMPSA-AAm (r1 = 0.50 ± 0.01, r2 = 1.02 ± 0.01) [5], AMPSA-AAm (r1 = 0.42 ± 0.03 r2 = 1.05 ± 0.06) [6], AMPSA-AN (r1 = 0.50 ± 0.05, r2 = 0.54 ± 0.06) [7]. In the work [5] the interesting effect contradicting standard regularities of the classical theory of copolymerization have been discovered. It was shown that at investigated conditions (the total initial monomer concentration 1M, 50 0C) compositions of copolymers Na-AMPSA-AAm remained constant up to high conversion, though AAm considerably exceeds Na-AMPSA in activity. It should be noted that analogous results have been obtained for copolymerization of AAm with N-vinylpyrrolidone [8], sodium vinylsulfonate [9] and dimethylaminoethyl methacrylate sulfate (DMAEMS) [10] in aqueous solutions. It was shown that for the copolymerization of Na-AMPSA with AAm increasing the ionogenic monomer concentration and introducing the external electrolyte in a solution lead to appreciable increase of the overall polymerization rate and reaction order with respect to Na-AMPSA [11]. The latter one is equals 0.82 at initial concentration of monomeric salt up to 0.32 M and 1.81 at the concentration from 0.32 to 0.71 M. The increased reaction order with respect to monomer (1.5) was observed also in the homopolymerization of AMPSA in water [12].
The reasons of peculiar behavior of polymerization systems on the basis of Na-AMPSA and AAm remain debatable. The authors [11] have put forward the hypothesis of prevailing influence of electrostatic factors. The increase of ionic strength of solutions leads to the decrease of dissociation degree of a sulfomonomer, therefore the concentration of free ions in solutions decreases. This results in reducing the repulsion of the sulfomonomer from the same charged polymeric radical, and the copolymer is enriched with an ionogenic monomer. However, in copolymerization of nonionic monomers with ionogenic ones the increase of initial concentrations does not always leads to copolymer enrichment with ionogenic units [13]. In radical homopolymerization of AMPSA in water when increasing initial monomer concentration the lowering of termination and propagation rate constants has been observed [14]. Consequently, the influence of electrostatic factors is not always prevalent.
Last years more and more data appear about the influence of self-assembly of (meth)acrylic monomers on processes of their polymerization in homogeneous aqueous solutions. It was traditionally considered that the monomers which do not have long hydrophobic fragments, for example, AAm and others are not capable of forming multimolecular assemblies in water. Nevertheless, I. Laćik, P. Pascal and coworkers in series of their works showed that the monomer self-assembly is the principal cause of both unusual extreme dependence of a propagation rate constant kp on initial monomer concentration in the homopolymerization of acrylic acid in water [15, 16] and extreme dependence of kp on temperature in the homopolymerization of AAm [17]. Furthermore, for a number of ionogenic vinyl monomers (for example, N,N-dimethylaminoethylmethacrylate (DMAEM) salts [18]), appreciable surface activity in aqueous solutions has been discovered which indicates the formation of multimolecular assemblies. In our previous work the self-assembly of monomers was shown to be a necessary condition of proceeding spontaneous polymerization in concentrated aqueous solutions of N-dimethylaminoalkyl (meth)acrylamides salts [19] and DMAEM salts [20].
In copolymerization, varying the composition of the assemblies one can control the composition of copolymers formed inside them. One of control methods is changing the ratio of monomers to solvent.
When investigating a number of copolymerization processes the self-assembly of (meth)acrylic monomers was taken into consideration. For example, while studying copolymerization of sodium styrene sulfonate (Na-StS) with methyl methacrylate (MMA) or 2-hydroxyethyl methacrylate (2-HEM) in aqueous solutions it was shown that above certain initial concentrations of Na-StS in the system with MMA the formation of combined monomer assemblies occurs, whereas in the system with 2-HEM, on the contrary, the destruction of assemblies of Na-StS is observed [21]. Besides, in the copolymerization of DMAEMS with AAm or AN the composition of copolymers was found to be dependent on initial concentration of the monomers and the initiator [13]. The influence of initiator is connected with the effect of preferential sorption of monomeric molecules by propagating macroradicals (model “bootstrap”) [22–24]. Such sorption can be considered as assembly process with participation of monomers and macroradicals.
The purpose of this work is to investigate the effect of monomer and initiator concentrations, monomer ratio on the composition and compositional heterogeneity of polymers in copolymerization of Na-AMPSA with AAm or AN in aqueous solution.
Experimental section
Materials
The starting monomers (AMPSA and AAm) and potassium persulfate were commercially available (Aldrich) and used without further purification. The initiator 4,4'-azobis(4-cyanovaleric acid) (ACV) was recrystallized from water–methanol mixture. The industrial AN was purified by the double distillation before use. Na-AMPSA was obtained by adding of an equivalent amount of 20 % solution of NaOH to the aqueous solution of the monomer. Scheme 1 shows the chemical structure of the monomers used.
Techniques
The 1H NMR and 13C NMR spectra were recorded in D2O on a Bruker DRX500 spectrometer; tetramethylsilane was used as a reference. The individual concentrations of monomers in reaction mixtures were determined by the liquid chromatography (Na-AMPSA and AAm) and gas–liquid chromatography (AN) using absolute calibrations. Chromatograph Praha with UV detector (254 nm) and steel column (150 × 4.6 mm) filled with modified silica gel Silasorb C18 (particle size 10 μm) was used for the liquid chromatography analysis. Acetonitrile was used as the eluent. Chromatograph “Chromos GH-1000” equipped with flame-ionization detector and steel column (length 2 m, diameter 3 mm) filled with 10 % PEGA on chromatone N-AW was used for gas–liquid chromatography analysis; carrier gas (nitrogen) consumption was 2∙10-3 m3∙hr-1; the evaporator temperature was 60 °C, the chromatographic oven temperature was 40 0C. The conductivity of the solutions of monomers was measured using PRL T57-21 conductometer with graphite electrodes, operating at 3500 Hz. The specific viscosity of the aqueous solutions of monomers was measured using Ubbelohde viscometer with capillary diameters of 0.56, 0.73 and 0.99 mm at 25 °C. Molecular weight of copolymers was defined using Mark-Kuhn-Houwink equation with the coefficients found in the work [25].
Synthesis of copolymers
The kinetics of copolymerization of Na-AMPSA with AN and AAm was studied at 50–70 °C in the presence of ACV and potassium persulfate in water. Total initial concentration of monomers was varied from 0.5 to 3.0 M. Monomer ratio in the feed was varied from 1÷9 to 9÷1. During the copolymerization process the composition of products was controlled by comonomers consumption. After the polymerization was finished the reaction products were precipitated into acetone, followed by removal of the remaining starting monomers through the multiple washing. After the washing, polymers were dried under vacuum (20 °C, 20 mmHg) until constant weight was achieved.
A typical procedure for the synthesis of copolymers is as follows:
-
Poly(acrylonitrile-co-sodium-2-acrylamido-2-methylpropane sulfonate).
To the solution of AMPSA (2.247 g, 0.0109 mol) in water (3.75 ml) 2.18 g of 20 % aqueous solution of NaOH (0.0109 mol), 0.578 g (0.0109 mol) of AN and 0.0058 g of potassium persulfate was added. Reaction mixture was then cooled and pure nitrogen gas was bubbled for 10 minutes through the solution to remove dissolved oxygen. Resulting solution was then kept at 50 °C for 5 hours. After the end of synthesis the product was poured into acetone; the precipitate was filtered and dried under vacuum. The polymer yield was 2.59 g (85 %). The isolated product was lacking double bonds. 13C NMR: , ppm 176.6-173.8 (−CONH-), 122.6-120.2 (−C = N), 58.2 (−CH2SO3), 52.8 (−C(CH3)2-), 43.9-42.1 (−CH2- in AMPSA unit), 36.8-33.4 (−CH- in AMPSA unit, -CH2- in AN unit), 28.2 (−CH- in AN unit), 26.8 (CH3-).
Results
Effect of polymerization conditions on the composition and compositional heterogeneity of products in copolymerization of Na-AMPSA with AN or AAm in aqueous solutions
In the first stage of this work the effect of monomer ratio on the copolymerization kinetics and the composition of products in the system Na-AMPSA-AN was studied.
The dependence of initial rate of copolymerization on initial comonomer ratio Na-AMPSA:AN is shown in Fig. 1. The rate of copolymerization nonlinearly increases with increase of the mole fraction of Na-AMPSA in the reaction mixtures, this occurring especially noticeably at the mole fraction of Na-AMPSA in the feed more than 0.4.
At the same time an interesting regularity is observed, though increase of sulfonated monomer mole fraction in reaction mixture causes considerable increase in process rate, but relative enriching the copolymer with this monomer does not occur (Table 1).
The values of the calculated comonomers reactivity ratios (Table 2) show that AN is a more reactive monomer than Na-AMPSA. During the copolymerization process the values of r1 and r2 considerably decrease, their ratio remaining constant (r2:r1 ≈ 3) in investigated range of conversion (10–60 %). It follows from this that to describe the process the classical Mayo-Lewis equation cannot be used. Therefore, investigated systems can be referred to so-called “peculiar” systems [26]. For such systems, using of reactivity ratios r1 and r2 is invalid as it does not allow one to appropriately predict the copolymer composition or its microstructure.
One of the monomers (Na-AMPSA) being a strong electrolyte, changing its initial concentration leads to the proportional change in effective ionic strength of solution. It occurs both while varying the comonomers ratio and while changing their total concentration. In this connection, to estimate the influence of electrostatic factors on the process behavior, the experiments with addition of external electrolytes have been carried out. As it is seen in Table 1, in the copolymerization with addition of NaCl the composition of products changes differently depending on the initial monomers ratio. So, in experiments with mole ratio of Na-AMPSA:AN varying from 7:3 to 2:8 the increase of sulfonated monomer units content in copolymers was observed when the ionic strength of solutions increased. On the contrary, the product was considerably enriched with nitrile units in experiments with more significant excess of Na-AMPSA (8:2 and 9:1). Such ambiguous influence does not allow one to conclude that electrostatic effects prevail in all cases.
As mentioned above, the polymerization of some vinyl monomers in aqueous solutions is affected by the self-assembly of monomers. The self-assembly of molecules is known to be more intensive in concentrated aqueous solutions. Therefore, in the number of experiments we varied the initial monomer concentration (the ratio of monomers was constant). It is also known that the assembly of comonomers with propagating macroradicals in organic medium depends on their molecular weight [25]. In this connection, in another series of experiments we changed the molecular weight of growing macromolecules by varying the initial concentration of the initiator.
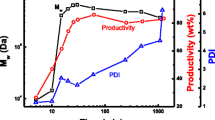
As it is shown in Figs. 2 and 3, the increase of total monomers concentration can result in amazing increase of ionogenic monomer fraction in the product for both monomer pairs (Na-AMPSA-AAm and Na-AMPSA-AN). This increase amounts to 15 % in copolymer with AN and 40–50 % in copolymer with AAm (at conversion of 10–15 %). It should be noted that in the system with AAm such effect was achieved upon increasing the total initial monomers concentration from 0.5 to 1.5 M (i.e. ionic strength increased only by 0.5 M). Besides, as it is well shown in Fig. 3, the copolymer composition remained constant with increasing the conversion at initial concentration of 1 M (as well as in the work [7]). However, this effect was not observed at other initial monomer concentrations.
Dependence of AAm units content in copolymers m2 (mol%) on conversion in copolymerization of Na-AMPSA with AAm. [Na-AMPSA]:[AAm] = 5:5, [K2S2O8] = 0.3 mol%; [M1 + M2]0, M = 0.5 (1), 1.0 (2), 1.25 (3), 1.5 (4); T = 50 °C. m2 is calculated as shown in Fig. 2
The initial copolymer composition and dynamics of its change with conversion are also essentially influenced by the molecular weight of growing macroradicals. The data presented in Table 3 show that according to the general regularities of the radical polymerization the decrease of molecular weight of copolymer formed is observed when the initiator concentration increases, it being accompanied by the dramatic reducing of nonionic units content in the product. To study the influence of molecular weight on the composition of copolymers formed, the experiments with a variation of the initiator concentration have been carried out (Figs. 4, 5). The especially pronounced effect of the initiator concentration on the products composition appears at high monomers concentration for pair Na-AMPSA-AN (Fig. 4). Decrease of molecular weight of copolymers formed (due to increase of the initiator concentration in reaction mixture) results in significant increase of Na-AMPSA fraction in copolymers (up to 20 %). It is interesting to note that in relatively diluted solutions the influence of initiator concentration is levelled (Fig. 4, curve 1). On the contrary, in case of copolymerization of Na-AMPSA-AAm the above effect was observed also in relatively diluted solution (Fig. 5).
Influence of the initiator (K2S2O8) concentration on copolymers composition. [Na-AMPSA]:[AN] = 5:5, [M1 + M2]0, M = 0.5 (1), 1.0 (2), 2.0 (3), T = 50 °C. m2 is calculated as shown in Fig. 2
Dependence of AAm units content in copolymers m2 (mol%) on conversion in copolymerization of Na-AMPSA with AAm. [Na-AMPSA]:[AAm] = 5:5, [M1 + M2]0 = 0.5 M, [K2S2O8], mol% = 0.3 (1), 0.5 (2), 0.7 (3); T = 50 °C. m2 is calculated as shown in Fig. 2
A series of experiments with a variation of initial initiator concentration has confirmed also conclusions about limited character of composition constancy effect with increase of conversion during copolymerization for system Na-AMPSA–AAm. For system Na-AMPSA–AN such effect was not observed in all experiments.
Self-assembly of investigated monomers in aqueous solutions
In this stage of the work the processes of monomers self-assembly in aqueous solutions have been investigated in a wide range of concentrations (under inhibition of radical polymerization). To study the monomer self-assembly, viscosimetric, conductometric methods and H1 NMR-spectroscopy have been used.
Dependences of both specific viscosity and electrical conductivity on concentration have been obtained for individual solutions of AMPSA, Na-AMPSA, AAm and AN. As it is clear from Figs. 6–8, AMPSA, Na-AMPSA and AAm display appreciable tendency towards self-organization. It is evidenced by the nonlinear character of dependences of both specific conductivity and viscosity on the concentration. We noted several “critical” concentrations on these dependences. Two of them (near 0.8 and 3.0 %) appear as inflection points on the dependence of the electrical conductivity on the concentration of AMPSA (Fig. 7, curve 1). Other “critical” concentrations (4, 10, 16, 22 and 26 %) correspond to a deviation of the viscosity-concentration dependence from the linearity (curve 2). The presence of several inflection points testifies to numerous structural changes of monomer assemblies formed during the strengthening of solutions.
The data of NMR-spectroscopy investigations of AMPSA solutions at concentration more than 10 % can be the evidence of the intensification of monomer molecules interaction at the increase of the solution concentration. So, according to the data of 1H NMR (Table 4) at the increase of Na-AMPSA concentration the signals of all monomer protons shift into the weak field and the line width of their signals increases, which is usually connected with the decrease of the molecular mobility [28, 29]. Earlier similar effects in the spectrum 1H NMR have been observed by us when increasing the concentration of aqueous solutions of quaternary salt of DMAEM with dimethyl sulfate (DMAEM·DMS).

In comparison with ionogenic monomer, AAm molecules assemble in more concentrated solutions (Fig. 8, curve 1), and its viscosity-concentration dependence curve has three inflection points (near 6, 20 and 32 %). It is necessary to note that AN does not display tendency to form multimolecular assemblies in the range of its water solubility (up to 7 %). It is evidenced by the linear character of the viscosity-concentration dependence (curve 2).
The 1H NMR-spectroscopy data show that introduction of AN in dilute aqueous solution of Na-AMPSA does not lead to appreciable changes in position and half-width of proton signals of Na-AMPSA (Table 5). Consequently, AN does not essentially influence the assemblies of Na-AMPSA in these conditions (they are insufficiently hydrophobic, and AN molecules do not penetrate into the region of these hydrophilic assemblies). For more concentrated solutions of Na-AMPSA, an addition of AN causes essential decrease of half-width of proton signals of Na-AMPSA (Table 5), their values in this case becoming close to those in dilute solution (Table 4). These data indicate that in enough concentrated solutions AN molecules are capable of penetrating into assemblies of Na-AMPSA “loosening” them, i.e. to reduce packaging density of Na-AMPSA molecules. They are also evidence of enhancement of hydrophobicity of Na-AMPSA assemblies in concentrated solutions.

Thus, experimental data obtained show that investigated monomers incline to self-assembly. Na-AMPSA and AAm display appreciable tendency towards individual self-assembly in aqueous solutions even at relatively low concentrations. When increasing the monomer concentration in water structural changes of these assemblies occur, this must influence the processes of radical copolymerization of specified monomers. Hydrophobic AN does not form homoassemblies, it actively penetrating into relatively hydrophobic regions of other assemblies. As a result in the system Na-AMPSA–AN monomers form heteroassemblies at enough high concentrations.
Discussion
The experimental data show that investigated systems (Na-AMPSA-AN and Na-AMPSA-AAm) are referred not to classical ones, obeying the Mayo-Lewis scheme, but to so-called “peculiar” systems, in which reactivities of monomers change during copolymerization or at variation of initial process conditions. This is expressed in the following phenomena: (1) variability of r1 and r2 values during copolymerization of Na-AMPSA with AN (Table 2); (2) the presence of concentration effects (changing the initial concentration of monomers or initiator results in significant change in the copolymer composition); (3) the strong influence of additives of electrolytes and etc.
Considering the reasons of this “peculiarity”, one can estimate the results obtained by us from positions of the hypotheses listed in the introduction which have been put forward by various researchers having studied polymerization in systems ionogenic monomer–nonionic monomer in aqueous solutions. The hypothesis [10–12], that the ability of comonomers to penetrate into hydrophobic regions of growing macroradicals suppresses other factors affecting the process, is based on the constancy of copolymers composition up to enough high conversion (50–60 %). However, our data show that this constancy is observed not for all pairs of ionogenic and nonionic monomers (in copolymerization of Na-AMPSA with AN this phenomenon is absent) and has a local character (for the copolymerization of Na-AMPSA with AAm its presence depends on initial concentration of both monomers and initiator). So, there was no effect for the system with the most hydrophobic monomer (AN), consequently, it is not connected with the ratio of comonomer hydrophobicities. It is unlikely that the ratio of comonomers penetrating into macroradical coil from the bulk is constant during the experiment. This ratio should be influenced by changing the comonomer ratio and concentration of polymeric molecules during the reaction.
On the contrary, supporters of the electrostatic approach to consideration of ionogenic monomers copolymerization suppose that in all cases with increase of ionic strength of a solution the copolymer enrichment with only charged units (i.e. with ionogenic comonomer) should be expected [13]. However, our experiments concerning the copolymerization of Na-AMPSA with AN in the presence of additives of NaCl and without ones have shown that the composition of products can differently change depending on the initial monomer ratio in solutions of external electrolyte. Besides, as it is clear from Fig. 4, one can easily achieve the less content of ionogenic units in polymers obtained at greater ionic strength of a solution by varying the initiator concentration.
Two above hypotheses also do not allow one to explain the dramatic increase of total copolymerization rate with increase of sulfonated monomer concentration in reaction mixture above certain value.
Complex analysis of both discovered concentration effects in copolymerization and data concerning the influence of concentration on physical properties of monomeric solutions makes it possible to conclude that monomer self-assembly should be taken into account along with electrostatic and hydrophobic factors defining “peculiar” character of the polymerization of investigated systems. Indeed, if initial concentration of monomers is an important parameter defining process behavior to a considerable degree, it is logical to assume that prepolymerization interaction of monomeric molecules in aqueous solutions significantly influences the processes of radical copolymerization.
Let us consider the obtained results from these positions. In assemblies the rate of radical homopolymerization is known to be considerably higher than in bulk [30], therefore, it is logical to assume that when binding a part of comonomers into stable assemblies copolymerization will proceed more readily in these formations.
Such approach allows one to explain the dramatic acceleration of reaction with increase of fraction of actively assembling sulfomonomer in the feed. As it is shown in Fig. 1, the concentration of Na-AMPSA at which considerable acceleration of copolymerization reaction occurs practically coincides with the beginning of deviation of the viscosity-concentration dependence from the linear character.
As it is seen in Figs. 2 and 3, as initial concentration of monomers increases the relative enrichment of copolymerization products with sulfonated monomer units occurs. This fact conforms not only with increasing the ionic strength of solutions but also with data about high assembly activity of Na-AMPSA. The hypothesis of monomer self-assembly explains the ambiguous influence of NaCl additives on composition of Na-AMPSA-AN copolymer. NaCl enhances an ionic strength of solution, but at the same time it can enrich monomer assemblies of Na-AMPSA with AN as a result of salting-out effect. Besides, the correlation between the ionic strength of solution and ionogenic units content in copolymer is absent in our experiments. It will be also shown below that increase in initial monomer concentration can lead to the enrichment of copolymers with nonionic units; in these cases the assembly obviously opposes the electrostatic effect and prevails over it.
The strong effect of the initiator in copolymerization processes in organic solutions (the influence of initiator concentration on the copolymer composition) was shown to be connected with assembly of comonomer molecules with growing macroradicals [26]. Until recently this effect had not been considered for aqueous systems.
The water-soluble (met)acrylic polymers containing short hydrophilic side groups (−COOH, -CONH2 etc.) are considered as the compounds possessing surface activity. In aqueous solution they aspire to formation of microaggregates like micelles [31]. There is a hydrophobic hydrocarbon chain of polymer inside these micelle-like aggregates and hydrophilic groups are located outside. Appearance of such aggregates is experimentally determined in aqueous solutions, for example, in investigation of fluorescence spectra of polyacrylamide [32].
The data presented in Figs. 4–5 show that the preferential sorption of monomeric molecules by propagating macroradicals actively appears in copolymerization of investigated systems. Molecular weight of growing macroradicals appreciably effects the copolymer composition. While decreasing the initiator concentration in the system both molecular weight and hydrophobicity of the growing macroradicals increase. Thus, more hydrophobic molecules of nitrile penetrate into macroradical assemblies more readily in comparison with the sulfomonomer. This results in enriching the copolymers with AN units (Fig. 4). The hydrophobicity of macromolecular coils reduces in dilute solutions; therefore the effect of the initiator diminishes.
Hydrophobic monomers are absent in pair Na-AMPSA-AAm. In this case the influence of molecular weight on copolymers composition can be connected with easier penetration of uncharged AAm into the macromolecular coil (due to the hydrogen bond formation). The charged molecule of Na-AMPSA with the large amide substituent penetrates into the regions of macroradicals having high molecular weight with difficulty. The degree of repulsion of the sulfomonomer from the same charged macroradical decreases due to the increase of ionic strength in concentrated solutions. This results in decreasing the effect of the initiator.
Thus, our data show that the processes of both monomers self-assembly and assembly of monomers with growing macroradicals strongly effect the composition of copolymers in systems Na-AMPSA-AN and Na-AMPSA-AAm. Initially prereaction monomer assemblies form in these systems. Their role especially enhances with the increase of the concentration of assembling monomers: AMPSA and AAm. The polymerization rate is much higher in these assemblies than in bulk. After the beginning of polymerization process the appearance of a high-molecular product in reaction mixture leads to the transformation of initial monomer assemblies into more hydrophobic monomeric-polymeric assemblies. Both types of assemblies significantly influence the compositions of copolymers formed (along with true reactivities of monomers). The degree of monomer self-assembly and compositions of assemblies depend on initial concentrations of monomers and an initiator.
It is of interest to estimate the general regularities and features of the influence of both monomers and initiator initial concentrations in copolymerization of assembling ionogenic and nonionic (meth)acrylic monomers in water. For this, in Table 6 the above mentioned regularities have been compared with those obtained earlier for systems DMAEMS-AAm and DMAEMS-AN [15], N-(3-dimethylamino)propyl methacrylamide sulfate (DMPMAS)—AAm and DMPMAS-AN [33].
As it is clear from Table 6, the presence of dependence of copolymer composition on both initial concentrations of monomers and the initiator is common for all systems. It should be noted that effect of monomer self-assembly appears to the greatest degree in systems Na-AMPSA-AAm, DMPMAS-AAm and DMPMAS-AN (the copolymer composition can change by 25–50 %). The initiator concentration more strongly influences the composition of copolymers Na-AMPSA-AAm, DMAEMS-AN, DMPMAS-AN (maximum change in copolymer composition observed by us was 30–40 %).
At the same time there are no uniform tendencies of copolymers composition changing with change of initial concentrations of monomers and initiators. In some systems copolymers are enriched with nonionic units, in others—with ionogenic ones.
These differences are due to the fact that ionogenic monomers differ by the nature of charged groups, amide or ether groups presence and hydrocarbon fragments structure. So the ratio of abilities of ionogenic and nonionic monomers to assemble changes. This results in formation of assemblies with different compositions. Therefore, the character of concentration effects changes.
Conclusions
The following specific effects were observed in copolymerization of investigated monomer pairs in aqueous solutions:
-
(1)
the increase of sulfonated monomer fraction in the feed leads to nonlinear increase in copolymerization rate for the system Na-AMPSA–AN;
-
(2)
the increase of total monomers concentration in copolymerization of Na-AMPSA with AN or AAm causes increasing the ionogenic units content in copolymers;
-
(3)
the decrease of the initiator concentration results in enriching the copolymers with nonionic units;
-
(4)
the introduction of the external electrolyte differently affects the composition of the copolymers Na-AMPSA–AN, depending on the ratio of the monomers: the sulfonated monomer units content increases at mole ratio varying from 7:3 to 2:8, and decreases in case of 8:2 and 9:1.
Experimental data show that the variation of the initial monomer concentration allows one to control the compositional heterogeneity of copolymers. The concentration effects are explained by the formation of assemblies monomer–monomer and monomer–macroradical. In these assemblies favorable conditions are created for the reactions of chain propagation. The composition of the assemblies influences the composition of copolymerization products.
References
McCormick CL (1985) Water-soluble random and graft copolymers for utilization in enhanced oil recovery. J Macromol Sci: Part A—Chem 22(5–7):955–982. doi:10.1080/00222338508056647
Budanova YE, Shvetsov OK, Maer ZA (2001) Copolymerization of acrylamido sulfonic acids with acrylamide and acrylonitrile in concentrated neutral aqueous solutions. Russ J Appl Chem 74(7):1215–1219. doi:10.1023/a:1013099825077
Nart Z, Kayaman-Apohan N (2011) Preparation, characterization and drug release behavior of poly(acrylic acid–co-2-hydroxyethyl methacrylate-co-2-acrylamido-2-methyl-1-propanesulfonic acid) microgels. J Polym Res 18(5):869–874. doi:10.1007/s10965-010-9483-4
Kabiri K, Zohuriaan-Mehr M, Mirzadeh H, Kheirabadi M (2010) Solvent-, ion- and pH-specific swelling of poly(2-acrylamido-2-methylpropane sulfonic acid) superabsorbing gels. J Polym Res 17(2):203–212. doi:10.1007/s10965-009-9306-7
McCormick CL, Chen GS (1982) Water-soluble copolymers. IV. Random copolymers of acrylamide with sulfonated comonomers. J Polym Sci: Polym Chem Ed 20(3):817–838. doi:10.1002/pol.1982.170200319
Travas-Sejdic J, Easteal A (2000) Study of free-radical copolymerization of acrylamide with 2-acrylamido-2-methyl-1-propane sulphonic acid. J Appl Polym Sci 75(5):619–628. doi:10.1002/(sici)1097-4628(20000131)75:5<619::aid-app 4>3.0.co;2-e
Aggour YA, Bekhat G, Atia A (2000) J Polym Mater 17(2):193–197
Gromov VF, Bune EV, Barabanova AI (1995) Polym Sci A 37(11):1098–1102
Barabanova AI, Gromov VF, Bune EV (1994) Vysokomolek Soed A 36(6):901–907, Russ
Gromov VF, Bogachev YS, Bune EV (1993) Vysokomolek Soed A 35(1):7–12, Russ
Kurenkov VF, Utikeeva AR (2000) Polym Sci A 42(4):372–377
Kumar R, Srivastava A, Behari K (2003) Polym Prepr 44(1):1251–1252
Shirshin K, Kazantsev O, Sivokhin A, Khokhlova T (2007) Concentration effects in copolymerization of sulfuric acid salt of N, N -dimethylaminoethyl methacrylate with acrylonitrile and acrylamide in aqueous solutions. Russ J Appl Chem 80(8):1404–1408. doi:10.1134/s1070427207080289
Beuermann S, Buback M, Hesse P, Junkers T, Lacík I (2006) Free-radical polymerization kinetics of 2-acrylamido-2-methylpropanesulfonic acid in aqueous solution. Macromolecules 39(2):509–516. doi:10.1021/ma051187n
Lacík I, Beuermann S, Buback M (2004) PLP-SEC study into the free-radical propagation rate coefficients of partially and fully ionized acrylic acid in aqueous solution. Macromol Chem Phys 205(8):1080–1087. doi:10.1002/macp.200300251
Lacík I, Beuermann S, Buback M (2003) PLP–SEC study into free-radical propagation rate of nonionized acrylic acid in aqueous solution. Macromolecules 36(25):9355–9363. doi:10.1021/ma030365e
Pascal P, Winnik MA, Napper DH, Gilbert RG (1993) Pulsed laser study of the propagation kinetics of acrylamide and its derivatives in water. Macromolecules 26(17):4572–4576. doi:10.1021/ma00069a024
Shibalovich VG, Efimova DY, Nikolaev AF (2000) Int Polym Sci Tech 27(10):76–80
Kazantsev OA, Shirshin KV (2004) Spontaneous polymerization of (meth)acrylamides in concentrated aqueous solutions. Polymer 45(15):5021–5029. doi:10.1016/j.polymer.2004.05.031
Kazantsev OA, Kuznetsova NA, Shirshin KV (2003) Polym Sci A 45(4):338–345
Neumann MG, Schmitt CC, Maciel H, Goi BE (2006) The photoinitiated copolymerization of styrenesulfonate with methacrylate monomers in hydrotropic medium. J Photochem Photobiol A: Chem 184(3):335–339. doi:10.1016/j.jphotochem.2006.04.034
Harwood HJ (1987) Makromol Chem Macromol Symp 10/11:331–354
Semchikov YD, Smirnova LA, Knyazeva TY, Bulgakova SA, Sherstyanykh VI (1990) Dependence of copolymer composition upon molecular weight in homogeneous radical copolymerization. Eur Polym J 26(8):883–887. doi:Doi:10.1016/0014-3057(90)90162-w
Semchikov YD, Slavnitskaya NN, Smirnova LA, Sherstyanykh VI, Sveshnikova TG, Borina TI (1990) The influence of preferential sorption upon the copolymerization of vinylpyrrolidone with vinyl acetate. Eur Polym J 26(8):889–891. doi:Doi:10.1016/0014-3057(90)90163-x
Valueva SV, Kipper AI, Lyubina SY (1992) Vysokomolek Soed A 34(12):35–44, Russ
Myagchenkov VA, Frenkel’ SY (1978) Russ Chem Rev 47(7):665–683
Tüdős F, Kelen T, Földes-Berezhnykh T, Turcsányi B (1975) Evaluation of high conversion copolymerization data by a linear graphical method. React Kinet Catal Lett 2(4):439–447. doi:10.1007/bf02062350
Egorov VV, Ksenofontova OB, Batrakova EV (1991) Kolloidnyi zhurnal 53(12):351–356, Russ
Valdebenito A, Encinas MV (2010) Effect of solvent on the free radical polymerization of N, N-dimethylacrylamide. Polym Int 59(9):1246–1251. doi:10.1002/pi.2856
Egorov VV, Zubov VP (1987) Russ Chem Rev 56(1153–1165)
Khokhlov AR, Berezkin AV, Khalatur PG (2004) Computer modeling of radical copolymerization under unusual conditions. J Polym Sci, Part A: Polym Chem 42(21):5339–5353. doi:10.1002/pola.20451
Gromov VF, Bune EV, Teleshov EN (1994) Russ Chem Rev 63(6):507–518
Sivokhin A, Kazantsev O, Shirshin K, Samodurova S (2010) Effect of association processes on polymerization of N-[3-(dimethylamino)propyl]methacrylamide salts in aqueous solutions. Russ J Appl Chem 83(11):2041–2046. doi:10.1134/s1070427210110285
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kazantsev, O.A., Shirshin, K.V., Sivokhin, A.P. et al. Copolymerization of sodium 2-acrylamido-2-methylpropane sulfonate with acrylamide and acrylonitrile in water: an effect of conditions on the compositional heterogeneity. J Polym Res 19, 9886 (2012). https://doi.org/10.1007/s10965-012-9886-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-9886-5