Abstract
Ab initio calculations based on density functional theory have been used to comparatively investigate the electronic and magnetic properties of two non-magnetic ions (Cu, K)-doped magnesium sulfide. The results obtained indicate that the ferromagnetic phase is always energetically favorable than the paramagnetic one. It is also found that these ternary alloys are half-metallic ferromagnets with a total magnetic moment of 1.00 μB per supercell. The ferromagnetism is induced by hybridization between Cu 3d and its nearest neighboring S 3p in Cu-doped MgS, while it originates in the spin polarization of the p shell of anions S in K-doped MgS. Our results make these compounds attractive materials for possible spintronics devices.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Diluted magnetic semiconductors (DMSs) have been extensively studied due to their potential applications in the field of spintronics [1,2,3]. In particular, half metallic DMSs are attracting a considerable amount of attention, for which only one of the two spin channels presents a gap at the Fermi level (EF), while the other has a metallic character, leading 100% carrier spin polarization at EF. For practical microelectronic device applications, half metallic (HM) ferromagnets should exhibit ferromagnetism at room or higher temperatures [4]. During the past years, significant research efforts have been made toward studying the room temperature ferromagnetism in transition metal (TM)-doped II–VI and III–V semiconductors [5,6,7,8,9,10,11,12,13]. However, the origin of ferromagnetism in these alloys continues to be a subject of debate. This is because the TM-doped semiconductors usually suffer from the problems of the precipitates or secondary phase formation [14, 15]. To avoid these controversies, many research works have been focused on investigating the ferromagnetism of the intrinsic nonmagnetic element-doped semiconductors, such as Cu-doped ZnO [16, 17], GaN [18], and AlN [19, 20]. Besides Cu, other intrinsic nonmagnetic elements, like K, C, and Sr, can also lead to ferromagnetism in AlN [21], ZnO [22, 23], and III–V [24], respectively. More interestingly, most of these studies indicate the possibility of fabricating the room temperature ferromagnets, which provide an opportunity to study new mechanisms of ferromagnetism and open new ways to find advanced spintronic materials.
Due to their wide band gaps and low dielectric constants, the magnesium chalcogenides, MgX (X = S, Se, and Te) have received enormous interest from both experimental and theoretical points of view. These semiconductors can be used in blue and ultraviolet wavelength optics and high temperature electronics [25,26,27]. Among magnesium chalcogenides, magnesium sulfide (MgS) has a very large band gap, in excess of 4.5 eV, and it is an excellent barrier material with most other II–VI semiconductors [28]. MgS exists in abundance in the earth’s crust [29]; it crystallizes in the rock-salt structure as ground state under ambient pressure [30]. In addition, the zinc blende structure is realized by means of epitaxial growth over GaAs substrate either by metallo-organic vapor phase epitaxy (MOVPE) [31, 32] or by molecular beam epitaxy (MBE) [33]. Although many theoretical and experimental reports have been carried out for MgS, only few works have reported on the magnetism of MgS-based DMS. Liu et al. [34] using pseudopotential method have predicted the presence of the character half-metallic in C-doped alkaline earth chalcogenides X4CY4 (X = Mg, Ca, and Sr, Y = O and S) with NaCl structure.
In the present work, the influence of K and Cu as nonmagnetic dopants on the half-metallicity and electronic structure of zinc blende MgS is examined by first principle calculations. The theoretical analysis of the electronic structures provides us a clear understanding of the governing mechanisms behind K and Cu dopant-induced HM ferromagnetism. The paper is organized as follows. In Section 2, we briefly describe our method of calculation. Section 3 contains all results and discussions. Finally, we conclude with a summary in Section 4.
2 Method
To perform the calculations, we have employed the full potential of linear augmented plane wave method within the density functional theory (DFT) [35], as implemented in the wien2K package [36]. We considered different compositions, namely 25%, 12.5%, and 6.25% which correspond to supercell containing 8, 16, and 32 atoms, respectively, for Mg1 − xCuxS and Mg1 − xKxS systems. The generalized gradient approximation (GGA) proposed by Perdew, Burke, and Ernzerhof (PBE) [37] is used to treat the exchange and correlation functional. The basis function was expanded to RMT Kmax = 8, where RMT is the muffin-tin radius and Kmax is the maximum modulus for the reciprocal lattice vectors. The maximum value of partial waves inside the atomic sphere is l = 10. Full relativistic approximation is used for core electrons, and scalar relativistic approximation is used for valence electrons. Muffin-tin radii (RMT) of 2 bohr are chosen for all atoms. Brillouin zone integrations are performed using 5 × 5 × 5 k-points of Monkhorst-Pack [38] (MP) mesh for the supercell calculations. During calculations, the atomic coordinates are fully relaxed until maximum force on a single atom is less than 2.10−3 Ry/a.u., and convergence of energy is set at 10−5 Ry/cell.
3 Results and Discussions
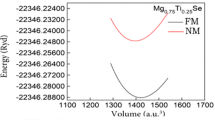
To explore the ground state properties, we first optimize the total energy as function of cell volume of studied DMS compounds for both ferromagnetic (FM) and paramagnetic (PM) phases. The calculated total energies are fitted with Murnaghan’s equation of state [39]. The optimized lattice parameter, bulk modulus, its pressure derivative, and total energy difference ∆E between PM and FM states at their equilibrium lattice constants are tabulated in Table 1. In all cases, the FM phase is favorable in energy than the corresponding PM phase.
Table 2 shows the calculated magnetic properties of the studied alloys at their optimized equilibrium lattice constants for various concentrations of dopants. For all investigated compounds, the total magnetic moment is exactly 1.00μB. An integer value of magnetic moment is a characteristic feature of half-metallic ferromagnetism. The total magnetic moment mtotal in supercell consists mainly of the following three parts: the mCu/K, mS, and minterstitial; however, the mMg is found negligible. For x = 0.0625, the magnetic moments are mainly localized within the Cu/K atom and its four nearest neighboring S atoms with the mCu (mK) is larger (smaller) than that of the S atoms. The magnetic moments on other S atoms away from (Cu/K)S4 tetrahedron are weak and not listed in Table 2.
As the trends in Mg1−xYxS (Y = Cu and K) alloys at different doping compositions are similar, in the following, we focus the discussion on the properties of doped ternary Mg0.9375Y0.0625S systems at the equilibrium lattice parameters. Figures 1 and 2 present the spin-dependent band structures along the high symmetry directions of the Brillouin zone for each compound. The band structures of both materials show half-metallic behavior. The minority spin electrons exhibit metallic natures, while the majority spin channels display a band gap of 2.88 eV for MgCuS and 3.13 eV for MgKS, revealing their semiconducting natures. The carriers are thus 100% polarized, which is the ideal case for spin injection applications. For MgKS, near the Fermi levels, there is a flat (undispersed) band in the whole Brillouin zone for both spin channels. This flat band is formed mainly from the S 3p orbitals. For the sake of comparison of magnetism mechanisms in Y-doped MgS DMS, we first analyze the total density of states (DOS) and partial DOS of Cu and its one nearest neighboring S for MgCuS. As can been seen in Fig. 3, the asymmetrical DOS between the spin up and spin down channels near the Fermi level (EF) shows the magnetic properties of such doped system. The density of states between − 4 and − 0.6 eV is mostly ascribed to the Cu d and S p states with relatively small contribution of Cu s and Cu p states. States around EF arise primarily from the Cu 3d orbitals, hybridized strongly with S 3p orbitals. The character of dominating states at and near EF can be elucidated by crystal field theory, in which the cation d states are split into triply degenerate t2g and doubly degenerate eg states, whereas the anion p states transform as t2p representation. The states with the same t2 symmetry for spin up (or spin down) between the anion and cation can couple with each other forming lower bonding and higher antibonding states, known as the p-d coupling, while the eg states cannot bond by symmetry and do not take part in the hybridization process. The contribution of these symmetry states toward total spin-up DOS is shown in Fig. 4. Therefore, the induced S t2 states can couple only Cu t2g states having the same symmetry. Consequently, the p-d coupling mechanism is responsible for the HM ferromagnetism in this compound.
Having discussed the origin of ferromagnetism in MgCuS, we now turn our attention to the MgKS compound. The spin-resolved total and partial density of state of MgKS are given in Fig. 5. It reveals that the anionic and cationic states contribute differently to the majority spin system. The states ranging from − 1 eV and EF are mainly composed of S 3p states with a slight contribution from K 3p and K 3d states. However, there is a K s peak at − 0.52 eV, which is different from TM-doped MgS [40, 41]. Also, from the DOS, a sharp peak is found at the top of the valence states. The Fermi level is located at the peak of minority spin states, i.e., in the gap of the majority spin states. One can clearly see that the peak of the DOS can be attributed to the almost dispersionless flat band shown in Fig. 2. It has been argued by other authors [42, 43] that the origin of this flat band is an interference effect between cationic t2g-character and anionic p-character and that the flat band is essential for HM ferromagnetism. In the present case, these conditions are fulfilled (Figs. 5 and 6). However, most t2g states are not occupied and lie at higher energies, and thus, K d splitting does not play an important role in FM K-doped MgS. The spin splitting mainly originates from S p states and is situated close to EF, which provides the main magnetic moment, and the hybridization between S p and K p and K t2g states leads to the small magnetic moment of the K atom. This is consistent with the results obtained in NaN and KN [44] and Sr-doped III–V [24] from first principle calculations.
The general picture of ferromagnetism that applies to MgCuS and MgKS systems can also be viewed in the charge density contour of spin up at the Γ point in Fig. 7 and Fig. 8, respectively. The fully filled majority spin at around − 2.55 eV are bonding Cu (t2g)-S(p) states, reflecting the covalent p-d bonding (i.e., the density lobes point toward the nearest neighbors, see Fig. 7a), while the antibonding Cu (t2g)-S(p) states are close to EF (i.e., the density lobes point between the nearest neighbor atoms, see Fig. 7b). The occupied states of MgKS close to the EF are strongly localized around the S atom, reflecting the weak polarized covalent bonds of S p orbitals and K electrons.
4 Summary
We have employed the first principle calculation to investigate the electronic and magnetic properties of Mg1 − xYxS (Y = K, Cu) alloys for x = 0.0625, 0.125 and x = 0.25 in their ordered zinc blende structure. We found that the structure within ferromagnetic configuration is always the stable ground state. All doped ternary systems exhibit half metallic character with a band gap for the majority spin channel and a partially filled band for the minority spin channel. It was observed that the total magnetization is 1.00 μB per dopant atom, and it remains independent on the dopant content. Also, it is revealed by means of partial DOS that the p-d coupling between cation and its neighboring anions plays a crucial role in forming the ferromagnetism Cu-doped MgS, while for K-doped MgS, the magnetism owns its origin to the spin polarization of S p states. With no magnetic element in these ternary alloys and the 100% spin polarization of the carriers, we expect Cu- and K-doped MgS to be promising half-metallic DMSs free from magnetic precipitates.
References
Ohno, H.: Science. 281, 951 (1998)
Wolf, S.A., Awschalom, D.D., Buhrman, R.A., Daughton, J.M., von Molnar, S., Roukes, M.L., Chtchelkanova, A.Y., Treger, D.M.: Science. 294, 1488 (2001)
Cui, X.Y., Medvedeva, J.E., Delley, B., Freeman, A.J., Stampfl, C.: Phys. Rev. B. 78, 245317 (2008)
Jungwirth, T., Sinova, J., Masek, J., Kucera, J., MacDonald, A.H.: Rev. Mod. Phys. 78, 809 (2006)
Liu, C., Yun, F., Morkoç, H.: J. Mater. Sci. Mater. Electron. 16, 555 (2005)
Pearton, S.J., Norton, D.P., Ip, K., Heo, Y.W., Steiner, T.: J. Vac. Sci. Technol. B. 22, 932 (2004)
Akhtar, M.S., Malik, M.A., Riaz, S., Naseem, S.: Mater. Chem. Phys. 160, 440 (2015)
Kumar, D., Antifakos, J., Blamire, M.G., Barber, Z.H.: Appl. Phys. Lett. 84, 5004 (2004)
Frazier, R.M., Thaler, G.T., Leifer, J.Y., Hite, J.K., Gila, B.P., Abernathy, C.R., Pearton, S.J.: Appl. Phys. Lett. 86, 052101 (2005)
Dhar, S., Brandt, O., Trampert, A., Däweritz, L., Friedland, K.J., Ploog, K.H., Keller, J., Beschoten, B., Güntherodt, G.: Appl. Phys. Lett. 82, 2077 (2003)
Chitta, V.A., Coaquira, J.A.H., Fernandez, J.R.L., Duarte, C.A., Leite, J.R., Schikora, D., As, D.J., Lischka, K., Abramof, E.: Appl. Phys. Lett. 85, 3777 (2004)
Granville, S., Ruck, B.J., Preston, A.R.H., Stewart, T., Budde, F., Trodahl, H.J., Bittar, A., Downes, J.E., Ridgway, M.: J. Appl. Phys. 104, 103710 (2008)
Zhi, Z.Y., Chun, H.M.: Chin. Phys. Lett. 21, 1632 (2004)
Park, J.H., Kim, M.G., Jang, H.M., Ryu, S., Kim, Y.M.: Appl. Phys. Lett. 84, 1338 (2004)
Kaspar, T.C., Droubay, T., Heald, S.M., Engelhard, M.H., Nachimuthu, P., Chambers, S.A.: Phys. Rev. B. 77(R), 201303 (2008)
Ye, L.-H., Freeman, A.J., Delley, B.: Phys. Rev. B. 73, 033203 (2006)
Buchholz, D.B., Chang, R.P.H., Song, J.-Y., Ketterson, J.B.: Appl. Phys. Lett. 87, 082504 (2005)
Wu, R.Q., Peng, G.W., Liu, L., Feng, Y.P., Huang, Z.G., Wu, Q.Y.: Appl. Phys. Lett. 89, 062505 (2006)
Ran, F.Y., Subramanian, M., Tanemura, M., Hayashi, Y., Hihara, T.: Appl. Phys. Lett. 95, 112111 (2009)
Jia, W., Han, P., Chi, M., Dang, S., Xu, B., Liu, X.: J. Appl. Phys. 101, 113918 (2007)
Han, R., Yuan, W., Yang, H., Du, X., Yan, Y., Jin, H.: J. Magn. Magn. Mater. 326, 45 (2013)
Pan, H., Yi, J.B., Shen, L., Wu, R.Q., Yang, J.H., Lin, J.Y., Feng, Y.P., Ding, J., Van, L.H., Yin, J.H.: Phys. Rev. Lett. 99, 127201 (2007)
Zhou, S., Xu, Q., Potzger, K., Talut, G., Grötzschel, R., Fassbender, J., Vinnichenko, M., Grenzer, J., Helm, M., Hochmuth, H., Lorenz, M., Grundmann, M., Schmidt, H.: Appl. Phys. Lett. 93, 232507 (2008)
Adli, W.: J. Supercond. Nov. Magn. 30, 1775 (2017)
Okuyama, H., Nakano, K., Miyajima, T., Akimoto, K.: Jpn. J. Appl. Phys. 30, L1620 (1991)
Wang, M.W., Phillips, M.C., Swenberg, J.F., Yu, E.T., McCaldin, J.O., McGill, T.C.: J. Appl. Phys. 73, 4660 (1993)
Wang, M.W., Swenberg, J.F., Phillips, M.C., Yu, E.T., McCaldin, J.O., Grant, R.W., McGill, T.C.: Appl. Phys. Lett. 64, 3455 (1994)
Wang, M., Li, X., Gao, M., Pan, H., Liu, Y.: J. Alloys Compd. 603, 158 (2014)
Sanchez, A.M., Olvera, J., Ben, T., Morrod, J.K., Prior, K.A., Molina, S.I.: Appl. Phys. Lett. 89, 121907 (2006)
Peiris, S.M., Campbell, A.J., Heinz, D.L.: J. Phys. Chem. Solids. 55, 413 (1994)
Uesugi, K., Obinata, T., Suemune, I., Kumano, H., Nakahara, J.: Appl. Phys. Lett. 68, 844 (1996)
Konczewicz, L., Bigenwald, P., Cloitre, T., Chibane, M., Ricou, R., Testud, P., Briot, O., Aulombard, R.L.: J. Cryst. Growth. 159, 117 (1996)
Bradford, C., O’Donnell, C.B., Urbaszek, B., Balocchi, A., Morhain, C., Prior, K.A., Cavenett, B.C.: Appl. Phys. Lett. 76, 3929 (2000)
Liu, J., Chen, L., Dong, H.-N., Zheng, R.-L.: Appl. Phys. Lett. 95, 132502 (2009)
Hohenberg, P., Kohn, W.: Phys. Rev. B. 136, B864 (1964)
Blaha, P., Schwarz, K., Madsen, G.K.H., Kvasnicka, D., Luitz, J.: WIEN2k, an Augmented Plane Wave Program for Calculating Crystal Properties. Vienna University of Technology, Vienna (2001)
Perdew, J.P., Burke, K., Ernzerhof, M.: Phys. Rev. Lett. 77, 3865 (1996)
Monkhorst, H.J., Pack, J.D.: Phys. Rev. B. 13, 5188 (1976)
Murnaghan, F.D.: Proc. Natl. Acad. Sci. U. S. A. 30, 244 (1944)
Gous, M.H., Meddour, A., Bourouis, C.: J. Magn. Magn. Mater. 422, 271 (2017)
Tanveer, W., Mahmood, Q., Faridi, M.A., Yaseen, M., Ramay, S.M., Mahmood, A.: J. Supercond. Nov. Magn. 30, 3481 (2017)
Yao, K.L., Jiang, J.L., Liu, Z.L., Gao, G.Y.: Phys. Lett. A. 359, 326 (2006)
Sieberer, M., Redinger, J., Khmelevskyi, S., Mohn, P.: Phys. Rev. B. 73, 024404 (2006)
Zhang, Y., Qi, Y., Hu, Y.: J. Magn. Magn. Mater. 324, 2523 (2012)
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adli, W. Prediction of Half-Metallic Ferromagnetism in Cu- and K-Doped MgS: A Comparative Study. J Supercond Nov Magn 33, 3107–3112 (2020). https://doi.org/10.1007/s10948-020-05558-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-020-05558-3