Abstract
Temperature sensitive hydrogels were prepared by free radical polymerization of N-isopropylacrylamide (NIPAM) and 2-acrylamido-2-methylpropanesulphonic acid (AMPSA) in presence of polyethylene glycol (PEG) as macroinitiator. The aim of the work reported here was to investigate temperature sensitive swelling and deswelling behaviors of the hydrogels in water in order to investigate the effect of various amounts of AMPSA. The result indicated that the incorporation of a hydrophilic ionizable comonomer (AMPSA) affects the temperature sensitivity, swelling/deswelling, morphology and network structure of the hydrogels. The volume fraction in the swollen state (V2m), the number average molecular weight between cross-links (\( {\overline M_c} \)), the number of segments between the cross-linked point (N), polymer-solvent interaction parameter (χ), enthalpy (ΔH) and entropy (ΔS) were determined using the Flory-Rehner theory at equilibrium swelling. The negative values of ΔH and ΔS showed that the prepared hydrogels had lower critical solution temperature (LCST). The Flory-Rehner theory of swelling equilibrium was qualitatively satisfied with the experimental data of hydrogels at different temperature.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrogels are basically consisting of elastic network systems that have ability to hold large amount of water or biological fluids in the interstitial space of the network and retain their network structure after swelling. Environmentally-sensitive hydrogels are also called smart hydrogels, which can respond to many physical, chemical or biochemical stimuli, including temperature, electric fields, solvent compositions, light, pressure, sound and magnetic fields, pH, ions, and specific molecular recognition [1–4]. In the past two decades, much attention has been paid to syntheses and applications of smart hydrogels. They are extensively used in the field of medicine, pharmacy, biological sensor, tissue engineering and actuators [5–9]. In all of their applications, the controlled drug delivery is the most prominent area [10, 11].
The swelling behavior of dried hydrogels results from the disentanglement of polymers or hydration of hydrophilic groups caused by the diffusion of water through glassy polymers. The degree of swelling of the hydrogel particles is significantly influenced by the distribution of the cross-link density [12]. As a hydrogel, the behavior or state of water is also important to understand the chemical or physical association between the polymer phases and the water. There are three types of water in hydrogels: free water, freezing bound water, and non-freezing bound water. Free water can move through the network without attractive or repulsive interactions between the polymeric networks. Freezing bound water or intermediate water is regarded as transitional water, which can switch between non-freezing bound water and free water, and it interacts weakly with the polymer chains. However, non-freezing bound water is joined into polymers through hydrogen bonds. When polymers are provided with a large number of hydrophilic groups, hydrogels can hydrate, and therefore contain a great amount of bound water. So the hydrophilicity of the polymers can significantly affect the swelling and transporting behavior of the hydrogels [1, 7].
Poly (N-Isopropylacrylamide) (PNIPAM) is one of the most studied thermosensitive hydrogel and exhibits a lower critical solution temperature (LCST) ~32 °C and undergoes a reversible volume change in response to the change in surrounding temperature. When the temperature is below the LCST, the hydrogel is swollen, whereas at the temperature above LCST it begins to shrink and collapsed. The LCST of the derivatives of polyacrylamides may vary according to the different substitution groups. The hydrogel based on pure PNIPAM are opaque and inhomogeneous and have relatively low swelling ratio and gel strength as comparison to other conventional hydrogels [11]. The hydrogels with high absorption capacity, swelling rate and high mechanical gel strength are the certain properties needed for some specific applications. It is already previously reported that the LCST of acrylamide polymers can be tuned by radical copolymerization of appropriate comonomers [6]. The swelling characteristic of PNIPAM can be improved by copolymerizing with hydrophilic and ionizable comonomers like 2-acrylamido-2-methylpropane sulphonic acid (AMPSA) [13, 14] and acrylic acid (AA) [15]. Sulphonic acid containing hydrogels are the strong polyelectrolyte gels with high degree of ionization and show high degree of swelling properties. The incorporation of anionic monomers in the PNIPAM backbone shifts the LCST transition to higher values. The most desirable transition temperature is as near as possible to the physiological temperature of 37 °C. According to the reported results [16], the shape of the Qv /T curve is changed in accordance with the ionic comonomer content in the copolymers; the equilibrium degree of swelling (Qv) is higher and the LCST value is shifted to higher temperatures, from 35 to 40 °C. Hence, the copolymer hydrogels of NIPAM and ionic comonomer have been studied extensively [17–19].
To evaluate the feasibility of hydrogels for biomedical applications it is necessary to know the structure and properties relationship of Hydrogels in solvent. The basic parameters that define the structure and properties of hydrogels are volume fraction in the swollen state (V2m), the number average molecular weight between cross-links (\( {\overline M_c} \)), the number of segments between the cross-linking point (N), correlation length (ζ) or pore size[20]. In this work, we prepared PEG-b-Poly (NIPAM-co-AMPSA) copolymer hydrogels with different mole percent of AMPSA as ionic comonomer using PEG as macroinitiator. It is reported that the PEG macroinitiaor is used to prepare monodispersed hydrogel and the incorporation of PEG in hydrogel modulates phase transition behavior, the network pore size and facilitates diffusion of water in hydrogel [21]. Incorporation of PEG chains also allows tunability of the hydrophilic/hydrophobic balance of these hydrogel networks, thereby controlling their deswelling characteristics [22]. Furthermore, when polymers are provided with a large number of hydrophilic groups, hydrogels can hydrate, and therefore contain a great amount of bound water. So the hydrophilicity of the polymers can significantly affect the swelling and transport behavior of the hydrogels. The Flory-Rehner theory of swelling equilibrium was used to calculate the volume fraction in the swollen state (V2m), the number average molecular weight between cross-links (\( {\overline M_c} \)), the number of segments between the cross-linked point (N) and polymer-solvent interaction parameter (χ).
Experimental
Materials
The monomer N-isopropylacrylamide(NIPAM), 2-acrylamido-2-methylpropane sulphonic acid(AMPSA) from Sigma-Aldrich, and PEG-6000 from Merck Specialties Pvt. Ltd, India were used as received. An extra pure N, N-methylenebisacrylamide (NMBA) from SRL (India) was used as cross linker. Ammonia ceric (IV) nitrate, 99.99 % pure from Sigma-Aldrich was dried in oven at 105 °C for 1 h prior to use. Double-distilled water drawn from a Millipore purification system was used.
Preparation of hydrogel
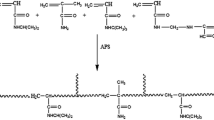
Thermoresponsive hydrogels were prepared by free radical copolymerization of NIPAM and AMPSA using PEG as macroinitiator in presence of NMBA as cross-linking agent in water at 25 °C. The measured amount of N-isopropylacrylamide, 2-acrylamido-2-methylpropane sulphonic acid and NMBA as cross-linker were dissolved in distilled water. The monomer molar ratio of NIPAM and AMPSA were taken as 100/0, 92/2, 96/4, 94/6 and 90/10 in the distilled water with the total amount of monomers 1.5 g. In this solution a macroinitiator solution prepared by the mixing of 10 ml solution of PEG (1 g) in water and 4 ml of 0.1 M (NH4)2Ce(NO3)6 solution was added and stirred for 5 min. Total volume of the solution was kept 25 ml. The solution was then transferred to a 12 cm long glass tube with 1.5 cm diameter and sealed the tube with rubber caps. The polymerization was carried out at 25°C for 24 h under nitrogen atmosphere. After polymerization the hydrogels were cut into 2 cm length and put in distilled water. The hydrogels were held in water at room temperature for 2 days and the water was replaced with fresh water to remove unreacted monomers. Swollen hydrogels were dried in oven at 50°C and these dried hydrogel were used for experiments.
Fourier transform infrared spectroscopy (FTIR) measurements
The dried gel was ground to a suitable size of powder and was then pressed into a tablet with potassium bromide. The structural characterization of the samples was carried out on Fourier-transform infrared spectrophotometer model FTIR-8700 SHIMADZU Japan in the range of 4400–400 cm-1.
Swelling properties measurements
The dried hydrogels were left to swell in distilled water at different temperature for 24 h in a constant temperature water bath. Swollen gels were removed from swelling medium at regular interval of time and dried superficially with filter paper, weight and placed in the same medium. The measurements were continued until a constant weight was reached for each sample. The average value of three measurements was taken for every sample. The equilibrium swelling ratio (Qv) and the volume fraction of the polymer network in the swollen gel at the equilibrium state (V2m) were calculated as follows:
Where m s and m d are the mass of the swollen hydrogel at the equilibrium state and dry gel, ρ 2 and ρ 1 are densities of dry gel and solvent respectively. The density of dry gel was determined by a Pycnometer using nonsolvent and the values of ρ 2, ρ 1 were 1.268 and 1.0 g/mL respectively.
Swelling and deswelling kinetics measurement
To study the swelling kinetics, dried hydrogels were left to swell in distilled water at 20 °C for 24 h in a constant temperature water bath. The weight changes of hydrogels were recorded at regular time intervals. The swelling ratio (SR) is defined as:
Where w t and w d are the weight of swollen and dry hydrogels at time‘t’ respectively.
To study the deswelling kinetics, equilibrated swollen hydrogels in distilled water at 20 °C were transferred into distilled water at 60°C. The weight changes of hydrogels were recoreded at regular time intervals. Water retention (WR) % is defined as:
Where W e W t and W d are the weight of equilibrated swollen hydrogel at 20°C, at regular time intervals and the dry hydrogel respectively.
Morphological analysis
The surface morphology of the dried hydrogels was studied by using scanning electron microscope (LEO 435 VP, Zeiss, Germany) operated at 15 kV. Coating was carried out under reduced pressure in an inert argon gas atmosphere (Agar Sputter Coater P7340).
Results and discussions
FTIR analysis
FTIR spectra of the homopolymer of NIPAM, PEG-b-NIPAM and PEG-b- Poly (NIPAM-co-AMPSA) hydrogels samples are shown in Fig. 1. In the PEG-NIPAM spectrum, the broad peak at 3433 cm-1 is for overlapping of stretching peaks of –N-H from PNIPAM and -O-H from PEG [23]. The band at 1651 cm-1, 1543 cm-1 and 1375 cm-1 are assigned to amide I, amide II, and methyl group in CH (CH3)2 respectively, the band at 1109 cm-1 is for C-O stretch of the PEG unit. All the bands mentioned above are found in the spectrum of PEG-b-Poly (NIPAM-AMPSA) copolymer with addition of significant band at 1041 cm-1 assign to SO3 symmetric stretch of AMPSA units. The characteristic peaks of NIPAM and AMPSA monomer at 1620 cm-1 is for C = C and 1410 cm-1 for CH2 = disappeared for all the spectrums [24].
Temperature responsive characteristics of hydrogels
The temperature dependence of equilibrium swelling ratio of the hydrogels with different AMPSA content in water at the range of 20-60 °C is shown in Fig. 2a. The
LCST or volume phase transition of hydrogel occurs continuously or discontinuously according to copolymer composition and structure. It can be defined as sharp change in the swelling ratio with respect to change in temperature [25, 26]. The hydrogels exhibit change in water content as a function of temperature with respect to AMPSA content in the feed composition. The water content inside the hydrogel decreases slowly above the LCST of PNIPAM due to hydrophobic interaction of PNIPAM chain present in the hydrogels. The result shows that an increase in AMPSA content in the feed composition the Qv value of the hydrogel increases, which is due to the increase in electrostatic repulsion between the same charged chain, results in the expansion of the network structure. Due to presence of negatively charged AMPSA comonomer in the polymerization system, there is strong electrostatic repulsion among the sulfonate anion (− SO -3 ) results in the expansion of network structure of hydrogels and have high water absorption properties. The equilibrium swelling ratio and gel transition temperature of the copolymer gel increase with increasing AMPSA content in the feed composition. A computer programme was used to determine the volume phase transition temperature of hydrogels differentiating the equilibrium swelling ratio at different temperature as shown in Fig. 2b. The volume phase transition temperature (VPT-T) of hydrogels are indicated at the mininum of the curves. By the induction of AMPSA, the volume phase transition temperature of hydrogels increased from 36 to 40 and 45 °C for HG-102 & HG-106 respectively where as for HG-110 thermosensitivity behaviour is weak and volume phase transition temperature increased to more than 65 °C.
Further, the Qv values of the hydrogel are affected by change in temperature in the surrounding medium. At lower temperature i.e. below LCST of PNIPAM the hydrogel swells due to the formation of intermolecular hydrogen bond with surrounding water molecules and shrinks at higher temperature i.e. above LCST of PNIPAM due to breakage of hydrogen bond and hydrophobic interaction. The amount of water content inside the hydrogel decreases slowly during shrinkage and the rate of water release can be controlled incorporating hydrophilic or ionic comonomer in the copolymer. The result shows that the temperature sensitive property of hydrogels is more prominent for the hydrogel having lower than 6 mol% of AMPSA in the feed composition. The swelling and shrinkage state of hydrogels are shown schematically in Fig. 3.
Swelling and deswelling kinetics of hydrogel
The swelling kinetics of the hydrogels was measured gravimetrically at definite time intervals. Figure 4 shows the swelling kinetics for hydrogels with different mole percent of AMPSA in the feed composition. The results show that both the swelling ratio and rate of swelling increased with increasing AMPSA content in the hydrogels. The hydrogel containing high amount of AMPSA absorbed higher amount of water due to increase in hydrophilicity.
The Fig. 5 shows the rate of shrinkage of hydrogels with different composition at 60 °C. The data shows that HG-100 and HG-102 exhibit faster rate of shrinkage compared to HG-106 and HG-110. Within 20–25 min HG-100 and HG-102 release 90 % and 76 % of water respectively whereas HG-106 and HG-110 release 75 & 15 % of water in 90 min. The macroporous structure and temperature sensitivity property influence on the rate of shrinkage characteristics of the hydrogel. The macroporous structure provides more channels for water to diffuse out from swollen hydrogels and the hydrophobicity behavior of PNIPAM above LCST favors the shrinkage process [27].
SEM analysis of hydrogels
The SEM micrographs in Fig. 6 Show that the surface morphology of PEG-b-Poly (NIPAM-co-AMPSA) gels prepared at 25°C markedly changes with AMPS contents in the feed composition. The roughness and porosity on the surface of the gels decreases as it have high water retention properties. The roughness and pore size decrease due to intramolecular interaction between AMPSA and PEG present in the hydrogels. Therefore, hydrogels display swelling and shrinkage behavior due to presence of pores in the gels and water molecules can easily diffuse in or out of the samples. This behavior is predominant for the sample having AMPSA content in the feed composition less than 6 mol%.
Network structure analysis
For the characterization of the network structure, the equilibrium-swelling ratio of ionic hydrogel was evaluated to determine the molecular weight between cross-links (\( {\bar{M}_c} \)). The swelling experiments were done gravimetrically until a constant swelling value was attained for the samples. The volume fraction of polymer (V2m) and the equilibrium volume swelling (Qv) were calculated by Eqs. 1 and 2. The Flory-Rehner equation [27] of swelling equilibrium for ionic hydrogel is used for calculation of \( {\bar{M}_{cE}} \).
Where V 2m is the volume fraction of the polymer in the equilibrium swollen hydrogel; χ is the polymer-solvent interaction parameter; ρ is the density of the polymer network; V 2r is the volume fraction of the polymer network after preparation; V 1 and \( {\bar{V}_r} \) are the molar volume of the solvent and polymer repeating units respectively; f i is the molar fraction of charged units in the network.
The Eq. 4 is simplified to a linear equation like,
The swelling data of hydrogels are used to plot the graph between A and B from Eq. 5 (Fig. 7). The \( \overline {{M_{cE}}} \) and χ values are obtained from the slope and intercept of straight line are listed in Table 1.
The number of segments between the crosslinked points (N) were calculated as [28]:
Where v1 is the molar volume of a segment, which is taken as the molar volume of water (18 mL/mol).
It is assumed that all crosslinker molecules (MBA) participate in forming effective crosslins during preparation of hydrogels. The theoritical \( \overline {{M_c}} \) value of hydrogels were calculated as [29]:
Where X is the crosslinker ratio i.e. mole of crosslinker/mole of monomers.
The results obtained show that the \( \overline {{M_{cE}}} \) and χ values are affected by the amount of AMPSA present in hydrogels. An increasing mole fraction of AMPSA in the feed composition, the \( \overline {{M_{cE}}} \) and N values decrease i.e. cross-links density increases as shown in Fig. 8. This behavior can be explained as ion pairs attract each other due to dipole-dipole interaction and physical cross-links in the hydrogels thus, cross-link density increases. The behavior is simillar to results reported elsewhere by different authors [30, 31]. The experimental results are supported by the decrease in pore size of hydrogels with increasing AMPSA content in the feed composition as shown in SEM micrograph Fig. 6. The theoritical \( \overline {{M_{cE}}} \) values for hydrogels HG-100 & HG-102 are higher than their experimental values, which indicate that the crosslinker are wested during polymerzation reaction. Figure 9 is the schematic representation of cross-linked hydrogels (a) is the lightly cross-linked (b) is the highly cross-linked.
Polymer-solvent interaction parameter
The polymer-solvent interaction parameter (χ) is one of the basic structural parameter of hydrogels. This parameter depends on various factors i.e. temperature, nature of polymer chains and solvent. The temperature dependence polymer-solvent interaction parameter (χ) can be calculated from the Flory-Rehner equation by using swelling data of hydrogels at different temperatures and expressed as a series expansion in power of the cross-linked polymer volume fraction (V 2m ) .
Where the coefficient χ1χ2 and χ 2 are the function of temperature and the molecular characteristics of polymer-solvent system. At low temperature that is for high swelling ratio χ is reduce to χ1 and χ1 is given by equation as [32].
Where ΔG, ΔH, ΔS are the change in the free energy, enthalpy and entropy of hydrogels in water.
The variation of χ parameter as a function of 1/T for different hydrogels is shown in Fig. 10. The χ values of all hydrogels at low temperature are located around 0.5. This assumes that the hydration of hydrogels depends on the hydrogen bonding interaction between water molecules and amide groups, thus the enthalpy and entropy contribution to χ parameter is constant.
The values of ΔH and ΔS appearing in the χ1 parameter were calculated from the slope and intercept of straight line obtained between χ vs 1/T at low temperature for all hydrogels. The values of ΔH and ΔS are given in Table 1. The negative values of enthalpy and entropy of hydrogels characterized that the polymer solvent system possesses the lower critical solution temperature (LCST) [33]. The value of enthalpy decreases with increasing AMPSA content in the feed composition where as entropy values slightly decrease and free energy values are increased. Thus, the enthalpy is the factor that controlled the temperature sensitivity of hydrogels.
Conclusions
The temperature sensitive characteristics of the aqueous solution of the PEG-b-(NIPAM-co-AMPSA) polymeric hydrogel were studied. The swelling ratio, volume transition temperature and breadth of the phase transition of PEG-b-NIPAM hydrogel are higher than the pure NIPAM hydrogel [13]. Further, the incorporation of hydrophilic AMPSA in the polymer chains has increased these properties significantly. The increasing in VPT-T was attributed to the discontinuous isopropyl amide pendant groups of P (NIPAM-co-AMPS) backbone in the hydrogel matrix. This effect is important in potential applications such as biomedical devices and in reversible systems. The experimental swelling data of hydrogels at different temperatures in water is in good agreement with the modified Flory–Rehner approach based on the affine network model. The \( {\overline M_c} \) and N values decrease i.e. cross-links density increases with increasing AMPSA content in the feed composition of the hydrogels and this result is supported by decrease in pore size of hydrgels in the SEM images. In addition, this approach signifies that the sensitive dependence of the χ parameter on both the polymer volume fraction and temperature is taken into account.
References
Saunders BR, Daly NLE, Hu STX, Stepto R (2009) Adv Colloid Interface Sci 147–148:251–262
Kasgöz H (2005) Colloids Surf A 266:44–50
Sannino A, Madaghiele M, Lionetto MG, Schettino T, Maffezzoli A (2006) J Appl Polym Sci 102:1524–1530
Chen J, Park H, Park K (1999) J Biomed Mater Res 44:53–62
Gemeinhart RA, Park H, Park K (2001) J Biomed Mater Res 55:54–62
Serra L, Doménech J, Peppas NA (2006) Biomaterials 27:5440–5451
Mullarney MP, Seery TAP, Weiss RA (2006) Polymer 47:3845–3855
Bajpai SK, Singh S (2006) React Funct Polym 66:431–440
Ngadaonye JI, Geever LM, Cloonan MO, Higginbotham CL (2011) J Polym Res 18:2307–2324
Nart Z, Kayaman-Apohan N (2011) J Polym Res 18:869–874
Couahlan DC, Quilty FP, Corrigan OI (2004) J Control Release 98:97–114
Acciaro R, Gilanyi T, Verga I (2011) Langmuir 27:7917–7925
Varaprasad K, Ravindra S, Narayana Reddy N, Vimala K, Mohana Raju K (2010) J Appl Polym Sci 116:3593–3602
Kabiri K, Zohuriaan-Mehr MJ, Mirzadeh H, Kheirabadi M (2010) J Polym Res 17:203–212
Zhang J, Chu LY, Cheng CJ, Mi DF, Zhou MY, Ju XJ (2008) Polymer 49:2595–2603
Kalagasidis-Krusic M, Ilic M, Filipovic J (2009) Polym Bull 63:197–211
Tasdelen B, Kayaman-Apohan N, Guven O, Baysal B (2005) Radiat Phys Chem 73:340–345
Erbil C, Yildiz Y, Uyanik N (2000) Polym Int 49:795–800
Bajpai AK, Shukla SK, Bhann S, Kankane S (2008) Prog Polym Sci 33:1088–1118
Turan EM, Demirci S, Caykara T (2008) J of Polym Sci: Part B: Poly Phy 46:1713–1724
Kalagasidis-Krusic M, Velickovic SJ, Griffiths PC, Filipovic J (2010) Polym Int 59:256–262
Kim KH, Kim J, Jo WH (2005) Polym Comm 46:2836–2840
Zhang C, Easteal AJ (2007) J Appl Polym Sci 104:1723–1731
Kim SY, Lee SC (2009) J Appl Polym Sci 133:3460–3469
Wu XS, Hoffmann AS, Paul Y (1992) J Appl Polym Sci 30:2121–2129
Emik S, Gurdag G (2006) J Appl Polym Sci 100:428–438
Zhang XZ, Yang YY, Chung TS, Ma KX (2001) Langmuir 17:6094–6100
Gundogan N, Melekaslan D, Okay O (2002) Macromolecules 35:5616–5622
Okay O, Sariisik SB, Zor SD (1998) J Appl Polym Sci 70:567–575
Turan E, Demirci S, Caykara T (2008) J Appl Polym Sci Part B: Polym Phy 46:1713–1723
Sen M, Sari M (2005) Euro Polym J 41:1304–1314
Erman B, Flory PJ (1986) Macromolecules 19:2342–2353
Jeria OM, Pizarro GDC, Marambio OG, Huerta M, Geckeler KE (2006) Appl Polym Sci 100:1735–1741
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saikia, A.K., Mandal, U.K. & Aggarwal, S. Network structure and temperature dependence swelling behavior of PEG-b-Poly (NIPAM-co-AMPSA) hydrogels in water. J Polym Res 19, 9871 (2012). https://doi.org/10.1007/s10965-012-9871-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-012-9871-z