Abstract
Equations set up for the transfer of neutral solutes from water to water–ethanol mixtures can also be used to correlate the transfer of ions and ionic species, as log10 P, where P is the partition coefficient. Only two additional terms are required in the equations, one for anions and one for cations. The extended equations can fit log10 P values for anions and cations with a standard deviation of about 0.2 to 0.3 log units for transfer of 41 anions and cations from water to various water–ethanol mixtures from 10 % ethanol to 100 % ethanol by volume. The log10 P values for carboxylate anions and protonated amine cations as obtained from the variation of pK a with solvent are quite compatible with log10 P values for simple anions and cations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Properties of the water–ethanol solvent system have long been of interest. Bates [1] has reviewed acid–base properties and Hyne [2] has reviewed effects on reaction rates in this system. Effects on ionic mobilities have also been reviewed, [3] and properties of non-electrolytes [4] and electrolytes [5] in water–ethanol mixtures have been discussed. The transfer of ions from water to water–ethanol mixtures has been of particular interest and both Blandamer and co-workers [6] and Marcus [7] have set out lists of Gibbs energies of transfer of ions. Most of the data on transfers from water to water–ethanol mixtures have been obtained from the solubilities of electrolytes, as described by Marcus [7]. We have recently obtained descriptors for ions and ionic species, and have shown that it is possible to construct equations for the transfer of neutral molecules, ions and ionic species from water to a number of nonaqueous solvents [8–10]. We define ‘ionic species’ as anions derived from neutral carboxylic acids or phenols by removal of a proton, and cations derived from amines by addition of a proton. We have also set out equations for the transfer of non-electrolytes from water to water–ethanol mixtures [11], and in view of the interest in ions in water–ethanol mixtures, we wished to ascertain if it was possible to obtain equations for the transfer of non-electrolytes, ions and ionic species from water to water–ethanol mixtures. If so, then the coefficients in such equations might lead to additional insights into the nature of the water–ethanol mixtures.
2 Methodology
We start with our well-known equation for the partition of neutral molecules (non-electrolytes) from water to another solvent or solvent system,
In Eq. 1, the dependent variable is log10 P, where P is the water to solvent partition coefficient for a series of non-electrolytes in a given water to solvent system. The independent variables are descriptors as follows [12, 13]. E is the non-electrolyte (or solute) excess molar refractivity in units of (cm3⋅mol−1)/10, S is the solute dipolarity/ polarizability, A and B are the overall or summation solute hydrogen bond acidity and basicity, and V is the solute McGowan characteristic volume in units of (cm3⋅mol−1)/100. Equation 1 has been applied to the partition coefficients of non-electrolytes between water and a very large number of organic solvents [14], and has also been used indirectly to predict the solubility of solutes in a variety of solvents, including water and ethanol [14].
For partition of non-electrolytes from water to water–ethanol mixtures, we constructed [11] Eqs. 2–11. The numbers in parenthesis in the equations refer to the volume % ethanol in the solvent and these are the compositions of the water–solvent mixtures that we shall use throughout this work.










In order to include ions and ionic species in equations of the type of Eq. 1, we devised two additional descriptors, J + for anions and J − for cations, leading to the general equation, Eq. 12 where j + and j − are the corresponding coefficients.
Thus Eq. 2 can be transformed into Eq. 12 for the transfer from water to ethanol of ions and ionic species as well as non-electrolytes. Of course, this requires knowledge of partition coefficients of ions and ionic species.
The data used to obtain partition coefficients for ions were obtained from the compilations of Gibbs energies of transfer by Blandamer et al. [6] and by Marcus [7]. For ionic species, we used the pK a method described by us previously [8]. For anions derived from carboxylic acids, this involves use of Eq. 13 where P(A−) is the partition coefficient from water to a solvent of the carboxylate anion, P(HA) is the partition coefficient of the neutral carboxylic acid, P(H+) is the partition coefficient of the hydrogen ion, and pK a(aq) and pK a(s) are the pK a values of the carboxylic acid in water and the given solvent.
We devised a similar equation for the determination of partition coefficients of protonated amine cations,
In Eq. 14, BH+ refers to the protonated amine and B to the neutral amine. It is important to note that all values for single ions, including log10 P(H+) in Eqs. 13 and 14, have to be based on some particular convention. We used the convention that log10 P for Ph4As+ or Ph4P+=log10 P for Ph4B−, the so-called TATB convention employed by Blandamer et al. [6] and by Marcus [7].
3 Results and Discussion
The values of Gibbs energies of transfer of ions listed by Blandamer et al. [6] and by Marcus [7] are generally in agreement, and we used both sets of data to obtain ΔG ∘ and then log10 P on the molar scale at rounded-off volume % ethanol. An exception was Pr4N+ where the disagreement was so large that we did not use either set of data.
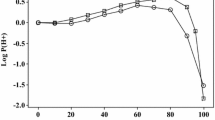
In order to obtain values for ionic species from Eqs. 13 and 14, we need values of log10 P(H+) and log10 P for the neutral species. Blandamer et al. [6] and Marcus [7] list values of the Gibbs energy of transfer of H+ at various water–solvent compositions. The two sets of data are in good agreement, as shown in Fig. 1, where we also show our interpolated values at rounded-off water–ethanol compositions. The actual values are in Table 1. As before [8], we calculated log10 P for transfer from water to water–ethanol mixtures for the neutral species from known descriptors and Eqs. 2–11.
Values of pK a for monocarboxylic acids in water–ethanol mixtures have been determined by Grunwald and Berkowitz [15], Spivey and Shedlovsky [16], Frohlinger et al. [17], Dash and Pattanaik [18], Azab et al. [19], and Thuaire [20]. For acetic acid, they are all in agreement [15–20] and we have used them to obtain values at rounded-off vol-% ethanol. There are less data for formic acid [15], propanoic acid [15, 18] and butanoic acid [15, 18]. For chloroacetic acid there are available date from Niazi [21] as well as from Grunwald and Berkowitz [15], and from Dash and Pattanaik [18].
Thuaire [20] has determined pK a values for a number of substituted benzoic acids; data are available for benzoic acid [15, 20, 22, 23] and for several substituted benzoic acids as well as phenylacetic acid [18]. We only studied acids for which pK a values could be obtained across the entire composition range, 0–100 %, and for which we had the necessary descriptors for the neutral and ionic forms. The exceptions were N-chloroacetamide and N-bromoacetamide for which Menard and Lessard [24] had measured pK a values from 0–80 %. We included these acids in order to ascertain if the present method was general enough to include nitrogen-acids as well as the usual oxygen-acids. The log10 P values obtained through Eq. 13 are in Table 2; values for simple anions are also given in Table 2. Details of the pK a values used, and the partition coefficients of the neutral species and the ionic species are given in Table S1 (see Electronic Supplementary Material).
There is much less data on pK a values of protonated amines in the water–ethanol system. Gutbbzahl and Grunwald [25] and also Reynaud [26] have provided most of the experimental data, but comparatively few amines were studied across the whole composition region. Oszczapowicz and Czuryłowska [27] have determined pK a values for protonated imidazole across the whole composition range, and fortunately, we have descriptors for the neutral and protonated forms [8]. Reynaud [26] measured pK a for pyridine and p-bromoaniline up to 50 % ethanol, and using pK a in ethanol of 4.00 and 4.50 respectively [28], we interpolated the intervening values. Values of log10 P for the protonated amines obtained through Eq. 14 are in Table 2. Details of the pK a values and partition coefficients of the neutral bases and the protonated bases are in Table S2 (see Electronic Supplementary Material).
We decided to use exactly the same set of anions and cations for all the water–solvent compositions, except for N-chloroacetamide and N-bromoacetamide in 100 % ethanol, as mentioned above. This meant the omission of a number of ions for which we did not have data across the whole composition range or which showed marked inconsistencies at various compositions. These included the perchlorate ion and the anions derived from propanoic and butanoic acid. We were left with 26 anions and 15 cations as shown in Table 2. The coefficients c–v in Eq. 12 are exactly the same as those in Eq. 1, and so log10 P values for the ions must fit Eq. 12 with only one extra term for the anions and one extra term for the cations. The descriptors for the anions and cations were taken as before [8], except that those for the anions of formic acid, N-chloroacetamide and N-bromoacetamide were calculated from the equations given in Ref. [8]. The full set of descriptors for anions and cations is in Table 3.
We have 15 cations and 26 anions that have to be fitted to Eqs. 2–11. Results are in Table 4 where we give the obtained values of j + and j − together with the statistics of the fit in terms of the standard deviation, SD, of the observed and fitted values. The standard deviations average around 0.2 to 0.3 log units which can be considered reasonable. For transfer from water to a number of pure solvents, similar SD values were obtained [8]. We can conclude that the general equation, Eq. 12, previously applied to transfers from water to pure solvents applies just as well to transfers from water to water–solvent mixtures. Note that the values of j + and j − for ethanol itself in Table 4 obtained with a total of 39 ions or ionic species are slightly different to those we found before [8], with a total of 19 ions or ionic species (j +=−3.170 and j −=3.085).
The log10 P values for the ionic species, viz the carboxylate anions and the protonated amine cations, are quite compatible with log10 P values for the simple ions listed in Table 3. Notable exceptions are the acetate anion for which the log10 P values from the compilations of Blandamer et al. [6] and of Marcus [7] are completely different from those we calculate by the pK a method. The data given by Marcus [7] are actually those of Blandamer et al. [6] and so we just compare values from the latter workers to those we obtained from the pK a method, see Fig. 2.
Comparison of log10 P values for the acetate ion. ■, Experimental values from solubilities [6]; ●, experimental values by the pK a method; ○, calculated values (this work)
There is the most remarkable disagreement between log10 P values from solubility measurements and those from the pK a method—even the sign of log10 P differs. No anion in Table 2 has a positive log10 P value for transfer to 100 % ethanol (except \(\mathrm{BPh}_{4}^{-}\)). The solubility measurements were made using potassium acetate [6]. In order to obtain log10 P for (K++ acetate−), it is essential that the same solid phase is in equilibrium with the saturated aqueous solution and the various saturated water–ethanol solutions. However, the solid phase in equilibrium with a saturated solution in water at 298 K is known [29] to have the composition 2CH3CO2K⋅3H2O, so that it is not surprising that solubility measurements on potassium acetate yield incorrect values of log10 P(K++ acetate−) and hence log10 P (acetate−) for transfer to water–ethanol mixtures.
The specific terms associated with partition of cations and anions are j + J + and j − J −. However, it must be noted that other terms make a contribution to the overall log10 P value. We can illustrate this by comparison of the partition of ionic species and the corresponding neutral species, term by term, giving transfers from water to ethanol as an example. In Table 5 we compare the benzoate anion with neutral benzoic acid. Although the j − J − term for the benzoate anion is very large, it is not as large numerically as the b B term that results from the extremely large hydrogen bond basicity (B=2.88) of the benzoate anion. In the case of the p-toluidine protonated cation, the j + J + term is about the same as the s S term. The a A term is not so important because the p-toluidinium cation is not such a very strong hydrogen bond acid (A=1.98).
For some of the protonated amines, the log10 P values exhibit a maxima against the volume % ethanol around 80 or 90 %, as shown in Fig. 3 for protonated p-toluidine. However, log10 P for protonated pyridine shows only a very small maximum at 50 % ethanol. In Fig. 3, the calculated log10 P values are also shown.
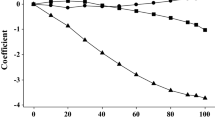
The two new solvent parameters are j + and j −. They vary quite regularly with solvent composition as is shown in Fig. 4, with a slight decrease in j − at 90 % ethanol. There have been numerous experimental studies of the variation of properties with solvent composition in the water–ethanol system. Excess enthalpies of mixing, velocity of sound, sound absorption coefficients, activation enthalpies for the t-butyl chloride solvolysis, viscosity, and the Walden product for the lithium ion show marked maxima or minima in the water-rich region, but vary smoothly with solvent composition in the ethanol-rich region [3, 4]. Indeed, the variation of log10 P for the hydrogen ion, Fig. 1, is the only property we have encountered with a pronounced maximum or minimum in the ethanol-rich region. Values of log10 P for the rather hydrophobic cations show weak maxima at various solvent compositions, for example Et4N+ at 50 %, anilineH+ at 70–80 % and 4-bromoanilineH+ at 90 %, but log10 P for the rather hydrophobic anions decreases regularly over the entire composition range, see Table 1. This is not reflected in the coefficients j + and j −; the former plot an almost perfect straight line, and the latter shows slight deviations from linearity against vol-% ethanol. As regards the additional parameters for cations and anions, so far we have been unable to connect j + and j − with other solvent properties; they remain empirical solvent parameters.
4 Conclusions
In conclusion, we have been able to show that our previous equations for the transfer of neutral molecules, ions and ionic species from water to nonaqueous solvents can be applied just as well to transfers from water to aqueous ethanol. This is the first time that a single equation has been applied for the transfer of neutral molecules and ions from water to any aqueous organic solvent. The two additional terms required for transfer of ions and ionic species vary quite regularly with solvent composition over the entire composition range. The equations that we have constructed allow for the first time predictions of log10 P for transfer of protonated amine cations and carboxylate anions from water to aqueous ethanol for numerous such species for which we have already determined the required solute parameters.
Electronic Supplementary Material
Tables S1 and S2 contain all the pK a values in the water–ethanol mixtures, partition coefficients for the neutral species and calculated partition coefficients for anionic and cationic species.
References
Bates, R.G.: Equilibrium properties of acids and bases in amphiprotic mixed solvents. In: Covington, A.K., Jones, P. (eds.) Hydrogen-Bonded Solvent Systems, pp. 49–86. Taylor and Francis, London (1968)
Hyne, J.B.: The effects of pressure and temperature on reaction rates in aqueous binary solvent systems. In: Covington, A.K., Jones, P. (eds.) Hydrogen-Bonded Solvent Systems, pp. 99–113. Taylor and Francis, London (1968)
Kay, R.L., Cunningham, G.P., Evans, D.F.: The effect of solvent structure on ionic mobilities in aqueous solvent mixtures. In: Covington, A.K., Jones, P. (eds.) Hydrogen-Bonded Solvent Systems, pp. 249–260. Taylor and Francis, London (1968)
Franks, F.: The properties of aqueous solutions of non-electrolytes. In: Franks, F. (ed.) Physico-Chemical Processes in Mixed Aqueous Solvents, pp. 50–70. Heineman, London (1967)
Feakins, D.: Ionic solvation in mixed aqueous solvents. In: Franks, F. (ed.) Physico-Chemical Processes in Mixed Aqueous Solvents, pp. 71–90. Heineman, London (1967)
Blandamer, M.J., Briggs, B., Burgess, J., Elvidge, D., Guardado, P., Hakin, A.W., Radulovic, S., Hubbard, C.D.: Transfer chemical potentials for ions, solubilities of salts and kinetics of reactions involving inorganic complex ions at ambient pressure and 298.2 K in binary aqueous mixtures containing ethanol and propan-2-ol. J. Chem. Soc. Faraday Trans. I 84, 2703–2721 (1988)
Marcus, Y.: Gibbs energies of transfer of anions from water to mixed aqueous organic solvents. Chem. Rev. 107, 3880–3897 (2007)
Abraham, M.H., Acree, W.E. Jr.: Equations for the transfer of neutral molecules and ionic species from water to organic phases. J. Org. Chem. 75, 1006–1015 (2010)
Abraham, M.H., Acree, W.E. Jr.: Solute descriptors for phenoxide anions and their use to establish correlations of rates of reaction of anions with iodomethane. J. Org. Chem. 75, 3021–3026 (2010)
Abraham, M.H., Acree, W.E. Jr.: The transfer of neutral molecules, ions and ionic species from water to ethylene glycol and to propylene carbonate; descriptors for pyridinium cations. New J. Chem. 34, 2298–2305 (2010)
Abraham, M.H., Acree, W.E. Jr.: Partition coefficients and solubilities of compounds in the water–ethanol solvent system. J. Solution Chem. 40(7), 1279–1290 (2011)
Abraham, M.H.: Scales of hydrogen bonding: their construction and application to physicochemical and biochemical processes. Chem. Soc. Rev. 22, 73–83 (1993)
Abraham, M.H., Ibrahim, A., Zissimos, A.M.: The determination of sets of solute descriptors from chromatographic measurements. J. Chromatogr. A 1037, 29–47 (2004)
Abraham, M.H., Smith, R.E., Luchtefeld, R., Boorem, A.J., Luo, R., Acree, W.E. Jr.: Prediction of solubility of drugs and other compounds in organic solvents. J. Pharm. Sci. 99, 1500–1515 (2010)
Grunwald, E., Berkowitz, B.: The measurement and correlation of acid dissociation constants for carboxylic acids in the system ethanol–water. Activity coefficients and empirical activity functions. J. Am. Chem. Soc. 73, 4939–4944 (1951)
Spivey, H.O., Shedlovsky, T.: Studies of electrical conductance in alcohol–water mixtures. II. The ionisation constant of acetic acid in ethanol water mixtures at 0, 25, and 35 °C. J. Phys. Chem. 71, 2171–2175 (1967)
Frohlinger, J.O., Gartska, R.A., Irwin, H.W., Steward, O.W.: Determination of ionization constants of monobasic acids in ethanol–water solvents by direct potentiometry. Anal. Chem. 40, 1408–1411 (1968)
Dash, U.N., Pattanaik, E.R.: Conductometric determination of dissociation constants of carboxylic acids in various mixed solvents. Egypt. J. Chem. 37, 17–24 (1994)
Azab, H.A., Ahmed, L.T., Mahmoud, M.R.: Potentiometric determination of the dissociation constants of some monocarboxylic acids in various hydroorganic mixtures. J. Chem. Eng. Data 40, 523–525 (1995)
Thuaire, R.: Sur la dissociation des acides benzoiques substitues dans les melange eau–ethanol. J. Chim. Phys. 67, 1076–1087 (1970)
Niazi, M.S.K.: Conductivities and thermodynamic dissociation constants for chloroacetic acid in binary mixed solvent systems at 298.15 K. J. Chem. Eng. Data 38, 527–530 (1993)
Rubino, J.T., Berryhill, W.S.: Effects of solvent polarity on the acid dissociation constants of benzoic acids. J. Pharm. Sci. 75, 182–186 (1986)
Sarmini, K., Kenndler, E.: Capillary zone electrophoresis in mixed aqueous-organic media: effect of organic solvents on actual ionic mobilities, acidity constants and separation selectivity of substituted aromatic acids. II. Ethanol. J. Chromatogr. A 811, 201–209 (1998)
Menard, H., Lessard, J.: Acidities of some N-haloamides (ZCONHX) in water and ethanol–water mixtures at 25 °C. J. Chem. Eng. Data 23, 64–65 (1978)
Gutbbzahl, B., Grunwald, E.: The effect of solvent on equilibria and rate constants. II. The measurement and correlation of acid dissociation constants of anilinium and ammonium salts in the system ethanol–water. J. Am. Chem. Soc. 75, 559–565 (1953)
Reynaud, R.: Etudes experimentale de l’ionisation de quelqes amines aromatiques ou heterocycliques et des acides acetique et benzoique dans dix families de solvants mixtes. Bull. Soc. Chim. Fr. 1967, 4597–4604 (1967)
Oszczapowicz, J., Czuryłowska, M.: The pK a values of the conjugate acid of imidazole in water–ethanol mixtures. Talanta 31, 559–560 (1984)
Bell, R.P.: The Proton in Chemistry, Methuen & Co. Ltd., London (1959)
Seidell, A.: In: Linke, W.F. (ed.) Solubilities of Inorganic and Metal–Organic Compounds, vol. II, 4th edn. American Chemical Society, Washington (1965)
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Below are the links to the electronic supplementary material.
10953_2012_9822_MOESM1_ESM.doc
Equations for the Partition of Neutral Molecules, Ions and Ionic Species from Water to Water–Ethanol Mixtures (DOC 607 kB)
Rights and permissions
About this article
Cite this article
Abraham, M.H., Acree, W.E. Equations for the Partition of Neutral Molecules, Ions and Ionic Species from Water to Water–Ethanol Mixtures. J Solution Chem 41, 730–740 (2012). https://doi.org/10.1007/s10953-012-9822-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10953-012-9822-7