Abstract
The structural, heat capacity, and magnetic properties of Y2Mo2(1-x)Ti2xO7 were investigated in this work. Y2Mo2(1-x)Ti2xO7 maintains the pyrochlore structure, but lattice constants are observed to decrease continuously with the Ti4+ doping. The magnetic measurements indicate a magnetic phase transition at low temperature for all Ti-doped samples, and the Ti4+ doping suppresses the transition temperature. This is ascribed to the diluted effect of nonmagnetic Ti4+ substitution of magnetic Mo4+. The heat capacity data confirm that the low-temperature magnetic phase is spin glass in the Ti4+-doped Y2Mo2(1-x)Ti2xO7 system. The M(H) data reveals the coexistence of antiferromagnetic and ferromagnetic interactions at low temperatures.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Pyrochlore oxides have attracted a lot of scientific interest over the past two decades [1,2,3,4]. In pyrochlore oxides A2B2O7, the rare earth cations and the transition metal cations generally occupy A and B sites, respectively. And the A- and B-site cations form the three-dimension array of corner-shared tetrahedra, respectively. When magnetic metal cations with the inherent antiferromagnetic (AFM) interaction occupy the vertexes of tetrahedra, spins encounter the magnetic frustration. This spin frustration originating from the structure leads to highly degenerate ground states. In some geometrically frustrated antiferromagnets, the degenerate magnetic ground states are lifted by the internal or external factors such as the lattice distortion, the strain, the next-nearest-neighbor interaction, and the ionic doping, which generates a rich variety of magnetic phases including spin glass, spin liquid, spin ice, antiferromagnetism [5,6,7].
In Y2Mo2O7, nonmagnetic Y3+ and magnetic Mo4+ ions occupy A and B sites, respectively. The magnetic interaction between the nearest neighbor Mo4+ ions at B sites is AFM, so the magnetic Mo4+ ions in Y2Mo2O7 meet the geometrical spin frustration [8]. In the past years, physical properties of Y2Mo2O7 and its doping compounds have been investigated in some references [9,10,11]. It has been established that Y2Mo2O7 behaves as a nearly ideal spin glass from nonlinear susceptibility, heat capacity, AC susceptibility, elastic and inelastic neutron scattering, muon-spin relaxation, and neutron spin echo data [10,11,12,13]. The other metal ions doping in Y2Mo2O7 would change magnetic property due to the change of structure and generation of chemical disorder [14]. The magnetic property in the Ti4+-doped Y2Mo2O7 was investigated in reference [15]. In this work, the effect of Ti4+ doping in Y2Mo2O7 was investigated. The Ti4+ doping does not change the structure except slightly increasing the lattice constant in the system. Spin glass is confirmed to persist in the Ti4+-doped Y2Mo2O7, but the transition temperature is suppressed with the Ti4+ doping.
2 Experimental
Polycrystalline samples of Y2Mo2(1-x)Ti2xO7 were prepared by using the standard solid-state method. Firstly, the stoichiometric reactants of Y2O3 (99.99 wt%), MoO2 (99 wt%), and TiO2 (99.99 wt%) were thoroughly mixed. Then the mixtures were pressed into sheets and pre-fired at 1073 K under the flowing N2. Finally, they were sintered at 1523 K under the same atmosphere with the intermediate grinding. The structure was checked by using the x-ray diffractometer (XRD) with the Cu Kα radiation at room temperature. The XRD data were recorded in the 2θ range from 20° to 80°. The XRD data have been analyzed using Rietveld refinement program (GSAS). The FRIR and Raman spectra were employed to check the change of microstructure in the Ti4+-doped samples. The magnetic properties were measured by employing a superconducting quantum interference device magnetometer. Temperature dependence of magnetization was measured in the zero-field-cooling (ZFC) and the field-cooling (FC) modes under the magnetic field of 100 Oe. The AC susceptibilities at different frequencies were measured at the zero DC magnetic field and a constant AC field of 3.5 Oe from 4 to 200 K. The heat capacity at low temperatures was measured by using physical properties measurement system.
3 Results and Discussion
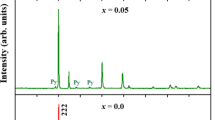
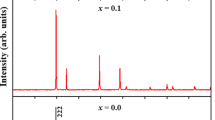
The experimental and the Rietveld refined XRD data of Y2Mo2(1-x)Ti2xO7 with x = 0.05, 0.10, 0.20, and 0.30 at room temperature were shown in Fig. 1. All Y2Mo2(1-x)Ti2xO7 samples present in the cubic phase with Fd3m symmetry and no impurity peaks are observed, which indicates that the Ti4+ doping does not change the crystal structure in this series of samples. Compared with the standard XRD spectra of Y2Mo2O7, the (222) peaks of Y2Mo2(1−x)Ti2xO7 with x = 0.05, 0.10, 0.20, and 0.30 shift − 0.004°, + 0.053°, + 0.144°, and + 0.155°. For x = 0.05, the (222) peak unexpectedly and slightly shifts to the low degree, which may ascribe to measurement inaccuracy from the large measurement step (0.033° per step). The Ti4+ doping shifts the (222) peak to the high degree, which means that the lattice constant becomes small. The experimental XRD data for all samples is well simulated by the Rietveld refinement. As shown in Table 1, the lattice constant (D) for Y2Mo2(1-x)Ti2xO7 decreases monotonically upon the substitution of Ti4+. This is due to that the ionic radius of Ti4+ (0.605 Å) is smaller than that of Mo4+ (0.65 Å). The bond lengths between different ions from Rietveld refinements for Y2Mo2(1−x)Ti2xO7 are shown in Table 2. When Ti4+ substitutes Mo4+, the bond lengths between most different ions in Y2Mo2(1−x)Ti2xO7 vary slightly (≤ 3.3%) except the Ti–O bond (up to 12%). The large change of the Ti–O bond length is related with the microstructure crystalline distortion resulting from the different ionic radii between Mo4+ and Ti4+.
Figure 2 shows magnetization as a function of temperature [M(T)] for Y2Mo2(1-x)Ti2xO7 measured under 100-Oe magnetic field. The ZFC and FC M(T) curves are coincident in the high-temperature region and show irreversible at low temperatures. The irreversibility temperature (Tirr) of Y2Mo2(1-x)Ti2xO7 is 20.2, 18.1, 14.1, and 12.0 K for x = 0.05, 010, 0.20, and 0.30, respectively. The inset of Fig. 2 exhibits that Tirr decreases almost linearly with the doping content of Ti4+. Obviously, the doping of Ti4+ suppresses the irreversibility temperature. In Y2Mo2O7, the observance of magnetic irreversibility is considered to originate from the thermodynamic spin glass [16, 17]. The generation of spin glass in Y2Mo2O7 is also confirmed by neutron powder diffraction, the heat capacity, and density functional theory calculations, and suggested to be the results of the spin-orbital coupling and random fluctuations in the Mo environment at the local level [18]. However, magnetic irreversibility behavior is also found in the antiferromagnetic and ferromagnetic systems. So, it could not be concluded that spin glass occurs in the Ti4+-doped Y2Mo2(1-x)Ti2xO7 system. Temperature dependence of the inverse magnetic susceptibility [χ−1 = (M/H)−1] is shown in Fig. 3. The curves are fitted through the Curie-Weiss law χ = C/(T − θCW), where C is the Curie constant and θCW is the Curie-Weiss temperature. The obtained θCWs for all samples are all negative, which indicates that the main magnetic interaction in Y2Mo2(1-x)Ti2xO7 is antiferromagnetic.
To further clarify the magnetic state in the Y2Mo2(1−x)Ti2xO7 system, we studied the isothermal magnetization as a function of a magnetic field [M(H)] measured at 5 K for Y2Mo2(1−x)Ti2xO7 (Fig. 4). The M(H) curves show almost linear behavior, which reflects the strong AFM interaction in this series of samples. But the M(H) curves also show a slight magnetic hysteresis in the low-field region. The typical M(H) curve of x = 0.20 as shown in the inset of Fig. 4. The magnetic hysteresis indicates that there is a weak ferromagnetic (FM) interaction in the system. Figure 5 shows the corresponding Arrott plot (M2 vs H/M) from the M(H) data [19]. For the materials with spontaneous magnetization, the Arrott plot will give a positive intercept in the high-field regime. On the other hand, a negative intercept in the Arrott plot implies an antiferromagnetic state [19, 20]. As seen in Fig. 5, the intercepts for all samples are negative, which indicates that the AFM interaction is predominated rather than the FM interaction in Y2Mo2(1-x)Ti2xO7. Therefore, the AFM interaction is predominated but the weak FM interaction also exists in Y2Mo2(1-x)Ti2xO7.
The coexistence of the AFM and FM interactions in this series of samples would be possible to induce spin glass in the system. To demonstrate whether or not spin glass occurs in Y2Mo2(1-x)Ti2xO7, we further measured temperature dependence of AC susceptibility under zero DC magnetic field and a constant AC field of 3.5 Oe from 4 to 200 K with different frequencies (f) of 10 Hz and 100 Hz. The real part of AC susceptibility curves [χ’(T)] with different frequencies are shown in Fig. 6. A pronounced peak is observed in the χ’(T) curves for all samples, and the corresponding temperature is the transition temperature. When frequency change from 10 to 100 Hz, the χ’(T) curve at 100 Hz is coincident with that of 10 Hz and no peak shift is found for each sample. In a conventional spin glass, when increasing frequency, peak shifts to a higher temperature [8]. But in the Y2Mo2(1−x)Ti2xO7, no peak shift is found, which is unlike the conventional spin glass. In some spin glass system, the behavior of peak shift could not be detected due to that this shift is smaller than the measurement accuracy. Therefore, the spin glass could not be excluded. The transition temperature obtained from the AC susceptibility is 22, 20, 16, and 14 K for x = 0.05, 0.10, 0.20, and 0.30, respectively.
We further made the heat capacity measurements as a function of temperature [Cp(T)] for Y2Mo2(1−x)Ti2xO7 with x = 0.05, 0.10, 0.20, and 0.30, shown in Fig. 7. The measured Cp(T) curves of Y2Mo2(1−x)Ti2xO7 (x = 0.05, 0.10, 0.20, and 0.30) is similar to that of Y2Mo2O7 [18]. To get the magnetic specific heat, Y2Ti2O7 was used as a subtraction from the lattice contribution at low temperatures because Y2Mo2O7 presents a similar crystalline structure to Y2Ti2O7. Figure 8 shows temperature dependence of the magnetic specific heat [Cm(T)] of Y2Mo2(1−x)Ti2xO7 (x = 0.05, 0.10, 0.20, and 0.30). A broad peak is observed at the transition temperature for all samples. Especially, the magnetic specific heat in the low-temperature region exhibits linear behavior. This phenomenon suggests a constant density of states of the low-temperature magnetic excitations and it is also claimed to be a common feature of spin glass [11, 21, 22]. A broad magnetic specific heat also extends up to high temperatures, which indicates that short-range order contributions persist at the high temperatures [11]. This also confirms spin glass. The coexistence of AFM and FM interactions in the frustrated magnet may be the origin of spin glass in this series of samples.
4 Conclusion
In summary, we have investigated the structural, heat capacity, and magnetic properties of the pyrochlore Y2Mo2(1-x)Ti2xO7. No structural phase transition is induced, but the lattice constant decreases continuously with Ti4+ doping. Spin glass persists in the Ti4+-doped Y2Mo2O7 in the low-temperature region. The Ti4+ doping suppresses the transition temperature. This is ascribed to the diluted effect of nonmagnetic Ti4+ substitution of magnetic Mo4+. The coexistence of AFM and FM interactions in the frustrated magnet may be the origin of spin glass.
References
Greedan, J.E.: Geometrically frustrated magnetic materials. J. Mater. Chem. 11(1), 37–53 (2000)
Harris, M.J., Bramwell, S.T., Mcmorrow, D.F., Zeiske, T., Godfrey, K.W.: Geometrical frustration in the ferromagnetic pyrochlore Ho2Ti2O7. Phys. Rev. Lett. 79(13), 2554–2557 (1997)
Attig J., Trebst S., Classical spin spirals in frustrated magnets from free-fermion band topology. Phys. Rev. B 96,085145 (2017)
Lacroix, C., Mendels, P., Mila, F.: Introduction to Frustrated Magnetism. Springer, Berlin Heidelberg (2011)
Gingras, M.J.P., Stager, C.V., Raju, N.P., Gaulin, B.D., Greedan, J.E.: Static critical behavior of the spin-freezing transition in the geometrically frustrated pyrochlore antiferromagnet Y2Mo2O7. Phys. Rev. Lett. 78(5), 947–950 (1997)
Gardner, J.S., Dunsiger, S.R., Gaulin, B.D., Gingras, M.J.P., Greedan, J.E., Kiefl, R.F., Lumsden, M.D., MacFarlane, W.A., Raju, N.P., Sonier, J.E., Swainson, I., Tun, Z.: Cooperative paramagnetism in the geometrically frustrated pyrochlore antiferromagnet Tb2Ti2O7. Phys. Rev. Lett. 82(5), 1012–1015 (1999)
Fukazawa H., Melko R., Higashinaka R., Maeno Y., Gingras M., Magnetic anisotropy of the spin-ice compound Dy2Ti2O7. Phys. Rev. B 65(5), 054410 (2002)
Ying, Y., Wang, L., Li, W., Qiao, L., Zheng, J., Yu, J., Cai, W., Jiang, L., Che, S., Zhang, L., Ling, L.: Spin glass in a geometrically frustrated magnet of ZnFe2O4 nanoparticles. J. Supercond. Nov. Magn. 31, 3553 (2018)
Greedan, J.E., Sato, M., Yan, X., Razavi, F.S.: Spin-glass-like behavior in Y2Mo2O7, a concentrated, crystalline system with negligible apparent disorder. Solid State Commun. 59(12), 895–897 (1986)
Miyoshi, K., Nishimura, Y., Honda, K., Fujiwara, K., Takeuchi, J.: Magnetic ordering of pyrochlore oxides R2Mo2O7 (R=Er–Nd, Y) by AC and DC magnetic measurements. Phys. B Condens. Matter. 284(7), 1463–1464 (2000)
Raju, N.P., Gmelin, E., Kremer, R.K.: Magnetic-susceptibility and specific-heat studies of spin-glass-like ordering in the pyrochlore compounds R2Mo2O7 (R=Y, Sm, or Gd). Phys. Rev. B. 46(9), 5405 (1992)
Dunsiger, S.R., Kiefl, R.F., Chow, K.H., Gaulin, B.D., Gingras, M.J.P., Greedan, J.E., Keren, A., Kojima, K., Luke, G.M., Macfarlane, W.A.: Muon spin relaxation investigation of frustrated antiferromagnetic pyrochlores A2B2O7. Hyperfine Interact. 104(1–4), 275–280 (1997)
Gardner, J.S., Ehlers, G., Bramwell, S.T., Gaulin, B.D.: Spin dynamics in geometrically frustrated antiferromagnetic pyrochlores. J. Phys. Condens. Matter. 16(11), S643 (2004)
Taguchi, Y., Ohgushi, K., Tokura, Y.: Optical probe of the metal-insulator transition in pyrochlore-type molybdate. Phys. Rev. B. 65(11), 115102 (2002)
Dunsiger, S.R., Kiefl, R.F., Chow, K.H., Gaulin, B.D., Gingras, M.J.P., Greedan, J.E., Keren, A., Kojima, K., Luke, G.M., Macfarlane, W.A.: Low temperature spin dynamics of geometrically frustrated antiferromagnets Y2Mo2O7 and Y2Mo1.6Ti0.4O7 studied by muon spin relaxation. J. Appl. Phys. 79(8), 6636–6638 (1996)
Gardner, J.S., Gingras, M.J.P., Greedan, J.E.: Magnetic pyrochlore oxides. Rev. Mod. Phys. 82(1), 53–107 (2010)
Gingras, M.J.P., Stager, C.V., Gaulin, B.D., Raju, N.P., Greedan, J.E.: Nonlinear susceptibility measurements at the spin-glass transition of the pyrochlore antiferromagnet Y2Mo2O7. J. Appl. Phys. 79(8), 6170–6172 (1996)
Silverstein, H.J., Fritsch, K., Flicker, F., Hallas, A.M., Zhou, H.D.: Liquidlike correlations in single-crystalline Y2Mo2O7: an unconventional spin glass. Phys. Rev. B. 89(5), 188–192 (2014)
Arrott, A.: Criterion for ferromagnetism from observations of magnetic isotherms. Phys. Rev. 108(6), 1394–1396 (1957)
Lamichhane, T.N., Taufour, V., Thimmaiah, S., Parker, D.S., Bud’Ko, S.L., Canfield, P.C.: A study of the physical properties of single crystalline Fe5B2P. J. Magn. Magn. Mater. 401, 525–531 (2015)
Meschede, D., Steglich, F., Felsch, W., Maletta, H., Zinn, W.: Specific heat of insulating spin-glasses, (Eu,Sr)S, near the Onset of Ferromagnetism. Phys. Rev. Lett. 44(2), C345–C347 (1980)
Wenger, L.E., Keesom, P.H.: Calorimetric investigation of a spin-glass alloy: CuMn. Phys. Rev. B. 13(9), 4053–4059 (1976)
Funding
This work was supported by the National Nature Science Foundation of China through Grant No. U1809215 and the Zhejiang Provincial Natural Science Foundation of China through Grant No. LY18E020016.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ying, Y., Zhang, W., Yu, J. et al. Structure and Magnetic Properties of the Ti-Doped Pyrochlore Molybdate Y2Mo2(1-x)Ti2xO7. J Supercond Nov Magn 32, 3563–3568 (2019). https://doi.org/10.1007/s10948-019-5132-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-019-5132-2