Abstract
Pubertal development has been separately linked to adolescents’ sleep problems and larger family functioning, but research connecting these inter-related processes remains sparse. This study aimed to examine how pubertal status and tempo were related to early adolescents’ sleep and their family functioning. Using longitudinal data from the Adolescent Brain and Cognitive Development study, the study’s sample (N = 4682) was 49.2% female, was an average of 9.94 years old at baseline, and was 60.1% white. Analyses in the current study modeled the indirect associations between pubertal change and changes in family conflict via adolescent sleep duration and variability of duration. The results suggested that pubertal status and tempo predicted shorter adolescent sleep durations and greater variability in those durations, which predicted residual increases in family conflict. The findings highlight the role of adolescents’ pubertal changes in their sleep and how such changes can negatively affect family functioning.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Early adolescents’ development occurs within the larger family system, which offers the potential for their intraindividual changes to influence other aspects of the family. The physiological changes associated with puberty are documented to significantly impact adolescents’ sleep (Crowley et al., 2018). Specifically, pubertal change is accompanied by changes in adolescents’ circadian cycles (Dahl & Lewin, 2002) and sleep drive, or the pressure of needing to sleep that accumulates during the day to increase their ability to sleep at night (Jenni et al., 2005). Recent data suggest that over 70% of adolescents are getting deficient sleep (i.e., poor-quality or short-duration sleep; Wheaton & Claussen, 2021), and adolescents’ experiences of sleep problems put them at risk for deleterious outcomes, including mental, physical, and academic problems. Highlighted by Becker’s et al. (2015) biopsychosocial and contextual model of adolescent sleep, adolescents are at the center of an epidemic of inter-related processes that includes deficient sleep and rising rates of mental health problems (Gregory & Sadeh, 2016; Owens, 2014). However, adolescent sleep problems affect more than just adolescents, so understanding how their sleep is linked to other aspects of the family (e.g., family conflict; El-Sheikh & Kelly, 2017) remains critical to understanding this developmental period. Accordingly, the current study examined the process of how pubertal status and change (i.e., tempo) potentially influences larger family functioning via adolescents’ sleep. Adolescence is generally marked by a relative increase in conflict or tension during family interactions (Steinberg & Morris, 2001), so understanding the consequences of early adolescents’ sleep deficiency during puberty on family functioning might illuminate important avenues for intervention in families negatively impacted during this time.
Pubertal Development and Adolescent Sleep
During puberty, adrenal and gonadal changes are associated with co-occurring changes to other biological, psychological, and social systems for the individual (e.g., Becker et al., 2015). Puberty, however, is a highly individualized process, and understanding when a child begins puberty (i.e., status) as well as the pace at which they progress through this period of development (i.e., tempo) is critical (Mendle et al., 2019). Whereas initial pubertal status (also referred to as timing) indicates children’s pubertal stage at baseline, tempo requires longitudinal assessments (Mendle et al., 2019). Children who enter puberty early relative to their peers or for whom the rate of pubertal change is relatively rapid (i.e., fast tempo) may experience challenges due to their lack of readiness for such changes (Dorn & Biro, 2011). Although there is considerable interindividual variation regarding the age at which one begins puberty, there are numerous factors that have been shown to covary with pubertal development. For instance, research suggests that family socio-economic status and weight are important influences on pubertal onset. Specifically, youth, especially girls, from lower socioeconomic contexts (James-Todd et al., 2010) and obese and overweight (i.e., body mass index greater than 25) youth tend to start puberty earlier than their counterparts (Jasik & Lustig, 2008).
One well-documented change during the transition into puberty for early adolescents is the change to their sleep patterns, specifically to their chronotype (e.g., Jenni et al., 2005), which also serves as a critical covariate when analyzing adolescent sleep. Extensive research shows that adolescents’ circadian phases shift to later in the day (i.e., phase delay) as puberty progresses, which leads to adolescents’ biological predispositions to develop evening chronotypes, or a need to stay up later and to want to wake up later the next day (Carskadon et al., 1993, 1997). Within the context of environmental and social constraints (i.e., early school start times), these changes result in adolescents’ deficient sleep (Crowley et al., 2018). Explanations for this phase delay include the lengthening of the endogenous circadian period as well as a decrease in sensitivity to morning light and an increase in sensitivity to evening light, although these latter hypotheses have not been supported (Akacem et al., 2018; Crowley et al., 2018). Despite the lingering questions as to what is causing the phase delay, recent research suggests that adolescents have greater exposure to bright, phase-delaying light in the evening, which may interact with other bioregulatory and emotion regulation systems, thereby delaying sleep onset and ultimately resulting in shorter, more variable sleep (Crowley et al., 2018).
Early adolescents are not monoliths, and important differences emerge in studying puberty and sleep. Recent longitudinal studies have demonstrated that chronotype and sleep durations may interact with pubertal changes in unique ways for different populations of adolescents. For instance, in a study of 966 children over the course of seven years, researchers found that changes in girls’, but not boys’, circadian patterns (i.e., eveningness preference) were uniquely associated with their development of secondary sex characteristics, suggesting that sleep-related behaviors are robustly linked to pubertal change (Foley et al., 2018). It should be noted, however, that despite the longitudinal nature of the assessments, sleep was only assessed twice via self-reports during the study (Foley et al., 2018). Building on research suggesting that girls, particularly Black girls, evidenced earlier pubertal development (Marceau et al., 2011; Susman et al., 2010), researchers found in a sample of 1239 youth that clinician-reported early pubertal status (i.e., early breast or pubic hair development) specifically in Black girls, but not their faster tempo, predicted shorter self-reported sleep durations across their 8-year study (Hoyt et al., 2018). In a cross-sectional sample of twins (N = 596), more advanced pubertal development was associated with longer sleep durations specifically for Hispanic and white girls (Lecarie et al., 2022). Notable methodological differences between the two studies included the use of parent-reported pubertal development in addition to actigraphy-based sleep assessments (Lecarie et al., 2022). Although it is challenging to draw over-arching conclusions across these studies, it appears possible that gender and ethnic/racial differences exist in how and when pubertal changes are linked with changes in sleep.
Regardless of why or for whom the circadian phase shift occurs, adolescents’ sleep needs generally do not change across the period of pubertal development (Crowley et al., 2018). Although the majority of adolescents do not obtain the recommended 8–10 h of sleep per night, when the sleep opportunity exists, they tend to average approximately 9.25 h (Wheaton & Claussen, 2021). Furthermore, adolescents’ changing schedules and engagement in evening activities often results in greater sleep duration variability (Fuligni et al., 2018), which, in addition to insufficient sleep, has been shown to impair their psychological, physical, and academic functioning (Gregory & Sadeh, 2016; Shochat et al., 2014).
Pubertal Development and Family Functioning
The pubertal transition, especially for early maturing children, places early adolescents at risk for both behavioral and emotional problems (Ge et al., 2002; Hummel et al., 2013). The risk may be even greater for children when they are more biologically sensitive to their environments due to higher stress reactivity (Ellis et al., 2011). For example, children who are highly reactive to stress and who experience lower quality parent-child relationships in early childhood (e.g., ages 3–5) tend to enter puberty earlier and experience faster pubertal tempo (Ellis et al., 2011). However, the dynamic associations between the family context and pubertal timing and tempo have largely examined family context as a predictor of pubertal development. Despite the focus on pubertal development as an outcome, family systems theory provides an alternative framework for understanding these phenomena as it stresses the interdependent nature of subsystems within families in addition to the perturbations that reverberate throughout the family when conflict arises (Becker et al., 2015; Minuchin, 1985). In this light, the role of pubertal change as an evocative change agent within the family remains an important phenomenon to examine.
Early studies of pubertal change within the family context found that pubertal maturation was linked with increased emotional distance and conflict between children and their parents (Haynie, 2003; Steinberg, 1987). Subsequent research examining pubertal change has highlighted the family environment as a potential context of risk, which can also play a role in children’s development. Specifically, in studies of African American boys (Klopack et al., 2020a) and girls (Klopack et al., 2020b), earlier puberty prospectively predicted harsh parenting one to two years later, which subsequently predicted delinquent behavior. Although these studies did not include adolescent sleep within their models, the family environment acted as a catalyst for poor outcomes, specifically in children who experienced early puberty. Alternatively, researchers have investigated pubertal development as a predictor of shifts to evening chronotype in both cross-sectional (Díaz-Morales et al., 2014) and longitudinal (Díaz Morales et al., 2023) samples of children ages 12 and older. Their results suggest that pubertal development co-occurred with increases in family conflict, which ultimately led to changes toward evening chronotypes. However, this area of research has been limited by its reliance on self-reported sleep behaviors (i.e., chronotype) and did not assess sleep quantity or variability or the rate of pubertal change (Díaz Morales et al., 2023). Given the epidemic of deficient sleep for adolescents (Wheaton & Claussen, 2021), understanding the determinants of adolescent sleep and its role in the lives of adolescents and their families remains an important gap to be filled.
Adolescent Sleep and Family Functioning
Although family functioning has been shown to strongly predict adolescent sleep (El-Sheikh & Kelly, 2017; Peltz et al., 2019), the opposite direction of influence has received relatively little attention. Multiple studies highlight the diverse impact of family dysfunction on adolescent sleep disturbance, with stronger links emerging particularly in lower SES families (El-Sheikh & Kelly, 2017). For instance, not only has family chaos been shown to predict adolescent sleep problems via issues in adolescents’ sleep hygiene practices (Billows et al., 2009), but it has also been a key predictor of adolescents’ mental health problems through its impact on their sleep (Peltz et al., 2019). In contrast, positive and more organized family environments tend to ensure better sleep for adolescents. This is evidenced by studies documenting better sleep outcomes for adolescents when parents enforce bedtimes (Peltz et al., 2020; Short et al., 2011), establish pre-bedtime routines (Bartel et al., 2015), or engender more positive home environments (Cousins et al., 2007; Doane et al., 2019).
The notion that an individual’s behavior or functioning can influence the larger family environment is a hallmark of family systems theory and emblematic of the interdependence of subsystems within the family (Minuchin, 1985). The most extensive research done in this area has focused on infants’ sleep (Meltzer & Montgomery-Downs, 2011), but research on older children, especially those experiencing sleep disturbances (e.g., sleep-onset delay, insufficient sleep), has shown that children’s sleep can also evoke changes in parental functioning (Boergers et al., 2007; Meltzer & Mindell, 2007). For instance, children’s sleep problems (e.g., bedtime resistance, night wakings) have been linked to poorer maternal sleep quality, greater maternal and paternal daytime sleepiness, and higher levels of maternal depressive symptoms (Boergers et al., 2007; Meltzer & Mindell, 2007). Furthermore, there is some evidence to support reciprocal effects between children’s (ages 8–11) sleep and maternal parenting and child-parent conflict (Bell & Belsky, 2008). Although adolescents’ sleep problems tend to have multiple determinants, extensive research highlights puberty as a critical factor in influencing the development of sleep disturbances (Crowley et al., 2018). Despite the potential for diverse mechanisms from which pubertal change might affect family functioning (e.g., mood, autonomy-seeking), there is a paucity of research examining the role of sleep in this process.
Current Study
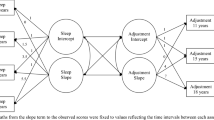
Pubertal development has been separately linked to adolescents’ sleep problems and larger family functioning, but research connecting these inter-related processes remains sparse. Understanding the processes through which pubertal development may impact families would thus provide a deeper understanding of both adolescent sleep and the context in which adolescents sleep. Accordingly, this study sought to examine the relative influence of pubertal change on family functioning via sleep duration/variability in early adolescence. To test these interrelations, the current study utilized path modeling (Fig. 1) in a sample of youth who took part in the Adolescent Brain and Cognitive Development (ABCD) study across the first three years of the study (i.e., baseline – age 10, 1-Year – age 11, and 2-Year follow-up – age 12). The model thus supported an examination of both the child’s initial pubertal status, in which development tends to occur earlier in girls, in addition to tempo (i.e., rate of change across three years) as predictors of both their sleep and family functioning. Given the significant links between children’s pubertal development and their age, sex, race, BMI, chronotype, and socio-economic status, the model included these constructs as covariates to adjust for their potential influence. Building on previous research, it was hypothesized that both more advanced pubertal status at baseline and higher rates of pubertal tempo would predict shorter sleep durations and greater variability in children’s sleep duration (Hypothesis 1); shorter sleep durations and greater variability would predict higher levels of family conflict (Hypothesis 2); and adolescent sleep would mediate the direct effect of pubertal status/tempo on family conflict (Hypothesis 3). Given the robust findings suggesting that pubertal development might be more pronounced in girls’ sleep, the current study explored this possibility through a secondary set of multi-group analyses comparing males to females.
Methods
Participants
The current sample of adolescents (N = 4682; 49.2% female) were part of the ABCD study (data release 4.0, 2021), who wore Fitbit Charge HR watches 24 months after baseline (Year 2; the only wave thus far to include objective sleep data). Out of the initial sample of 11,887 early adolescents at baseline, 5949 received and used Fitbit watches to record their sleep. Of this group, 4682 early adolescents with at least seven nights of recorded sleep (with a maximum of 31 nights) and who wore Fitbit on both weekdays and weekends were included in the current study. Data were accessed from the NIMH database, including baseline (T0; Mage = 9.94, SD = 0.62), Year 1 (T1; Mage = 10.98, SD = 0.66), and Year 2 (T2; Mage = 11.98, SD = 0.66) data. The analytic sample’s racial-ethnic composition was 60.1% white, 8.6% Black, 18.8% Hispanic, 2.4% Asian/Pacific Islander, and 10.0% Other. The families were economically diverse, with 13.7% reporting family incomes of <$35,000 and 13.4% with incomes of >$200,000. The demographic information of the analytic sample is similar to the full sample (N = 11,887), with the exception that the analytic sample had more white participants (60.1%) than the full sample (46.8%), demonstrated lower levels of pubertal development (measured with the Pubertal Development Scale, Petersen et al., 1988) over the course of the study (Baseline: Mnon = 8.4 vs. Manalytic = 8.3; Cohen’s d = 0.05; Year 1: Mnon = 9.2 vs. Manalytic = 9.1; Cohen’s d = 0.06; Year 2: Mnon = 10.7 vs. Manalytic = 10.5; Cohen’s d = 0.08), had slightly lower levels of family conflict study (Year 1: Mnon = 2.1 vs. Manalytic = 1.9; Cohen’s d = 0.11; Year 2: Mnon = 2.0 vs. Manalytic = 1.8; Cohen’s d = 0.11), had higher family income (Mnon = 6.9 vs. Manalytic = 7.7; Cohen’s d = 0.35) and parental education (Mnon = 3.6 vs. Manalytic = 4.0; Cohen’s d = 0.33), and were slightly older at baseline (Mnon = 9.90 vs. Manalytic = 9.94; Cohen’s d = 0.08).
Procedure
The ABCD study, which was launched in 2015 by the National Institutes of Health, is ongoing and originally aimed to recruit approximately 12,000 youth and their parents/guardians across 21 sites in the United States using multi-stage probability sampling (for a detailed sample description, see Garavan et al., 2018). Institutional review boards at participating universities approved all study procedures. Primary caregivers and youth provided consent to participate in the study. Participants had been selected to capture the variation in age, sex, ethnicity, socioeconomic status (household income), and urbanicity present in the U.S. population (retention strategies are detailed in Feldstein Ewing et al., 2018). Data from the ABCD study are publicly available and can be accessed via https://abcdstudy.org/scientists/data-sharing/. Based on best practices for capturing sleep via objective assessment (Acebo et al., 1999), the current study included children with a minimum of seven days of recorded sleep data.
Measures
Pubertal Development
To assess baseline pubertal status in addition to pubertal tempo (assessed at baseline, T1, and T2), children completed the 5-item Pubertal Development Scale (PDS; Petersen et al., 1988). The PDS produces a sex-normed composite score, which is composed of growth in height, growth of body hair, and skin changes for both boys and girls; breast growth and menstruation for girls; and deepening of voice and growth of facial hair for boys. Separate composite scores for adrenal and gonadal development were also formed based on a validated coding system (Shirtcliff et al., 2009). Scores on the PDS range from 5 to 20, with higher scores indicating more advanced physical/reproductive development. This extensively used measure has demonstrated high internal consistency and correlations with picture-based pubertal assessments, clinician-reported physical exams, and hormonal data (Shirtcliff et al., 2009).
Sleep
Both mean daily sleep duration and variability of daily sleep duration were assessed with the Fitbit Charge HR, which was worn daily on participants wrists across both week and weekend nights at T2 (range: 7–31 nights; M = 15.1, SD = 4.8). Fitbit Charge HR 2 devices use proprietary algorithms to continuously measure biobehavioral features at up to a 1-s sampling rate with photoplethysmography and an accelerometer to calculate sleep. Fitbit devices have been validated in developmental samples of children and adolescents with comparisons to polysomnography, the gold-standard in sleep assessment (Godino et al., 2020). Mean sleep duration represents the average total sleep time in minutes (i.e., wake-after-sleep-onset minutes removed) across all nights assessed, and variability of sleep duration (i.e., SD) refers to the nightly variation in total minutes slept across that same period.
Family Conflict
To assess family conflict, children completed the 9-item family conflict subscale from the Family Environment Scale (Moos & Moos, 1976) during the T1 and T2 assessments (r = 0.49, p < 0.001). These items assessed the level of conflict in the household (e.g., “We fight a lot in our family.”; “Family members often criticize each other.”; “Family members rarely become openly angry.” – reverse-scored), were rated on 2-point scales (True – 1, False – 0), and were summed so that higher scores indicated higher levels of conflict in the family environment (αT1 = 0.67; αT1 = 0.65).
Covariates
Six covariates were included in the model: child’s biological sex assigned at birth, family SES, race (white vs. non-white), child’s age at T2, BMI at T2, and chronotype at T2. Families’ SES was calculated by averaging the standardized values of total family annual income and caregivers’ years of education (r = 0.67, p < 0.001). Higher scores indicate higher family SES. Finally, chronotype was assessed using the 19-item Munich Chronotype Questionnaire, with higher values indicating greater preference for morning (Roenneberg et al., 2003).
Analytical Strategy
To examine whether sleep was a mediator in the association between pubertal status/tempo on residual change (T1 to T2) in family conflict, the current study employed path modeling in Mplus (v. 8.8; Muthén & Muthén, 2021) and used RMediation (Tofighi & Mackinnon, 2011), which employs bootstrapping to accommodate asymmetry in confidence intervals, to assess the significance of the indirect paths. Controlling for covariates, the mediational path model tested the hypotheses regarding the indirect effect of both initial (T0) pubertal status and pubertal tempo on residual changes in family conflict via children’s sleep duration and sleep duration variability. Based on the recommendations of Mendle and colleagues (2019), pubertal tempo was represented by the linear change of pubertal development ratings across three timepoints. To examine residual change over time in family conflict, the current study included T2 family conflict as the outcome within the model with T1 family conflict as one of its predictors. This modeling of the cross-lagged effect in family conflict allowed the pubertal variables and all control variables to predict residual change in family conflict. Finally, to assess differences between boys and girls, the current study included multigroup analyses and assessed significant differences via chi-square tests of difference.
Missing data ranged from 0.00 to 18.7% across all study variables. Little’s MCAR test was significant (χ2(181) = 508.3, p < 0.001), suggesting that data were not missing completely at random (MCAR). Thus, a correlation analysis was subsequently utilized to determine if missingness on modeled variables was related to observed data. Analyses were consistent with the assumption of data missing at random (Schafer & Graham, 2002). A full information maximum likelihood (FIML) algorithm was used to estimate missing data, and all study hypotheses were tested using maximum likelihood estimation with robust standard errors as it produces unbiased parameter estimates (Enders & Bandalos, 2001). Current best practices for determining model fit (e.g., Kline, 2011) suggest reporting multiple model fit indices. Overall model fit was assessed with the comparative fit index (CFI; Bentler, 1990; values above 0.90 indicating good fit), the root-mean-square error of approximation (RMSEA; Kline, 2011; values below 0.08 indicating good fit) and the standardized root-mean-square residual (SRMR; Hu & Bentler, 1999; values below 0.10 indicating good fit). Finally, non-independence of observations were expected due to clustering and stratification effects of participants within families and sites. Thus, the current analyses corrected for standard errors, and chi-square test of model fit using the Mplus STRATIFICATION and CLUSTER commands (Saragosa-Harris et al., 2022).
Results
Preliminary Analyses
Descriptive statistics for the sample and intercorrelations amongst the key variables are presented in Table 1. At baseline, 11.5% of boys and 10.6% of girls were considered “prepubertal” (i.e., average scores of 1 across the five indicators of the Pubertal Development Scale; Petersen et al. 1988). By the final wave of the current study (i.e., wave 2), 6.2% of boys and 1.3% of girls were reporting to be “prepubertal.” On average, children slept for 7 h and 26 min per night (SD = 37.5 min) across all nights assessed by the Fitbit, with weeknight sleep duration (M = 7 h and 25 min., SD = 39.6) being significantly shorter than weekend night (M = 7 h and 28 min., SD = 52.4), t(4638) = 3.51, p < 0.001, and with significantly less variability on weeknights (M = 63.3 min., SD = 33.0) compared to weekend nights (M = 66.6 min., SD = 43.1), t(4414) = 4.87, p < 0.001. Overall, the bivariate correlations were almost all significant and in the expected directions.
Primary Analyses
The path model demonstrated acceptable fit (X2(12) = 98.31; RMSEA = 0.04; CFI = 0.99; SRMR = 0.01). First, as shown in Table 2, more advanced initial pubertal status predicted both shorter sleep durations (β = −0.08, p < 0.001) and greater sleep variability (β = 0.12, p < 0.001). In addition, faster pubertal tempo predicted both shorter sleep durations (β = −0.10, p < 0.001) and greater sleep duration variability (β = 0.08, p < 0.001). Including the contribution of baseline predictors, this portion of the model explained approximately 12.6% of sleep duration and 9.1% of variability. Second, longer sleep duration predicted relative decreases in family conflict (β = −0.04, p < 0.05) and greater sleep variability predicted relative increases in family conflict (β = 0.07, p < 0.01) across a 1-year span (T1 to T2). Finally, controlling for the direct effects of pubertal status and tempo as well as the other variables in the model, children’s sleep duration partially mediated the effect of both initial pubertal status (indirect effect: B = 0.003, SE = 0.002; 95% Confidence Interval (CI) [0.001, 0.007]) and pubertal tempo (indirect effect: B = 0.004, SE = 0.002; 95% CI [0.001, 0.008]) on residual changes in family conflict. In addition, children’s sleep variability partially mediated the direct effect of both pubertal status (indirect effect: B = 0.006, SE = 0.002; 95% CI [0.002, 0.011]) and pubertal change (indirect effect: B = 0.004, SE = 0.002; 95% CI [0.001, 0.008]) on residual changes in family conflict. Although this model explained a relatively modest percentage of the variability in changes in family conflict (2.4%), taken together, these results suggest that pubertal status and tempo predict changes in the family environment partially via their association with children’s sleep duration and variability.
Secondary Analyses
To examine the possibility that children’s sex moderated the indirect effect of pubertal status/change on residual changes in family conflict via sleep duration/variability, the current study included a multigroup analysis comparing models across boys and girls (Table 2). Using chi-square tests of model difference, neither the individual paths nor the mediational paths emerged as significantly different across the groups. Despite previous research demonstrating differences between boys and girls in terms of pubertal development and its correlates (e.g., Foley et al., 2018), these results suggest that the processes through which pubertal development indirectly impacts family functioning through sleep remain largely the same across the two groups.
To ascertain if the models differed across mean sleep duration and sleep variability during the week or the weekend, the current study included separate models using data from either the weekday or the weekend (see Supplemental Table 1). The weekday model was virtually identical to the primary model, except that the indirect path linking initial pubertal status to family conflict via mean sleep duration was no longer significant (indirect effect: B = 0.003, SE = 0.002; ns). However, in the model using weekend sleep data, all mediation effects were non-significant except for the indirect path linking pubertal tempo to family conflict via mean sleep duration (indirect effect: B = 0.004, SE = 0.002; 95% CI [0.001, 0.007]).
Finally, to assess if the models differed across adrenal or gonadal development, separate models using pubertal data based on adrenal or gonadal processes were created (Shirtcliff et al., 2009; see Supplemental Table 2). The model using gonadal data replicated the primary model and all the indirect paths emerged as significant. In the adrenal model, the indirect path linking pubertal tempo to family conflict via sleep variability was no longer significant (indirect effect: B = 0.002, SE = 0.002; ns), while the other three mediational pathways replicated the primary model.
Discussion
Research linking adolescents’ pubertal changes to both their own behavior and to processes within the larger family system is needed to provide a more detailed understanding of both adolescent sleep but the environment in which they sleep. Whereas research has demonstrated clear links in which the family system impacts adolescents’ sleep and pubertal development, less is known about the capacity for adolescents’ intraindividual changes (i.e., puberty) to influence the aspects of their environment. The current study, therefore, sought to examine the potential links between early adolescents’ pubertal change and family functioning via their sleep. As depicted by the model and results, children’s pubertal development appears to affect family functioning partially via their sleep. These findings elucidate associations between these inter-related constructs (i.e., puberty, sleep, family)—seldomly examined within the same model—and provide a more holistic picture of early adolescents within a critical developmental context (i.e., the family). These findings thus speak to the evocative nature of pubertal change within the family by demonstrating that biological changes within the adolescent (i.e., puberty) and their consequences for sleep have the potential to influence other aspects of the family system.
Using a large, prospective, multi-wave design, the current study demonstrated that both adolescents’ sleep duration and variability in their sleep duration partially mediated the link between children’s baseline pubertal status (at approximately age 10) and their pubertal tempo across three years on residual changes in family conflict from age 11 to age 12. Results from the current study build on extensive research by not only demonstrating that adolescent sleep is influenced by pubertal change, but also that the influence of these developmental changes extends to the larger family system (Díaz Morales et al., 2023). Not only did more mature pubertal status at the start of the study predict shorter sleep duration and greater variability in sleep duration for early adolescents, but the faster pace of pubertal change (i.e., tempo) over three consecutive years was also associated with children’s reduced sleep duration and higher variability. Consistent with previous research in this area (e.g., Hoyt et al., 2018), the current findings further support the associations between pubertal change and sleep by incorporating an objective measurement of sleep (i.e., Fitbit). Furthermore, while most models within this area of research focus on family functioning as a predictor of children’s sleep (e.g., El-Sheikh & Kelly, 2017; Peltz et al., 2019), the model demonstrated that early adolescent sleep could also mediate the association between pubertal development and changes in family functioning.
The Evocative Nature of Pubertal Change
The association between pubertal change and changes in family conflict highlights the possibility that early adolescents’ intraindividual changes might provoke changes in the larger family system, a finding originally demonstrated in Steinberg (1987). Findings from the current study thus extend the research demonstrating that, consistent with younger children’s sleep behavior (e.g., infant sleep disturbance; McQuillan et al., 2019), adolescents are also active contributors to their home contexts and influence factors such as parental or family functioning. Consistent with previous research that demonstrated that daily increases in adolescents’ sleep disturbance predicted subsequent increases in parents’ psychological distress the next day (e.g., Peltz & Rogge, 2022), the current findings suggest that both adolescents’ sleep duration and the variability of that duration are strong predictors of higher levels of family conflict. This process is notable as pubertal development and its influence on adolescent sleep are natural and to be expected. However, connecting the biological changes inherent in puberty to a families’ larger functioning via adolescent sleep may not be obvious to parents. Our findings thus may have implications for families struggling with increasing conflict as their children enter adolescence. Specifically, they might add another potential avenue of intervention if they can effectively address their adolescent’s sleep needs. In this light, research has shown that parental enforcement of adolescents’ bedtimes can result in longer sleep durations for adolescents (Peltz et al., 2020). One challenge that remains, however, is that enforcing an adolescent’s bedtime can be a breeding ground for conflict, so parents and children must find ways to work collaboratively to establish effective sleep routines. Although the current study did not specifically examine the transactional nature of adolescent sleep and family functioning, considering research in younger children that has demonstrated bidirectionality with family processes (Peltz et al., 2019), it seems likely that proactively addressing adolescents’ sleep needs may yield more cohesive family interactions.
Previous research suggests that boys and girls experience different trajectories regarding pubertal change and sleep (e.g., Foley et al., 2018). Although girls typically enter puberty earlier than boys (e.g., Susman et al., 2010), boys and girls progress through puberty at different rates (Marceau et al., 2011). In addition, in girls, changes in bedtimes and waketimes, sleep duration, and chronotype (i.e., evening preference) have been shown to be specifically related to the development of secondary sex characteristics, such as earlier pubic hair development and faster breast development (Foley et al., 2018). As a result, the dynamics between adolescents’ sleep and pubertal development remain complex due to the multiple levels of change and the timeframes in which these changes occur. Previous research also suggests that the puberty-related changes in sleep, such as delayed sleep phase and disrupted sleep patterns, can appear prior to bodily changes associated with puberty (Sadeh et al., 2009). This suggests that sleep changes resulting from puberty might precede the physical development captured by pubertal development scales and thus necessitate biological assessments (e.g., hormone levels) to ascertain the direction of effects.
In light of the complexities in measuring pubertal development, it is important to acknowledge that a sizeable portion of the current study’s baseline sample—11.5% of boys and 10.6% of girls—were considered “prepubertal” at the start of the study. By the final wave of the current study (i.e., wave 2), 6.2% of boys and 1.3% of girls were reporting to be “prepubertal,” which highlights the considerable variability across the three years of pubertal assessment. Consistent with differing trajectories of pubertal development (Foley et al., 2018), girls in the current sample reported greater levels of physical development and greater variability at each of the three waves of the study period. In this light, it is noteworthy that the model did not demonstrate significant group differences when comparing boys to girls. It is possible that such differences were not as robust in the current study’s early adolescent sample (ages 10–12) due to the relatively short 3-year window of assessment. For instance, research has shown that significant associations between girls’, but not boys’, sleep changes and corresponding development of secondary sexual characteristics, as measured through Tanner staging, were demonstrated over a seven-year period (Foley et al., 2018). This study, however, did not measure sleep objectively (Foley et al., 2018), a relative strength of the current study. The timing of assessment may also have played a role. In contrast to the slightly older sample in the current study, gender differences were found in a cross-sectional study of early adolescents’ sleep in children (average age of 8.5 years) who had recently entered puberty (Lecarie et al., 2022), although none of the girls in this sample had started menstruation. Future studies, especially with the ABCD dataset that are released as participants mature, will provide greater clarity to the dynamic associations between puberty and sleep.
The current results suggest that early adolescent sleep partially mediated the link between puberty and family functioning. Despite the relatively small mediation effects, which may be a function of the size of ABCD’s dataset (Owens et al., 2021), the current findings highlight the possibility that early adolescents’ physical development and its association with their sleep might evoke changes in family relations. Such findings align with family systems theory, which suggests that changes in one family subsystem (i.e., adolescent functioning) have the potential to impact other areas of the family due to their interdependence (Minuchin, 1985). Family conflict tends to be multiply determined, but addressing adolescents’ sleep needs – much less the needs of all family members—should be considered as a useful point of intervention for reducing family conflict. In addition to the interdependent nature of subsystems within the family, family processes tend to unfold cyclically and generally emerge as transactional or bidirectional over time (Minuchin, 1985). Research has shown that in stressful family environments, higher levels of parental support are linked to longer sleep durations and less sleep variability for adolescents (Tsai et al., 2018). As such, it is likely that family members, especially adolescent-age children, who experience better sleep will have greater potential to effectively handle stressors and thus prevent more serious conflicts from impairing family functioning.
Limitations and Future Research Directions
Despite the strengths of the current study, which include a large, representative sample and objective sleep assessment, there are also several limitations. First, the questionnaire-based reports of pubertal development and family conflict were both self-reported by the child participants, which increases the potential for reporter bias and could inflate associations amongst variables due to shared method and reporter variance. With that said, large studies, such as the ABCD study, might have difficulty obtaining physician-based assessments of puberty or observational assessments of family functioning due to the size of the sample and the reluctance of children and families to participate in such assessment procedures. Second, the current study relied on pubertal assessments at only three timepoints. Despite its longitudinal measurement in the current study, pubertal change is not necessarily a linear process and that more measurement occasions of pubertal development might afford a more complex analysis of rates of change (i.e., quadratic change; Mendle et al., 2019). Finally, the current study only had access to one wave of objective sleep data, which precluded the examination of bidirectional relationships amongst the primary constructs. Research has shown that children’s sleep and family functioning have the potential to be bidirectionally related (El-Sheikh & Kelly, 2017; Peltz et al., 2016), and future studies should seek to extend the current analyses to ascertain the dynamic associations amongst sleep, pubertal change, and family functioning.
Although the current results are consistent with previous research, which demonstrated that changes in adolescents’ sleep patterns were associated with more advanced pubertal development and family conflict (Díaz Morales et al., 2023), future studies might consider including alternative pathways, such as children’s psychosocial functioning, to explain how pubertal change and adolescent sleep can impact families. For instance, previous studies have documented links between deviations in the timing and tempo of pubertal development and psychosocial problems (e.g., Beltz et al., 2014). Whereas pubertal timing and tempo have been less predictive of psychosocial functioning in boys, earlier pubertal timing and faster tempo show stronger links to internalizing symptoms in girls (Beltz et al., 2014; Marceau et al., 2011). Given the robust links between adolescent sleep and psychosocial functioning, it will be important for future research to develop more holistic models of adolescent development that can incorporate these diverse factors (Becker et al., 2015).
Conclusion
Adolescents’ pubertal changes, their sleep, and their family functioning are robustly linked, but research examining their associations as transactional processes remains sparse. Although the natural and expected biological changes to early adolescents’ sleep during puberty are well documented, the current study highlights the larger ramifications that these factors might have for family functioning. Specifically, adolescents who experienced a faster pace of pubertal change demonstrated shorter sleep durations and greater variability in their sleep durations, which ultimately predicted relative increases in family conflict across a 2-year period. Although some family conflict is to be expected as adolescents develop greater levels of autonomy and independence, early adolescents who experience high levels of sleep disturbance may be especially at risk for family dysfunction. In this light, families must take note that the individual changes that early adolescents go through during puberty are not theirs alone to bear.
References
Acebo, C., Sadeh, A., Seifer, R., Wolfson, A. R., Hafer, A., & Carskadon, M. A. (1999). Estimating sleep patterns with activity monitoring in children and adolescents: How many nights are necessary for reliable measures? Sleep, 22(1), 95–103. https://academic.oup.com/sleep/article/22/1/95/2731704.
Akacem, L. D., Wright, K. P., & LeBourgeois, M. K. (2018). Sensitivity of the circadian system to evening bright light in preschool-age children. Physiological Reports, 6(5), 1–10. https://doi.org/10.14814/phy2.13617.
Bartel, K. A., Gradisar, M., & Williamson, P. (2015). Protective and risk factors for adolescent sleep: A meta-analytic review. Sleep Medicine Reviews, 21, 72–85. https://doi.org/10.1016/j.smrv.2014.08.002.
Becker, S. P., Joshua, M. L., & Byars, K. C. (2015). Advancing a biopsychosocial and contextual model of sleep in adolescence: A review and introduction to the special issue. J Youth Adolescence, 44, 239–270. https://doi.org/10.1007/s10964-0140248-y.
Bell, B. G., & Belsky, J. (2008). Parents, parenting, and children’s sleep problems: Exploring reciprocal effects. British Journal of Developmental Psychology, 26(4), 579–593. https://doi.org/10.1348/026151008x285651.
Beltz, A. M., Corley, R. P., Bricker, J. B., Wadsworth, S. J., & Berenbaum, S. A. (2014). Modeling pubertal timing and tempo and examining links to behavior problems. Developmental Psychology, 50(12), 2715–2726. https://doi.org/10.1037/a0038096.
Bentler, P. M. (1990). Comparative fit indexes in structural models. Psychological Bulletin, 107(2), 238–246.
Billows, M., Gradisar, M., Dohnt, H., Johnston, A., McCappin, S., & Hudson, J. (2009). Family disorganization, sleep hygiene, and adolescent sleep disturbance. Journal of Clinical Child & Adolescent Psychology, 38(5), 745–752. https://doi.org/10.1080/15374410903103635.
Boergers, J., Hart, C., Owens, J. A., Streisand, R., & Spirito, A. (2007). Child sleep disorders: Associations with parental sleep duration and daytime sleepiness. Journal of Family Psychology, 21(1), 88–94. https://doi.org/10.1037/0893-3200.21.1.88.
Carskadon, M. A., Acebo, C., Richardson, G. S., Tate, B. A., & Seifer, R. (1997). An approach to studying circadian rhythms of adolescent humans. Journal of Biological Rhythms, 12, 278–289.
Carskadon, M. A., Vieira, C., & Acebo, C. (1993). Association between puberty and delayed phase preference. Sleep, 16(3), 258–262. https://academic.oup.com/sleep/article/16/3/258/2749376.
Cousins, J. C., Bootzin, R. R., Stevens, S. J., Ruiz, B. S., & Haynes, P. L. (2007). Parental involvement, psychological distress, and sleep: A preliminary examination in sleep-disturbed adolescents with a history of substance abuse. Journal of Family Psychology, 21(1), 104–113. https://doi.org/10.1037/0893-3200.21.1.104.
Crowley, S. J., Wolfson, A. R., Tarokh, L., & Carskadon, M. A. (2018). An update on adolescent sleep: New evidence informing the perfect storm model. Journal of Adolescence, 67(Apr), 55–65. https://doi.org/10.1016/j.adolescence.2018.06.001.
Dahl, R. E., & Lewin, D. S. (2002). Pathways to adolescent health: Sleep regulation and behavior. Journal of Adolescent Health, 31(6 SUPPL), 175–184. https://doi.org/10.1016/S1054-139X(02)00506-2.
Díaz Morales, J. F., Escribano, C., Puig-Navarro, Y., & Jankowski, K. S. (2023). Factors underpinning the shift to eveningness during early adolescence: Pubertal development and family conflicts. Journal of Youth and Adolescence, 52(3), 561–569. https://doi.org/10.1007/s10964-022-01708-z.
Díaz-Morales, J. F., Escribano, C., Jankowski, K. S., Vollmer, C., & Randler, C. (2014). Evening adolescents: The role of family relationships and pubertal development. Journal of Adolescence, 37(4), 425–432. https://doi.org/10.1016/j.adolescence.2014.03.001.
Doane, L. D., Breitenstein, R. S., Beekman, C., Clifford, S., Smith, T. J., & Lemery-Chalfant, K. (2019). Early life socioeconomic disparities in children’s sleep: The mediating role of the current home environment. Journal of Youth and Adolescence, 48(1), 56–70. https://doi.org/10.1007/s10964-018-0917-3.
Dorn, L. D., & Biro, F. M. (2011). Puberty and its measurement: A decade in review. Journal of Research on Adolescence, 21(1), 180–195. https://doi.org/10.1111/j.1532-7795.2010.00722.x.
Ellis, B. J., Shirtcliff, E. A., Boyce, W. T., Deardorff, J., & Essex, M. J. (2011). Quality of early family relationships and the timing and tempo of puberty: Effects depend on biological sensitivity to context. Development and Psychopathology, 23(1), 85–99. https://doi.org/10.1017/S0954579410000660.
El-Sheikh, M., & Kelly, R. J. (2017). Family functioning and children’s sleep. Child Development Perspectives, 11(4), 264–269. https://doi.org/10.1111/cdep.12243.
Enders, C. K., & Bandalos, D. L. (2001). The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Structural Equation Modeling, 8(3), 430–457. https://digitalcommons.unl.edu/edpsychpapers.
Feldstein Ewing, S. W., Chang, L., Cottler, L. B., Tapert, S. F., Dowling, G. J., & Brown, S. A. (2018). Approaching retention within the ABCD study. In Developmental cognitive neuroscience (Vol. 32, pp. 130–137). Elsevier Ltd. https://doi.org/10.1016/j.dcn.2017.11.004.
Foley, J. E., Ram, N., Susman, E. J., & Weinraub, M. (2018). Changes to sleep-wake behaviors are associated with trajectories of pubertal timing and tempo of secondary sex characteristics. Journal of Adolescence, 68, 171–186. https://doi.org/10.1016/j.adolescence.2018.07.017.
Fuligni, A. J., Arruda, E. H., Krull, J. L., & Gonzales, N. A. (2018). Adolescent sleep duration, variability, and peak levels of achievement and mental health. Child Development, 89(2), e18–e28. https://doi.org/10.1111/cdev.12729.
Garavan, H., Bartsch, H., Conway, K., Decastro, A., Goldstein, R. Z., Heeringa, S., Jernigan, T., Potter, A., Thompson, W., & Zahs, D. (2018). Recruiting the ABCD sample: Design considerations and procedures. Developmental Cognitive Neuroscience, 32, 16–22. https://doi.org/10.1016/j.dcn.2018.04.004.
Ge, X., Brody, G. H., Conger, R. D., Simons, R. L., & Murry, V. M. B. (2002). Contextual amplification of pubertal transition effects on deviant peer affiliation and externalizing behavior among African American children. Developmental Psychology, 38(1), 42–54. https://doi.org/10.1037/0012-1649.38.1.42.
Godino, J. G., Wing, D., de Zambotti, M., Baker, F. C., Bagot, K., Inkelis, S., Pautz, C., Higgins, M., Nichols, J., Brumback, T., Chevance, G., Colrain, I. M., Patrick, K., & Tapert, S. F. (2020). Performance of a commercial multi-sensor wearable (Fitbit Charge HR) in measuring physical activity and sleep in healthy children. PLoS One, 15(9), 1–16. https://doi.org/10.1371/journal.pone.0237719.
Gregory, A. M., & Sadeh, A. (2016). Annual Research Review: Sleep problems in childhood psychiatric disorders—A review of the latest science. Journal of Child Psychology and Psychiatry and Allied Disciplines, 57(3), 296–317. https://doi.org/10.1111/jcpp.12469.
Haynie, D. L. (2003). Contexts of risk? Explaining the link between girls’ pubertal development and their delinquency involvement. Social Forces, 82(1), 355–397. https://about.jstor.org/terms.
Hoyt, L. T., Deardorff, J., Marceau, K., Laurent, C. A., Windham, G. C., Greenspan, L. C., Pinney, S. M., Teitelbaum, S., Grimm, K. J., Hagan, M. J., Biro, F. M., Wolff, M. S., Kushi, L. H., & Hiatt, R. A. (2018). Girls’ sleep trajectories across the pubertal transition: Emerging racial/ethnic differences. Journal of Adolescent Health, 62(4), 496–503. https://doi.org/10.1016/j.jadohealth.2017.10.014.
Hu, L., & Bentler, P. M. (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal., 6(1), 1–55.
Hummel, A., Shelton, K. H., Heron, J., Moore, L., & van den Bree, M. B. M. (2013). A systematic review of the relationships between family functioning, pubertal timing and adolescent substance use. Addiction (Vol. 108, Issue 3, pp. 487–496). https://doi.org/10.1111/add.12055.
James-Todd, T., Tehranifar, P., Rich-Edwards, J., Titievsky, L., & Terry, M. B. (2010). The impact of socioeconomic status across early life on age at menarche among a racially diverse population of girls. Annals of Epidemiology, 20(11), 836–842. https://doi.org/10.1016/j.annepidem.2010.08.006.
Jasik, C. B., & Lustig, R. H. (2008). Adolescent obesity and puberty: The “perfect storm. Annals of the New York Academy of Sciences, 1135, 265–279. https://doi.org/10.1196/annals.1429.009.
Jenni, O. G., Achermann, P., & Carskadon, M. A. (2005). Homeostatic sleep regulation in adolescents. Sleep, 28(11), 1146–1454.
Kline, R. B. (2011). Principles and practice of structural equation modeling (3rd ed.). New York, NY, The Guilford Press.
Klopack, E. T., Sutton, T. E., Simons, R. L., & Simons, L. G. (2020a). Disentangling the effects of boys’ pubertal timing: The importance of social context. Journal of Youth and Adolescence, 49(7), 1393–1405. https://doi.org/10.1007/s10964-019-01141-9.
Klopack, E. T., Simons, R. L., & Simons, L. G. (2020b). Puberty and girls’ delinquency: A test of competing models explaining the relationship between pubertal development and delinquent behavior. Justice Quarterly, 37(1), 25–52. https://doi.org/10.1080/07418825.2018.1472291.
Lecarie, E. K., Doane, L. D., Clifford, S., & Lemery-Chalfant, K. (2022). The onset of pubertal development and actigraphy-assessed sleep during middle childhood: Racial, gender, and genetic effects. Sleep Health, 8(2), 208–215. https://doi.org/10.1016/j.sleh.2021.12.006.
Marceau, K., Ram, N., Houts, R. M., Grimm, K. J., & Susman, E. J. (2011). Individual differences in boys’ and girls’ timing and tempo of puberty: Modeling development with nonlinear growth models. Developmental Psychology, 47(5), 1389–1409. https://doi.org/10.1037/a0023838.
McQuillan, M. E., Bates, J. E., Staples, A. D., & Deater-Deckard, K. (2019). Maternal stress, sleep, and parenting. Journal of Family Psychology, 33(3), 349–359. https://doi.org/10.1037/fam0000516.
Meltzer, L. J., & Mindell, J. A. (2007). Relationship between child sleep disturbances and maternal sleep, mood, and parenting stress: A pilot study. Journal of Family Psychology, 21(1), 67–73. https://doi.org/10.1037/0893-3200.21.1.67.
Meltzer, L. J., & Montgomery-Downs, H. E. (2011). Sleep in the family. Pediatric Clinics of North America, 58(3), 765–774. https://doi.org/10.1016/j.pcl.2011.03.010.
Mendle, J., Beltz, A. M., Carter, R., & Dorn, L. D. (2019). Understanding puberty and its measurement: Ideas for research in a new generation. Journal of Research on Adolescence, 29(1), 82–95. https://doi.org/10.1111/jora.12371.
Minuchin, P. (1985). Families and individual development: Provocations from the field of family therapy. Child Development, 56(2), 289–302. https://doi.org/10.2307/1129720.
Moos, R. H., & Moos, B. S. (1976). A typology of family social environments. Family Process, 15(4), 357–371.
Muthén, L. K., & Muthén, B. O. (2021). Mplus user’s guide. Los Angeles, CA, Muthén & Muthén.
Owens, J. (2014). Insufficient sleep in adolescents and young adults: An update on causes and consequences. Pediatrics, 134(3), e921–e932. https://doi.org/10.1542/peds.2014-1696.
Owens, M. M., Potter, A., Hyatt, C. S., Albaugh, M., Thompson, W. K., Jernigan, T., Yuan, D., Hahn, S., Allgaier, N., & Garavan, H. (2021). Recalibrating expectations about effect size: A multi-method survey of effect sizes in the ABCD study. PLoS ONE, 16(Sept), 1–13. https://doi.org/10.1371/journal.pone.0257535.
Peltz, J., & Rogge, R. (2022). Adolescent and parent sleep quality mediates the impact of family processes on family members’ psychological distress. Sleep Health, 8(1), 73–81. https://doi.org/10.1016/j.sleh.2021.08.009.
Peltz, J., Rogge, R., & O’Connor, T. (2019). Adolescent sleep quality mediates family chaos and adolescent mental health: A daily diary-based study. Journal of Family Psychology, 33(3), 259–569.
Peltz, J. S., Rogge, R. D., & Connolly, H. (2020). Parents still matter: The influence of parental enforcement of bedtime on adolescents’ depressive symptoms. Sleep, 43(5), 1–11. https://doi.org/10.1093/sleep/zsz287.
Peltz, J. S., Rogge, R. D., Sturge-Apple, M. L., O’Connor, T. G., & Pigeon, W. R. (2016). Reciprocal influences among family processes and toddlers’ sleep problems. Journal of Family Psychology. https://doi.org/10.1037/fam0000202.
Petersen, A. C., Crockett, L., Richards, M., & Boxer, A. (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133.
Roenneberg, T., Wirz-Justice, A., & Merrow, M. (2003). Life between clocks: Daily temporal patterns of human chronotypes. Journal of Biological Rhythms, 18(1), 80–90. https://doi.org/10.1177/0748730402239679.
Sadeh, A., Dahl, R. E., Shahar, G., & Rosenblat-Stein, S. (2009). Sleep and the transition to adolescence: A longitudinal study. Sleep, 32(12), 1602–1609. https://doi.org/10.1093/sleep/32.12.1602.
Saragosa-Harris, N. M., Chaku, N., MacSweeney, N., Guazzelli Williamson, V., Scheuplein, M., Feola, B., Cardenas-Iniguez, C., Demir-Lira, E., McNeilly, E. A., Huffman, L. G., Whitmore, L., Michalska, K. J., Damme, K. S., Rakesh, D., & Mills, K. L. (2022). A practical guide for researchers and reviewers using the ABCD Study and other large longitudinal datasets. Developmental Cognitive Neuroscience, 55. https://doi.org/10.1016/j.dcn.2022.101115.
Schafer, J. L., & Graham, J. W. (2002). Missing data: Our view of the state of the art. Psychological Methods, 7(2), 147–177. https://doi.org/10.1037/1082-989X.7.2.147.
Shirtcliff, E. A., Dahl, R. E., & Pollak, S. D. (2009). Pubertal development: Correspondence between hormonal and physical development. Child Development, 80(2), 327–337. https://doi.org/10.1111/j.1467-8624.2009.01263.x.
Shochat, T., Cohen-Zion, M., & Tzischinsky, O. (2014). Functional consequences of inadequate sleep in adolescents: a systematic review. Sleep Medicine Reviews, 18(1), 75–87. https://doi.org/10.1016/j.smrv.2013.03.005.
Short, M. A., Gradisar, M., Wright, H., Lack, L. C., Dohnt, H., & Carskadon, M. A. (2011). Time for bed: Parent-set bedtimes associated with improved sleep and daytime functioning in adolescents. Sleep, 34(6), 797–800. https://doi.org/10.5665/sleep.1052.
Steinberg, L. (1987). Impact of puberty on family relations: Effects of pubertal status and pubertal timing. Developmental Psychology, 23(3), 451–460.
Steinberg, L., & Morris, A. S. (2001). Adolescent development. Annual Review of Psychology, 52, 83–110.
Susman, E. J., Houts, R. M., Steinberg, L., Belsky, J., Cauffman, E., Dehart, G., Friedman, S. L., Glenn, Roisman, I., & Halpern-Felsher, B. L. (2010). Longitudinal development of secondary sexual characteristics in girls and boys between ages 9 1 ⁄2 and 15 1 ⁄2 years. Arch Pediatr Adolesc Med, 164(2), 166–173.
Tofighi, D., & Mackinnon, D. P. (2011). RMediation: An R package for mediation analysis confidence intervals. Behavior Research Methods2, 43, 692–700.
Tsai, K. M., Dahl, R. E., Irwin, M. R., Bower, J. E., McCreath, H., Seeman, T. E., Almeida, D. M., & Fuligni, A. J. (2018). The roles of parental support and family stress in adolescent sleep. Child Development, 89(5), 1577–1588. https://doi.org/10.1111/cdev.12917.
Wheaton, A. G., & Claussen, A. H. (2021). Short sleep duration among infants, children, and adolescents aged 4 months–17 years— United States, 2016–2018. MMWR. Morbidity and Mortality Weekly Report, 70(38), 1315–1321. https://doi.org/10.15585/mmwr.mm7038a1.
Acknowledgements
The authors wish to express their gratitude to all of the children and their families who are participating in the ABCD study. We would also like to thank all of the participating institutions that have conducted the ABCD data collections and the National Institutes for Health for providing access to these data. We would also like to acknowledge NIH/NiDA for their support of this project (R01DA058334 & R01DA055630; PI-Oshri; P50 DA051361-01; Co-PI-Oshri).
Author information
Authors and Affiliations
Contributions
J.P. conceived of the study, managed the data, conducted the analyses, performed the statistical analyses, and drafted the manuscript; L.Z. provided support with the analyses and helped to draft a manuscript; J.S. helped to draft the manuscript; A.O. provided support with the analyses and helped to draft the manuscript; L.D. provided support with the analyses and helped to draft the manuscript. All authors read and approved the final manuscript.
Data Sharing and Declaration
The datasets generated and/or analyzed during the current study are available in the ABCD Data repository: https://nda.nih.gov/abcd/.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Ethical Approval
The study was approved by the ethics committee of the State University of New York at Brockport. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Peltz, J., Zhang, L., Sasser, J. et al. The Influence of Pubertal Development on Early Adolescent Sleep and Changes in Family Functioning. J. Youth Adolescence 53, 459–471 (2024). https://doi.org/10.1007/s10964-023-01882-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10964-023-01882-8