Abstract
Rapidly solidified Nd15Fe77B8 magnetic alloy powders have been produced by using melt-spinning method. Different wheel surface structures such as smooth and textured were tested to investigate the effect of wheel surface morphology on powder shape, microstructure, Curie temperature, and magnetic properties. Produced powders were subjected to surfactant-active ball-milling process for powder size reduction and to enhance magnetic and thermal properties. Generally, flaky-shaped powders were obtained with smooth surface wheels and spherical-shaped powders were produced with textured surface wheel. Microstructure of flaky-shaped powders consisted of polygonal fine equiaxed grains with an average grain size of 0.45 µm. Spherical-shaped powders had dendritic microstructure regardless of their sizes. Both flaky and spherical powders involved hard magnetic Nd2Fe14B phase located in the grain interior and a fine network of Nd-rich phase at the grain boundaries. Surfactant-active ball-milling process significantly contributed to magnetic and thermal properties of powders. The coercivity values of flaky-shaped powders milled for 90, 150, 210, 270, 330, and 390 min milling times were substantially higher than that of spherical-shaped powders milled for the same period of times. The Curie temperatures of Nd15Fe77B8 ingot alloy and spherical- and flaky-shaped powders were found as 279, 305, and 321 ∘C, respectively. On the other hand, the Curie temperatures of flaky- and spherical-shaped powders milled for 390 min were measured as 331 and 311 ∘C, respectively.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
NdFeB permanent magnets have a commercial importance in today’s industry due to their superior magnetic properties [1]. For instance, these magnets possess the highest magnetic energy product among other permanent magnets. This property makes their usage possible for various applications in many different devices and industries such as DC motors, generators, and hybrid automotive industry in addition to household applications [2,3,4]. The outstanding magnetic properties of these magnets especially emerge from Nd2Fe14B tetragonal intermetallic phase, which is solidified from liquid metal with the peritectic phase transformation [5]. Therefore, the control of this phase in Nd–Fe–B matrix requires experience. Besides that the magnetically hard Nd2Fe14B (ϕ) phase existed in grain interior, the Nd–Fe–B magnets also involve two other nonmagnetic phases; Nd-rich phase placed in the grain boundary and magnetically soft α-Fe phase [6, 7]. In order to get relatively good magnetic properties, the volume fraction of Nd2Fe14B grains, Nd-rich phase, and magnetically soft α-Fe phase in the Nd–Fe–B matrix needs to be optimized [5, 6].
Alternative methods to produce Nd-Fe-B powders have been extensively explored [7]. Among them, the rapid solidification processing by melt spinning has been one of the widely used techniques because of its high rate of production capacity and fine powders production capability. As it is well known, the magnetic quality of fabricated Nd–Fe–B magnets quite depends on the furnacing conditions and the cooling rate of melted alloy. Because of its relatively high cooling rate, which is due to the use of copper wheel as a coolant agent, the melt-spinning process has been the primary production route of NdFeB permanent magnets. Commonly, ribbon or flaky materials are first obtained in this method and fine particles are produced by comminuting ribbon or flake [8, 9]. It is seen that the comminuted melt-spun particles have isotropic magnetic properties which make them well suited for the manufacture of the bonded magnets [10]. In melt-spinning process, inductively melted liquid metal is ejected by gas pressure through a nozzle and then, the melted alloy is quenched onto the copper wheel rotating at variable speeds. In this way, relatively uniform ribbons with varying thicknesses from 15 to 60 µm (or flaky-shaped coarse powders) can be produced depending on the processing parameters [11,12,13].
In this study, the effect of wheel surface type on the properties of targeted Nd15Fe77B8 alloys such as morphology, microstructure, Curie temperature, and magnetic parameters was examined. For this purpose, the single-roller melt-spinning device and different surface types of wheels such as the smooth and the textured were used to produce Nd15Fe77B8 alloy powders. In the melt-spinning process, the smooth surface wheel is known to be a commonly used technique. The textured surface wheel will be used for the first time in the present study as a contribution to the scientific literature. In addition, the produced powders were ball milled in a surfactant-active atmosphere and various milling routines were experimented to reveal the effect of the milling time on the mean particle size and other size-dependent properties such as magnetism and Curie temperature.
2 Materials and Methods
Commercial magnetic alloy ingot with a nominal composition of Nd15Fe77B8 (at.%) was used to produce flaky- and spherical-shaped powders. The experimental study was carried out using a laboratory-scale single-roller stainless-steel melt-spinning device working in a high-vacuum atmosphere (10− 7 mbar). Small amounts of crushed Nd15Fe77B8alloy ingots (approximately 3 mm3) were placed in a hexagonal boron nitrite crucible with slit shape nozzle of 10 × 0.7 mm at the bottom. The Nd15Fe77B8alloy ingots were heated to 1400 ∘C (about 150 ∘C above the equilibrium liquid temperature) in vacuum atmosphere before melt-spinning trial. The temperature of the melt was controlled by an infrared thermometer positioned on top of the crucible. Quench wheel which has 40 mm width, 270 mm in diameter, and made of copper, was rotated using an external AC motor, and the tangential wheel speed was controlled by a digital control unit placed out of the chamber. The surface velocity of the quench wheel was kept constant as 52 m s− 1. The distance from the crucible end to the quench wheel surface was set as 0.8 mm which is sufficient to maintain a stable melt stream and prevent any physical contact between the crucible and melt pool. The temperature of the melt was monitored and controlled by an infrared temperature control device located near the crucible. Before each run, the chamber was evacuated up to 10− 7 mbar and backfilled with high-purity argon gas and then evacuated to a maximum vacuum to wipe out the air. The melted alloy was ejected through the nozzle onto the rotating wheel by introducing high purity (99.999%) argon gas flow with the pressure of 0.25 bar through the hexagonal boron nitride crucible. Molten alloy is disintegrated as droplets as soon as it touches the surface of rotating wheel. These droplets were undercooled and rapidly solidified during their contact with cooling wheel and free fall, hence fragmented pieces were obtained.
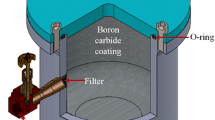
Produced Nd15Fe77B8 alloy powders were subjected to surfactant-assisted ball milling in a high-energy planetary ball-mill device of Fritsch Pulverisette 6 model at a rotational speed of 300 rpm. A milling jar with the capacity of 250 ml performing under a vacuum of 10− 3 mbar was designed and manufactured from tool steel, and the surface of the jar was hardened with boron-carbide material. The milling of melt-spun powders was performed in a protective surfactant-assisted atmosphere with the ball-to-powder weight ratio of 10/1 for milling times of 90, 150, 210, 270, 330, and 390 min, and tungsten carbide balls of 5-mm diameter were used. Oleic acid with a purity of 99.99% was introduced into the mill as surfactant-assisted agent together with hexane and heptane (99.99% purity) as solvents. The starting materials such as powders, surfactant-assisted material, solvents, and balls were charged to the jar, and milling was performed at room temperature.
Samples of melt-spun powders were cold mounted in a two-component epoxy resin. The mounted specimens were ground with 600, 800, 1000, and 1500 grid sandpapers. Then, these samples were polished with 3 and 1 µm diamond solution. Prepared samples were etched with the composition of 1.50 ml nitric acid and 50 ml ethanol. Microstructure of produced powders was examined by using a scanning electron microscope (SEM) of Zeiss EVO MA model. Elemental analysis of different phases was also made by using energy dispersive X-ray analyzer (EDX) attached to the SEM. The shape of the milled powders was examined with FEI Tecnai G2 F30 type transmission electron microscope (TEM) which operates at 300 kV. To observe the powders in TEM, the samples were dispersed in hexane solution with one or two drops of ethanol in it. The well-dispersed nanoparticles in hexane solution were placed over the microscopic copper grids (200 mesh size) and subsequently dried. The phases in the melt-spun powders were characterized by X-ray diffraction (XRD) apparatus of Panalytical X’pert3 Powder model with CuKα radiation. Thermal analysis of the produced powders was performed to determine the Curie temperature (TC) using the differential scanning calorimeter apparatus (DSC) of Linseis PT1600 model at a heating rate of 5 ∘C/min. Heating was carried out with flowing purified argon gas (99.999% purity) during the DSC analysis. The magnetic properties of powders were measured with a vibrating sample magnetometer of LDJ Electronics 9600 model using a maximum applied field of 1.5 T at room temperature. Figure 1 shows the flow chart diagram of the process for production of hard magnetic Nd15Fe77B8 alloy powders with different surface morphologies of wheels.
3 Results and Discussion
In meltspinning method, there are many process parameters such as wheel speed, melt superheat, gap distance between wheel surface and nozzle, ejecting gas pressure, vacuum pressure of the chamber, and wheel surface quality affecting the solidification rate of produced powders [14,15,16,17] Among them, the wheel speed is the most influential parameter affecting the cooling rate of powders. A lot of studies were performed in literature considering the effect of wheel speed at the solidification rate of powders in meltspinning procedure [15, 18, 19] In the present study, different from studies made in the literature, the surface structure of meltspinning wheel was changed from a smooth type to a textured form while other parameters were kept constant to search the effect of wheel surface on the shape and cooling rates of powders
Complete powder instead of ribbon was produced by using both smooth and textured types of wheels because of the brittle character of Nd15Fe77B8 alloy, in this study. Morphology of the produced powders is seen in Fig. 2. With the smooth surface wheel, the powder shape fluctuated and spherical, irregular, ligamental, fiber-like, and flaky-shaped powders were obtained (see Fig. 2a–d). Predominant shapes were sphere, ligament, and irregular for relatively small size of powders. When the powder size gets larger, the shape changes to flake. The width, the length, and the thickness of the flaky-shaped powders have been determined to be 10–240, 40–1020, and 5–48 µm, respectively. When textured surface wheel is used, the shape of the powders changes and the mostly spherical powders were obtained. The diameter of spherical powders extends from 10 to 350 µm (see Fig. 2e, f).
Produced Nd15Fe77B8 alloy powders by using both the smooth and the textured surface wheels were subjected to laser particle size measurement (with Malvern Master Sizer 2000) to compare particle size distribution. The results were represented in Fig. 3. Before laser particle size measurement, produced powders were initially subjected to sieve analysis to get sieve fractions of − 45 µm/pan, − 90/45 µm, − 125/90 µm, − 180/125 µm, − 250/180 µm, and − 355/250 µm. Each sieve fraction of powders was subjected to laser particle size measurement separately. As seen from Fig. 3, Nd15Fe77B8powders obtained by both the smooth and the textured surface wheels show a unimodal-type distribution. The mean particle sizes (d50) for − 45 µm/pan, − 90/45 µm, − 125/90 µm, − 180/125 µm, − 250/180 µm, and − 355/250 µm sieve fractions were 47.3, 95.3, 151.4, 218.4, 306.8, and 668.3 µm, respectively, for the powders produced by the smooth surface wheel. As can be noticed from mean particle sizes, the values were higher than their corresponding sieve fractions. This can be attributed to powder morphology given in Fig. 2. The shape of powders was spherical, irregular, ligamental, fibre like, and flaky. With this reason, fibre-like, ligamental, and flaky-shaped powders passed the sieve fractions with their longitudinal axes and complete surface of these powders could not be measured by sieve analysis. On the other hand, complete surfaces of powders could be measured with laser particle size analyzer and so, true results were achieved. As seen, there are significant differences between the results of sieve analysis and the laser particle size analyzer. The higher values were obtained with laser particle size analysis method. For example, the mean particle sizes of − 90/45 µm, − 125/90 µm sieve fractions were measured as 95.3 and 151.4 µm with laser particle size analysis process. On the other hand, the mean particle size of powders produced by using the textured surface wheel, the sieve analysis, and laser particle size analysis values give almost the same values because of the spherical shape of the powders. The mean particle sizes for − 45 µm/pan, − 90/45 µm, − 125/90 µm, − 180/125 µm, − 250/180 µm, and − 355/250 µm sieve fractions were obtained as 36.2, 80.5, 127.3, 191.4, 267.82, and 551.89 µm, respectively.
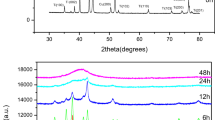
Figure 4 shows the XRD patterns of the Nd15Fe77B8 ingot alloy and melt-spun flaky- and spherically shaped powders produced by both the smooth and the textured surface wheels. As seen from the figure, the Nd15Fe77B8 ingot alloy consists of mostly magnetically hard Nd2Fe14B phase. In addition to Nd2Fe14B hard magnetic phase, there exist nonmagnetic Nd-rich phase and magnetically soft as α-iron phase. The XRD patterns of the flaky- and the spherically shaped powders involve magnetically hard Nd2Fe14B phase and nonmagnetic Nd-rich phase. Magnetically soft α-iron phase disappears from the powder microstructure as a result of rapid solidification process.
The size of flaky- and spherical-shaped Nd15Fe77B8 alloy powders were not appropriate to produce hard magnetic parts. It was reported in the literature that the small grain size of Nd2Fe14B phase and a structure with Nd2Fe14B grains surrounded by the paramagnetic Nd-rich phase is essential to get high values of Hc and Br for these magnets [20, 21]. For this reason, the produced melt-spun Nd15Fe77B8 alloy powders were subjected to ball-milling process for size reduction by using a newly designated vacuum jar. SEM images of the surfactant-active ball-milled particles of Nd15Fe77B8 alloy powders milled for different times are shown in Fig. 5. As stated above, there were two groups of starting powders: the first group of powders was mostly flaky shaped and obtained with smooth surface wheel, and the second group involved spherically shaped powders produced with textured surface wheel. Usually, two different deformation mechanisms take place in surfactant-assisted ball-milling process. In the first step, the large particles are broken due to excessive pressures on them. Secondly, the broken powders with smaller sizes tend to agglomerate in order to minimize their surface energy. Figure 5 reveals that the size reduction takes place quite rapidly even in the first stages of the milling process and the sizes of particles decrease from micron size to submicron for both two groups of powders. Nanoparticles are agglomerated in large clusters, and the clusters are getting larger as milling time increases. On the other hand, the presence of solvents (hexane and heptane) and surfactant (oleic acid) plays various roles in the milling process, including impeding cold welding of crushed particles, preventing oxidation and amorphization, and reducing contamination during milling [22]. When comparing particle sizes of flaky and spherical-shaped powders in the milling process, it is obvious that flaky-shaped powders have finer particle sizes than that of spherical powders. This can be explained in terms of powder shape. It is also evident that flaky-shaped powders have higher brittleness due to their thin cross-section, and they were more easily fractured in comparison with spherical powders. This difference was verified in Fig. 6 with high-resolution TEM images. As can be seen from the figure, the particle sizes of flaky-shaped powders were finer than that of spherical powders for the same milling times. Figure 7 shows the variation of mean particle sizes as a function of milling times for both the flaky and the spherical-shaped powders. The mean particle sizes of ball-milled flaky-shaped powders were 1.48, 1.23, 1.02, 0.82, 0.38, and 0.26 µm for 90, 150, 210, 270, 330, and 390 min milling times, respectively. The corresponding values of ball-milled spherical powders for the same milling times were 4.24, 3.39, 2.69, 2.01, 1.06, and 0.96 µm.
Figure 8a shows the back-scattered SEM micrographs of the Nd15Fe77B8 ingot alloy. Firstly, the microstructure of the starting Nd15Fe77B8 ingot alloy was examined to find out the grain size and phase distribution prior to melt-spinning production. As it can be seen, the alloy consists of dendritic microstructure having coarse grains with an average grain size of 50 µm and three distinct phases were observed in as-cast Nd15Fe77B8 ingot alloy. The majority of existing phases is Nd2Fe14B matrix phase. In addition to Nd2Fe14B, there are other phases of fine network of Nd-rich phase located on the grain boundaries and α-Fe phase in the form of dendrite like. Because of the adverse effect of α-Fe phase on the magnetic properties, the amount of this phase needs to be reduced to much lower levels or completely removed from the structure if possible. The presence of these phases in the microstructure of the alloy is easily identified by virtue of their EDX analysis, and the results were given in Fig. 8b–d. By EDX analysis, it was found that Nd2Fe14B phase contains 69.96 wt.% Fe, 26.46 wt.% Nd, and 3.58 wt.% B (Fig. 8b). The nominal composition of Nd2Fe14B phase given in literature [23, 24] was 72 wt.% Fe, 26 wt.% Nd, and 1 wt.% B. So, the results obtained by EDX analysis are in good correlation with the values given in the literature. As for the Nd-rich phase, it has 81.46 wt.% Nd, 16.42 wt.% B, and 2.12 wt.% Fe (Fig. 8c) and the magnetically soft α-iron phase contains 90.49 wt.% Fe, 5.37 wt.% B, and 4.14 wt.% Nd (Fig. 8d).
Figure 9 shows the back-scattered SEM micrographs of the melt-spun Nd15Fe77B8 alloy powders produced by the smooth surface wheel. As can be seen, the powder consists of polygonal fine equiaxed grains with an average grain size of 0.45 µm. SEM analysis revealed that microstructures of powders were uniform through their cross-section, and unlike some other studies made in literature [1, 5], there is no microstructural difference between disc side and air side surfaces of powders. The majority of the microstructure was assigned to the Nd2Fe14B phase, which is seen as dark regions in grain interiors. The remaining microstructure is a fine network of Nd-rich phase at the grain boundaries. Unlike the ingot alloy, the magnetically soft α-iron phase, which negatively affects hard magnetic properties, was not observed in the powder microstructure. As mentioned before, this can be attributed to the rapid solidification process of the flaky-shaped powders during the melt-spinning process. EDS analysis for grain interior and grain boundary regions was given in Fig. 9c, d, respectively. The elemental composition for grain interior region, which was thought to be Nd2Fe14B phase, was found as 70.61 wt.% Fe, 25.92 wt.% Nd, and 3.47 wt.% B (Fig. 9c). These values are very close to the nominal composition of Nd2Fe14B phase. Nd composition was obtained as 83.95 wt.% for Nd-rich grain boundary region (Fig. 9d).
Loosely speaking, ductile and light density alloys form as ribbon and brittle materials behave differently and are shaped as powder, fiber, and ribbon. As it is well known, the production of ribbon is an interim process and these ribbons are subjected to secondary processing routes such as high-energy ball milling to obtain useful powders. As stated before, the textured type of wheel surface to produce powder directly from molten alloy was experimented for the first time in this study. In the case of the textured wheel, the liquid metal adheres the wheel surface due to its rough surface texture and so it is atomized as powders. Spherical-shaped powders were produced with textured surface wheel because of the brittle character of Nd15Fe77B8 alloy and textured morphology of the wheel surface. As seen from Fig. 11, the microstructure of spherically shaped powders was dendritic. This microstructure was different from that of flaky-shaped powders which have a fine microstructure with polygonal equiaxed grains. Dendritic microstructure is explained in terms of the spherical shape of powders. The cooling rate of geometrical objects is expressed in terms of their solidification modulus. The term of modulus (M) is a general notion that expresses a comparable unit of mutual geometrical and physical quantities that determine the course of the given process. The modulus deals with the solidification process of castings of different geometry, and the determining quantities are the melt volume (V) and its cooling surface area (A) [25, 26]. The modulus is equal to V/A, and the higher the modulus, the lower the solidification rate. When comparing solidification modulus of sphere, cylinder, and cube, the modulus of sphere is 1.20, which is 1.35 times higher than that of the cylinder and cube, respectively. On the contrary, the solidification rates of the cylinder and cube shapes are 1.5 and 2.00 times higher than the sphere. As it is understood from these expressions, spherical powders produced by textured surface wheel have a much lower solidification rate than flaky-shaped powders produced by the smooth surface wheel. Relatively slow cooling rate explains dendritic microstructure of spherical-shaped powders produced with textured surface wheel. Figure 10a–d represents SEM microstructures of the spherically shaped powders with the sizes of 27, 82, 130, and 390 µm, respectively. As can be seen, all powders have dendritic microstructure. However, small deviations from the dendritic microstructure were seen with increasing powder size. It starts with needle-like and plate-like dendritic morphologies (27 and 82 µm size powders), then it transforms to a classical dendritic morphology (130 and 390 µm size powders). EDX analysis of powders was given in Fig. 10e, f. As it is seen from the figure, the microstructure of powders involves Nd2Fe14B phase and some small particles of Nd-rich phase. The elemental compositions for grain interior region (region A), which was thought to be Nd2Fe14B phase, were found as 70.09 wt.% Fe, 26.41 wt.% Nd, and 3.50 wt.% B (Fig. 10e). The composition of Nd-rich phase (region B) was found to be 87.95 wt.% (Fig. 10f).
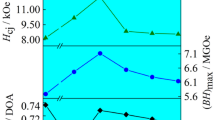
Magnetic hysteresis curves of Nd15Fe77B8 ingot alloy and melt-spun flaky and spherical-shaped powders are illustrated in Fig. 11. The hysteresis curve of Nd15Fe77B8 ingot alloy shows a constricted shape with relatively low coercivity. Such narrowed shape of hysteresis curve is a sign of coexistence of hard magnetic Nd2Fe14B and soft magnetic α-iron phases. The coercivity of melt-spun spherically shaped Nd15Fe77B8 alloy powders showed some increase in comparison with the ingot alloy. The highest coercivity value was obtained for flaky-shaped powders. This increase can be explained in terms of finely grained microstructure of these powders. Such a finely grained microstructure ensures increased hard magnetic properties such as the remanence magnetization and the coercivity [13]. The coercivity values of Nd15Fe77B8 ingot alloy and the melt-spun spherical and flaky-shaped powders are 710, 870, and 1215 Oe, respectively.
When the coercivity of spherical-shaped melt-spun Nd15Fe77B8 alloy powders was compared with those of the spherical-shaped powders produced by gas atomization in the literature, approximately the same results were obtained for the similar powder size range. For example, Seller et al. [10] stated that the coercivity value of spherical-shaped Nd30.1Fe69B0.91 (wt.%) alloy powders produced by gas atomization changed with the powder size, and they obtained the coercivity values of 900 and 600 Oe for 50–75 and 75–100 µm, size-ranged powders, repectively.
Melt-spun flaky- and spherical-shaped powders were subjected to surfactant-active ball-milling process for 90, 150, 210, 270, 330, and 390 min milling times. Different sizes of particles were obtained as a result of this procedure. Magnetic hysteresis loops for different milling times were recorded for the ball-milled flaky and spherical powders, and the results are presented in Figs. 12 and 13, respectively. The coercivity values are also included in Fig. 14 for comparison purposes. All loops show a single-phase-like magnetization behavior, indicating no secondary magnetic phases in the particles. Generally, the coercivity of ball-milled Nd15Fe77B8 particles increases with decreasing particle size. On the other hand, ball-milled flaky-shaped powders have higher coercivity values than that of the spherical powders. This phenomenon can be explained in terms of powder microstructure. The coercivity of Nd–Fe–B permanent magnets is extremely sensitive to their microstructures, especially the grain boundary structure. One well-known method to increase the coercivity of Nd–Fe–B magnets without using additional elements is to refine the grain size of the Nd2Fe14B phase [27]. The differences in optimum microstructures are probably related to the differences in magnetization-reversal mechanism. Changes of the domain direction in an applied field may be dominated either by domain nucleation or by domain growth which can be prevented by pinning. In optimum melt-spinning material, owing to the single-domain grain size, the reversal mechanism is generally perceived to be pinning controlled. The displacement of domain walls is suppressed by introducing many nonmagnetic inclusions or internal stresses which act as hindrances to domain wall motion. The best method, however, is to remove all domain walls from the material. This can be done by dividing the material into many fine particles or grains, the size of which is smaller than the critical size for single domain [28]. Due to the nanocrystalline grain size, there is an extremely large amount of grain boundary surface area per unit volume to provide pinning. An intergranular phase, which may be either amorphous or crystalline is usually found in melt-spinning magnets. It was generally believed that this intergranular phase is nonmagnetic and Nd enriched. This method improves the coercivity by magnetically de-coupling the adjacent grains [29]. Based on the above discussions and taking into consideration the microstructural differences of starting powder microstructures (given in Figs. 9 and 10 for the flaky and the spherical powders before the ball-milling process), we can conclude that flaky powders with finer microstructure resulted with higher coercivities. For the flaky-shaped powders, the coercivity values of 1814, 1890, 3330, 3570, 3567, and 4225 Oe were obtained for 90, 150, 210, 270, 330, and 390 min milling times, respectively. The corresponding values for spherical-shaped powders were 877, 914, 982, 1174, 1347, and 1715 Oe for 90, 150, 210, 270, 330, and 390 min milling times, respectively. On the other hand, the similar results were obtained by Cui et al. [30] for Nd15.5Fe78.5B6 alloy particles prepared by high-energy ball milling. They reported that the coercivity values of powders increased with increasing the milling time up to 5 h and then decreased beyond this time, and they obtained a maximum coercivity value of 3.500 Oe. In another study made by Liu et al. [31], the maximum coercivity value of 3.100 Oe was found for Nd2Fe14B alloy powders prepared by cryogenic grinding for 2 h. Remanence magnetization and coercivity values obtained in this study for the flaky- and the spherical-shaped powders, ball milled for 90, 150, 210, 270, 330, and 390 min milling times, were included in Table 1 for comparison purposes.
NdFeB-based magnets are widely used due to their extraordinary magnetic properties such as high coercivity, maximum energy product, and remanence magnetization. However, the relatively low Curie temperature (TC) of these magnets is the most important disadvantage for industrial application [32]. It was stated that the Curie temperature of NdFeB-based magnetic alloy can be increased to higher temperatures by adding alloying elements or increasing the amount of Nd2Fe14B hard magnetic phase in the microstructure [33]. Figure 15 represents the DSC curves of Nd15Fe77B8 ingot alloy, the flaky- and the spherically shaped powders produced by the smooth surface and the textured surface wheels. As can be seen from the figure, each curve has a tiny peak, and these peaks are defined as the Curie temperature point. On the other hand, the Curie temperatures of the flaky- and the spherically shaped as-spun powders increased significantly to higher temperatures. The Curie temperatures of Nd15Fe77B8 ingot alloy and the spherical and the flaky-shaped powders were found to be 279, 305, and 321 ∘C, respectively. The increase in Curie temperature for powders can be explained by the increased amount of Nd2Fe14B hard magnetic phase in the microstructures. As seen from the microstructure of the ingot alloy, given in Fig. 8a, there is a large amount of α-Fe phase because the peritectic transformation is not completed. Therefore, the low amount of N2Fe14B hard magnetic phase in the ingot alloy causes lower Curie temperature. When comparing Curie temperatures of the spherically and the flaky-shaped powders, the higher value was obtained at the flaky-shaped powders. This can be explained in terms of both relatively fine grain size microstructure and higher amount of hard magnetic phase in the flaky-shaped powders. On the other hand, surfactant-active ball-milling process significantly improves the Curie temperature of powders. DSC curves of surfactant-active ball-milled (390 min) spherical- and flaky-shaped Nd15Fe77B8 alloy particles are given in Fig. 16 for comparison purposes. As can be seen, the Curie temperatures of 318 and 331 ∘C were obtained after 390 min ball milling for the spherically and the flaky-shaped powders, respectively.
4 Conclusion
Rapidly solidified Nd15Fe77B8 magnetic alloy powders have been produced by using melt-spinning method. Different wheel surface structures such as the smooth and the textured were practised to investigate the effect of wheel surface morphology on powder shape, microstructure, and magnetic properties. Produced powders were subjected to surfactant-active ball-milling process for 90, 150, 210, 270, 330, and 390 min milling times for powder size reduction and to understand its affect on magnetic and thermal properties. The following conclusions are drawn from the present results;
-
1.
Generally, the flaky-shaped powders were obtained with the smooth surface wheel. Besides the flaky shape, different morphologies such as ligamental, spherical, and fibre-like powders depending on powder size were produced with the same wheel. On the other hand, the spherically shaped powders were produced with the textured surface wheel and powder shape was free of particle size.
-
2.
Microstructure of powders produced with the smooth surface wheel consists of polygonal fine equiaxed grains with an average grain size of 0.45 µm. On the other hand, the microstructure of powders was uniform through their cross-section and there were no microstructural differences between disc side and air side surfaces of powders. The spherically shaped powders produced with the textured surface wheel have dendritic microstructure regardless of their sizes.
-
3.
The microstructure of both the flaky and the spherical powders involves hard magnetic Nd2Fe14B phase located in grain interior and fine network of Nd-rich phase at the grain boundaries.
-
4.
The melt-spinning process enhances the magnetic properties of Nd15Fe77B8 alloy powders. Coercivity of powders increases significantly by using the smooth surface wheel, and the shape of hysteresis loops becomes more uniform.
-
5.
Surfactant-active ball-milling process significantly improves the magnetic properties for both the flaky and the spherical powders. The coercivity values of the flaky-shaped powders milled for 90, 150, 210, 270, 330, and 390 min milling times were substantially higher than that of the spherically shaped powders milled for the same period of times.
-
6.
The Curie temperature of Nd15Fe77B8 alloy increases significantly from ingot state to powder shape. The Curie temperatures of Nd15Fe77B8 ingot alloy and the spherically and the flaky-shaped powders were found as 279, 305, and 321 ∘C, respectively. On the other hand, the Curie temperatures of the spherically and the flaky-shaped powders were further increased to 311 and 331 ∘C, respectively, after a milling process for 390 min.
References
Pan, M.X., Zhang, P.Y., Ge, H.L., Wu, Q., Yu, N.J.: Effect of annealing temperature on microstructure and magnetic properties of α-Fe/Nd2Fe14B nanocomposite magnets. Mater. Sci. Technol. 30(7), 832–834 (2014). https://doi.org/10.1179/1743284713Y.0000000418
Harada, T., Kuji, T.: Crystallization of amorphous melt-spun Nd15Fe77Bx (x = 6–14) alloys. J. Mater. Res. 9(2), 372–376 (2011). https://doi.org/10.1557/jmr.1994.0372
Brown, D.N., Wu, Z., He, F., Miller, D.J., Herchenroeder, J.W.: Dysprosium-free melt-spun permanent magnets. J. Phys. Condens Matter 26(6), 064202 (2014). https://doi.org/10.1088/0953-8984/26/6/064202
Hua, Z.S., Wang, L., Wang, J., Xiao, Y.P., Yang, Y.X., Zhao, Z., Liu, M.J.: Extraction of rare earth elements from NdFeB scrap by AlF3–NaF melts. Mater. Sci. Technol. 31(8), 1007–1010 (2015). https://doi.org/10.1179/1743284714Y.0000000672
Ozawa, S., Saito, T., Motegi, T.: Effects of cooling rate on microstructures and magnetic properties of Nd–Fe–B alloys. J. Alloys Compd. 363(1–2), 268–275 (2004). https://doi.org/10.1016/s0925-8388(03)00461-4
Biswas, K., Hermann, R., Wendrock, H., Priede, J., Gerbeth, G., Buechner, B.: Effect of melt convection on the secondary dendritic arm spacing in peritectic Nd–Fe–B alloy. J. Alloys Compd. 480(2), 295–298 (2009). https://doi.org/10.1016/j.jallcom.2009.01.106
Sakaguchi, Y., Harada, T., Kuji, T.: Microstructural studies of Nd□Fe□B powders produced by gas atomization. Mater. Sci. Eng.: A 181, 1232–1236 (1994). https://doi.org/10.1016/0921-5093(94)90837-0
Volkmann, T., Gao, J., Strohmenger, J., Herlach, D.M.: Direct crystallization of the peritectic Nd2Fe14B1 phase by undercooling of the melt. Mater. Sci. Eng.: A 375–377, 1153–1156 (2004). https://doi.org/10.1016/j.msea.2003.10.208
Volkmann, T., Gao, J., Herlach, D.M.: Direct crystallization of the peritectic Nd2Fe14B1 phase by undercooling of bulk alloy melts. Appl. Phys. Lett. 80(11), 1915–1917 (2002). https://doi.org/10.1063/1.1461430
Sellers, C.H., Hyde, T.A., Branagan, D.J., Lewis, L.H., Panchanathan, V.: Microstructure and magnetic properties of inert gas atomized rare earth permanent magnetic materials. J. Appl. Phys. 81(3), 1351–1357 (1997). https://doi.org/10.1063/1.363871
Gögebakan, M., Uzun, O., Karaaslan, T., Keskin, M.: Rapidly solidified Al–6.5 wt.% Ni alloy. J. Mater. Process. Technol. 142(1), 87–92 (2003). https://doi.org/10.1016/s0924-0136(03)00466-7
Jassim, A.K., Hammood, A.S.: Single roll melt spinning technique applied as a sustainable forming process to produce very thin ribbons of 5052 and 5083 Al-Mg alloys directly from liquid state. Procedia CIRP 40, 133–137 (2016). https://doi.org/10.1016/j.procir.2016.01.079
Kramer, M., Lewis, L., Fabietti, L., Tang, Y., Miller, W., Dennis, K., McCallum, R.: Solidification, microstructural refinement and magnetism in Nd 2 Fe 14 B. J. Magn. Magn. Mater. 241(1), 144–155 (2002)
Kim, Y.-W., Yun, Y.-M., Nam, T.-H.: The effect of the melt spinning processing parameters on the solidification structures in Ti–30 at.% Ni–20 at.% Cu shape memory alloys. Mater. Sci. Eng.: A 438–440, 545–548 (2006). https://doi.org/10.1016/j.msea.2006.05.169
Tkatch, V.I., Limanovskii, A.I., Denisenko, S.N., Rassolov, S.G.: The effect of the melt-spinning processing parameters on the rate of cooling. Mater. Sci. Eng.: A 323(1–2), 91–96 (2002). https://doi.org/10.1016/S0921-5093(01)01346-6
Altieri, A.L., Steen, P.H.: Substrate heating in the planar-flow melt spinning of metals. J. Thermal Sci. Eng. Appl 6(4) (2014). https://doi.org/10.1115/1.4027809
Srinivas, M., Majumdar, B., Phanikumar, G., Akhtar, D.: Effect of planar flow melt spinning parameters on ribbon formation in soft magnetic Fe68.5Si18.5B9Nb3Cu1 alloy. Metall. Mater. Trans. B 42(2), 370–379 (2011). https://doi.org/10.1007/s11663-011-9476-7
Umadevi, K., Palit, M., Chelvane, J.A., Babu, D.A., Srivastava, A.P., Kamat, S.V., Jayalakshmi, V.: Effect of wheel speed on the structure, microstructure, magnetic, and electrical properties of Tb-Fe-Co ribbons. J. Supercond. Novel Magn. 29(9), 2455–2460 (2016). https://doi.org/10.1007/s10948-016-3559-2
Ekrami, A., Shahri, F., Mirak, A.: Effect of rare-earth elements and quenching wheel speed on the structure, mechanical and thermal properties of rapidly solidified AZ91 Mg melt-spun ribbons. Mater. Sci. Eng.: A 684, 586–591 (2017). https://doi.org/10.1016/j.msea.2016.12.105
Nothnagel, P., Müller, K.H., Eckert, D., Handstein, A.: The influence of particle size on the coercivity of sintered NdFeB magnets. J. Magn. Magn. Mater. 101(1), 379–381 (1991). https://doi.org/10.1016/0304-8853(91)90786-A
Kronmüller, H., Durst, K.D., Sagawa, M.: Analysis of the magnetic hardening mechanism in RE-feb permanent magnets. J. Magn. Magn. Mater. 74(3), 291–302 (1988). https://doi.org/10.1016/0304-8853(88)90202-8
Su, K.P., Liu, Z.W., Zeng, D.C., Huo, D.X., Li, L.W., Zhang, G.Q.: Structure and size-dependent properties of NdFeB nanoparticles and textured nano-flakes prepared from nanocrystalline ribbons. J. Phys. D: Appl. Phys. 46(24), 245003 (2013)
Choi, M., Kim, D., Yu, J., Kim, Y.: Improvement of the magnetic properties of Nd2Fe14B powders by dysprosium diffusion. Rev. Adv. Mater. Sci. 28(2), 134–140 (2011)
Mo, W., Zhang, L., Shan, A., Cao, L., Wu, J., Komuro, M.: Microstructure and magnetic properties of NdFeB magnet prepared by spark plasma sintering. Intermetallics 15(11), 1483–1488 (2007). https://doi.org/10.1016/j.intermet.2007.05.011
Havlicek, F., Elbel, T.: Geometrical modulus of a casting and its influence on solidification process. Arch. Foundry Eng. 11(4), 170–176 (2011)
Forno, I., Grande, M.A.: Influence of geometry and cooling rate on properties of sinter-hardened steels. Acta Metall. Slovaca 19(4), 271–281 (2013)
Hono, K., Sepehri-Amin, H.: Strategy for high-coercivity Nd–Fe–B magnets. Scripta Mater. 67(6), 530–535 (2012). https://doi.org/10.1016/j.scriptamat.2012.06.038
Chikazumi, S.: Mechanism of high coercivity in rare-earth permanent magnets. J. Magn. Magn. Mater. 54, 1551–1555 (1986). https://doi.org/10.1016/0304-8853(86)90925-X
Branagan, D.J., Hyde, T.A., Sellers, C.H., McCallum, R.W.: Developing rare earth permanent magnet alloys for gas atomization. J. Phys. D: Appl. Phys. 29(9), 2376 (1996)
Cui, B.Z., Zheng, L.Y., Li, W.F., Liu, J.F., Hadjipanayis, G.C.: Single-crystal and textured polycrystalline Nd2Fe14B flakes with a submicron or nanosize thickness. Acta Mater. 60(4), 1721–1730 (2012). https://doi.org/10.1016/j.actamat.2011.11.062
Lidong, L., Liu, J.P., Jian, Z., Weixing, X., Juan, D., Aru, Y., Wei, L., Zhaohui, G.: The microstructure and magnetic properties of anisotropic polycrystalline Nd2Fe14 B nanoflakes prepared by surfactant-assisted cryomilling. Mater. Res. Exp. 1(1), 016106 (2014)
Li, Y., Evans, H.E., Harris, I.R., Jones, I.P.: The oxidation of NdFeB magnets. Oxid. Metals 59(1), 167–182 (2003). https://doi.org/10.1023/a:1023078218047
Schultz, L., Wecker, J., Hellstern, E.: Formation and properties of NdFeB prepared by mechanical alloying and solid-state reaction. J. Appl. Phys. 61(8), 3583–3585 (1987). https://doi.org/10.1063/1.338708
Funding
This study was partially supported by The Scientific and Technological Research Council of Turkey (TÜBİTAK) through project no. 114M501.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Öztürk, S., İcin, K., Öztürk, B. et al. The Role of Wheel Surface Quality on Structural and Hard Magnetic Properties of Nd–Fe–B Permanent Magnet Powders. J Supercond Nov Magn 31, 3025–3041 (2018). https://doi.org/10.1007/s10948-018-4561-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-018-4561-7