Abstract
Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15,0.25,0.35) superconductors are prepared by a two-step solid-state reaction method, and their superconducting properties are studied by resistivity, susceptibility, FTIR, XRD, and excess conductivity analyses. The samples have shown an orthorhombic crystal structure in which the c-axis length and the volume of the unit cell increase with Sr doping. The normal-state resistivity is appreciably suppressed, and the zero resistivity critical temperature observed between 97 and 104 K. The magnitude of the superconductivity is enhanced in Sr-doped samples. In the Fourier transform infrared (FTIR) absorption measurements, the apical oxygen phonon mode is observed to be softened with Sr doping whereas the peak position of planar oxygen stays unaltered in Sr-incorporated samples. The excess conductivity analyses of Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15,0.25,0.35) samples have shown that the values of ξ c(0), the inter-layer coupling J, are not appreciably altered whereas the Fermi velocity v F of superconducting carriers and the phase relaxation increase with Sr doping. The values of parameters such as B c0(T), B c1(T), J c(0) are, however, enhanced with the doping of Sr in the final compound. These studies have shown that Sr doping promotes efficient transfer of the carriers from charge reservoir layer to the conducting CuO2 planes thereby promoting enhancement in the density of carriers and hence increase in the values of aforementioned superconductivity parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The crystal chemistry of cuprate high-temperature superconductors consists of a charge reservoir layer and number conducting CuO2 planes which are separated by Ca atoms. The role of the charge reservoir layer is to supply the carriers to the conducting planes whereas the superconductivity lies in CuO2 planes. The Ca atoms develop inter-plane coupling. We have extensively investigated the role of Ca atoms in developing inter-plane coupling by doping Be and Mg at the Ca sites [1, 2]. In the previous studies, we have observed that the partial substitution of Be and Mg at Ca sites resulted in a decrease in the c-axes length that provided direct evidence of increased inter-plane coupling. The enhancement of the inter-plane coupling is bought about by the small atomic size of the doped Be and Mg atoms at Ca sites. The enhancement of inter-plane coupling resulted into an increase in the T c(R = 0) and magnitude of diamagnetism of Cu0.5Tl0.5Ba2Ca n−1Cu n O2n+4−δ (n = 2,3,4,5) superconductors. Those studies confirmed the pivotal role of inter-plane coupling in the mechanism of high T c superconductors. In the current findings, we have tried to decrease the thickness of the Cu0.5Tl0.5Ba2O4−δ charge reservoir layer by doping Sr(2.15 Å) at the Ba(2.21 Å) sites. The thickness of Cu0.5Tl0.5Ba2O4−δ charge reservoir layer is 4.2 Å whereas the thickness of superconducting blocks containing a Ca atom at their center is 3.2 Å. The decrease in the thickness of the charge reservoir layer in current studies is brought about by doping a smaller sized Sr at the Ba sites. The effect of doped Sr atoms at the intrinsic superconductivity parameters is investigated by carrying excess conductivity analysis of conductivity data.
In previous studies, Sr-doped Tl0.6Pb0.4Ba2−xSr x Ca2 Cu3O9−δ (x = 0,0.10,0.20,0.30,0.40,0.50) samples were synthesized by a two-step solid-state reaction method [1]. Highest zero resistivity temperature was observed at 113 K for x = 0.5, and their X-ray diffraction (XRD) analysis showed a pseudo-tetragonal structure. Increase in c-axis lattice constant with the Sr content was observed. In another study, Sr-doped Y(Ba2−y Sry)Cu3O6+δ superconductors were prepared by a two-step solid-state reaction method at 950 °C [2]. In order to optimize the density of carriers, the samples were annealed in a flowing O2 environment for 20 h. An XRD scan of the annealed samples had shown an orthorhombic structure with zero resistivity temperature at 90 K for y = 0 and 84 K for y = 0.8 content of Sr. They had observed that chemical substitution of Sr2+ ions in fully oxygenated samples leads to a decrease in T c. Sr-doped YBa2(1−x)Sr x Th x Cu3O7−δ (x = 0.1,0.15.0.2 and 0.3) superconductors were prepared at 850° [3]. The XRD scans had shown that double-doped 123 superconductors have mainly single-phase orthorhombic crystal structure with some small extra peaks attributed to secondary phases like tetragonal and non-superconducting YBCO and Y2Cu2O5 phases. The replacement of Ba by Sr had caused a distortion in the perovskite structure and brought shrinking in three axes. Moreover, the orthorhombic splitting of the lattice parameters was also affected by Sr content and was correlated to a decrease in T c. Sr-doped Hg2(Ba1−ySry)2YCu2O8−δ superconductors were synthesized at high pressure (3.5–4 GPa) and temperature (900–1050°) [4]. An increase in T c was observed with an increase in “y” which reached 42 K for y = 1.0. The main phase was Hg-2212, but sometimes for Sr-rich compositions, Hg-1212 impurity phase was observed due to some reaction with gold capsule enhanced by Sr content. A decrease in “a” and “c” axis lengths was observed with an increase in Sr content. The structure of Hg-2212 belonged to space group I/4mmm. Sr-doped samples of La2−xSr x CuO4 superconductors were prepared under high pressure up to 20 kbar [5]. They observed that T o decreases with pressure. The T c of the samples for x ≤ 0.206 increases with the pressure until a critical pressure P c, but pressure dependence of T c becomes very small above P c. Sr-doped samples of Ba2Ca2+xCu3+yO z (T c = 126 K) superconductors were prepared under 5 GPa at 800–950° [6]. A slight decrease up to ∼ 121 K was observed due to Sr doping. With increased Sr concentration more than 0.40, a new superconducting phase was observed with Tc ∼ 106 K. The cation composition was (Ba + Sr)/Ca/Cu ∼ 2:1:2, and it was indexed with I4 symmetry with lattice constants “ a = 3.86 Å” and “c = 22 Å.” A series of compounds having compositions (Er0.76Ca0.24)Ba2−ySr y (Cu2.76Co0.24)O z (y = 0.0,0.2,0.4,0.6) and (Er1−x Ca x )Ba1.4Sr0.6(Cu2.76Co0.24)O z (x = 0.24,0.36,0.48) were prepared by a standard ceramic technique [7]. The XRD pattern showed that three Sr-doped samples (y = 0.0–0.6) remained orthorhombic, while two Ca-Sr doped samples (x = 0.36 and 0.48, y = 0.6) approached tetragonal structure. The XRD pattern revealed that Sr-doped Y-123 showed a decrease of the unit cell volume. A decrease in T c and oxygen content was observed with an increase in Sr content. The suppression in the T c and oxygen content was attributed to the arrangement of oxygen atoms in the basal plane. B.R. Hickey et al. prepared Sr-doped (La1−x Sr x )OFeAs (x = 0.10–0.30) and La(O0.9 F0.1−δ )FeAs samples by a two-step method [8]. It was revealed from the XRD analysis that all samples gave clear evidence that the main peaks were from (La1−x Sr x )OFeAs phase. All main peaks could be indexed by a tetragonal structure with lattice parameters “ a = b = 4.035 Å” and “ c = 8.7710 Å.” These cell parameters are a bit larger than those in parent phase LaOFeAs (a = b = 4.032 Å and c = 8.726 Å) which suggested that lattice expanded a bit with Sr substitution. Resistivity measurements showed that the maximum transition temperature was about 25 K at a doping level of x = 0.13 and no superconductivity was observed beyond x = 0.23. Sr-doped Sr2−x Ba x CuO3+δ (x ≤ 0.6) superconductors were synthesized under a high pressure of 6 GPa at 1000 °C [9]. Maximum T c was obtained in the undoped SrCuO3+δ sample. The XRD analysis was done, and it was found that for x ≤ 0.6, apparently all the samples were tetragonal single phase and belonged to space group I4/mmm. Single-phase superconducting samples of (Bi/Pb)2(Sr2−x Ca x )2Cu3O10 were synthesized a by solid-state reaction method [10]. DC electrical resistivity were carried out, and it was found that a prepared sample with x = 0.6, with optimally nominal oxygen content (over doped), showed T c of 107 K. The XRD analysis has shown that crystal belonged to a tetragonal system and had a space group of I4/mmm.
2 Experimental
The Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) samples were prepared using a solid-state reaction method. We started out with the precursor material of non-stoichiometric composition of form Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) and used Ba(NO3)2, Ca(NO3)2, SrCO3, and Cu(CN) as starting compounds. These materials were mixed for 1 h and fired twice at 860 °C in quartz boat for 24 h. The precursor material after firing was mixed with Tl2O3 to give Tl0.5 Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) as a final reactant composition. Thallium-mixed material was pressed under a pressure of 3.8 tons/cm2 to make disc-shaped pallets. These pallets were enclosed in a gold capsule and sintered at 860 °C for 10 min. The samples were characterized by resistivity, ac susceptibility, and Fourier transform infrared spectroscopic measurements. The FTIR absorption measurements were carried out by using a Nicolet 5700 Fourier transform infrared spectrometer (FTIR) in 400–700 cm−1 wave number range. The crystal structure of the samples was measured by X-ray diffraction scan using Bruker DX 8 Focus employing CuK α radiations of wavelength 1.54056 Å. The cell parameters were determined by check cell computer refinement program. The oxygen content in the final compound was optimized by carrying out post-annealing of the samples for 6 h in a tubular furnace in flowing oxygen atmosphere at 500°.
3 Results and Discussion
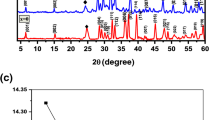
The X-ray diffraction scans of Tl0.5Cu0.5(Ba1−x Sr x ) Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) samples are shown in Fig. 1. Most of the diffraction lines are fitted to the orthorhombic crystal structure by following PMMM space group. The c-axis length and the volume of the unit cell of Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) samples increase with increased Sr content (Table 1). It is most likely that smaller sized Sr promotes relaxation of the lattice axes that would likely result into an increase in the thickness of the charge reservoir layer. The resistivity versus temperature measurements of Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) samples are displayed in Fig. 2. All the samples have shown metallic variations of resistivity from the room temperature down to onset of superconductivity. The room temperature resistivity is significantly reduced with increased Sr doping which shows that Sr incorporation induces a more metallic character in the final compound. Tl0.5Cu0.5(Ba1−x Sr x )Cu3O y (x = 0, 0.15, 0.25, 0.35) samples are shown onset of superconductivity around 113, 112, 113, and 113 K and T c(R = 0) at 97, 102, 96, and 104 K, respectively. The AC magnetic susceptibility measurements of Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) samples are shown in Fig. 3. The onset of superconductivity in these samples is observed around 110, 108, 99, and 103 K (Table 1). The magnitude of diamagnetism systematically increases with Sr doping.
The FTIR absorption measurements of Tl0.5 Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) samples are shown in Fig. 4. In the phonon spectra of these samples, three prominent absorption modes related to the vibrations of oxygen atoms are observed around 450, 555, and 570 cm−1. The former two modes are related to the vibrations of apical oxygen atoms whereas the last mode to the vibrations of CuO2 planar oxygen atoms. The former apical oxygen mode is slightly hardened whereas the later mode is softened (by 2 cm−1). The third mode stays almost unchanged at around 570 cm−1. The main route cause of this hardening/softening of apical oxygen modes is incorporation of Sr at the charge reservoir layer. The effect of Sr doping at the Ba sites on the intrinsic superconductivity parameters has also been investigated by carrying out excess conductivity analysis (FIC) of conductivity data of Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (y = 0, 0.15, 0.25, 0.35) samples.
3.1 Excess Conductivity Analyses (FIC) of Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) Samples
The excess conductivity analyses accomplished by using the equation Δσ (T) = Δσ RT ε −λD in the temperature regime around T c and beyond. This equation can be written in the form as follows:
where \(\varepsilon =\left [ {\frac {T-T_{c}^{\text {mf}}}{T_{c}^{\text {mf}}}} \right ]\) is the reduced temperature and λ D is the dimensional exponent. The dimensional exponent has values 0.3, 0.5, 1.0, and 2.0 for critical, three-dimensional (3D), two-dimensional (2D), and zero-dimensional (0D) conductivities, respectively [13–15]. The Lawrence and Doniach (LD) model used for the analyses of the polycrystalline samples is of the form [16]
Here, J = {2 ξ c(0)/d}2 is the inter-layer coupling and d as the thickness of superconducting layers (∼ 15 Å in current studies) and ξ c(0) is the coherence length along the c-axis. We have determined various crossover temperatures, such as T G, T 3D−2D, and T 2D−0D, from the log plot of the excess conductivity versus the reduced temperature, see Tables 2 and 3. These crossover temperatures are determined from the intersection of various dimensional exponents, i.e., λ cr refers to the slope below T G, λ 3D above T G, and λ 2D to the slope value above T 3D−2D and λ 0D corresponds to the slope of exponent value 2, respectively, Fig. 5a–d. The parameters such as ξ c(0), the inter-layer coupling J are not appreciably altered whereas the Fermi velocity v F of superconducting carriers and their phase relaxation time are enhanced with the doping of Sr in the final compound.
The Ginzburg number N G in the transition region and the temperature T G are determined from the intersection of critical and three-dimensional regimes. By employing the T G and the N G and using the Ginzburg-Landau theory, important superconducting parameters are determined by using the following equations [17–19]:
\(T_{\mathrm {c}}^{\text {mf}}\) is the mean field critical temperature and is determined from the point of inflection of the temperature of derivative of resistivity (d ρ/dT). The Ginzburg-Landau (GL) parameter is defined as κ = λ/ξ. The phase relaxation time of the Cooper pair is calculated by using the following equation [20]:
The coupling constant [\(\lambda =\frac {\hslash \tau _{\varphi }^{-1}}{2\pi k_{B} T}\)] is determined by using the phase relaxation time τ ϕ . The Fermi velocity of the carriers and the energy required to break apart the Cooper pairs are determined by using the following expressions; the value of the proportionality coefficient K ≅ 0.12 is used as in reference [17].
By using N G values in the critical regime of Tl0.5Cu0.5 (Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) samples, the B c(0), B c1(0), B c2(0), J c(0), and κ = λ/ξ are determined by using the aforementioned equations [10–12]. The values of B c0(T), B c1(T), and J c(0) are enhanced with the increased Sr doping (Table 2). The thermodynamic critical field B c is related to the free energy difference between the normal and superconducting electrons. The increase in the values of these parameters suggests that efficient transfer of the mobile charge carrier takes place which increases the Cooper pair density in the conducting CuO2 planes. Moreover, it is most likely that the flux pinning characters is enhanced with Sr doping in the final compound. The energy required to break the Cooper pairs apart into normal electrons, the magnetic field penetration depth λ p.d, and the Ginzburg-Landau (GL) parameter κ are suppressed with Sr incorporation. The phase relaxation time of the carriers increases with Sr doping.
4 Conclusion
Sr-doped Tl0.5Cu0.5(Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15, 0.25, 0.35) superconducting samples are synthesized at a normal pressure, and their superconducting properties are determined. A significant decrease in the room temperature resistivity is observed with the doping of Sr. All the samples have shown metallic variations of resistivity and zero resistivity critical temperature ranging from 97 to 104 K. Magnitude of the superconductivity is significantly enhanced with the doping of Sr in the final compound. The phonon modes related to vibrations of apical oxygen atoms are softened whereas the peak position of CuO2 planar oxygen mode stays unchanged showing that Sr is incorporated at the Ba sites. The excess conductivity analyses of conductivity data of these samples have shown an increase in the values of the phase relaxation time of the carriers τ ϕ and the Fermi velocity v F of superconducting carriers with the doping of Sr in the final compound. The \(V_{F} =\frac {\pi \xi _{C} {\Delta }} {\hslash } \) depends on Fermi-vector K F = (3π 2 N/V)1/3[n = N/V] which in turn depends on the density of the carriers. An increase in the values of v F shows that the carrier’s density in the superconducting CuO2 planes is enhanced with the doping of Sr at the Ba sites despite an increase in the volume of the unit cell. It confirms that doped Sr atoms induce efficient transfer of the charge carriers to the conducting planes. The values of parameters such as B c0(T), B c1(T), and J c(0) are enhanced with the doping of Sr in the final compound. The values of these parameters crucially depend on the thermodynamic critical field B c which is related to the free energy difference between the normal and superconducting electrons. The doped Sr at the Ba sites seems to promote an increase in the difference of free energy difference between normal and superconducting state and hence the values of these parameters. Moreover, the doped Sr atoms enhance the flux pinning characters that can be witnessed in the decrease of London penetration depth λ p.d and the Ginzburg-Landau(GL) parameter κ.
References
Khan, N.A., Khurram, A.A.: Appl. Phys. Lett. 86, 152502 (2005)
Khan, N.A., Husnain, G.: Phys. C 436, 51 (2006)
Jassim, K.A., Alwan, T.J.: J. Supercond. Nov. Magn. 22, 861–865 (2009)
Liu, R.S., Chang, C.Y., Chen, J.M., Liu, R.G.: J. Supercond. 11, 5 (1998)
Sekkina, M.M.A., Elsabawy, K.: J. Supercond.: Inc. Novel Magn. 4, 15 (2002)
Toulemonde, P., Odier, P., Bordet, P., Le Floch, S., Suard, E.: J. Phys.: Condens. Matter. 16, 4061–4076 (2004)
Yamada, N., Ido, M.: Phys. C 203, 240–246 (1992)
Hickey, B.R., Du, Z.L., Xue, Y.Y., Ross, D.K., Dezaneti, L.M., Sun, Y.Y., Wu, N.L., Chu, C.W.: J. Phys. Chem. Solids 60, 1655–1662 (1999)
Pansuria, K.M., Kuberkar, D.G., Baldha, G.J., Kulkarni, R.G.: J. Supercond. 10, 1 (1997)
Wen, H.H., Mu, G., Fang, L., Yang, H., Zhu, X.: Lett. J. Exploring Front. Phys. 82, 17009 (2008)
Gao a, W.B., Liu a, Q.Q., Yang a, L.X., Yua, Y., Li a, F.Y., Wanga, X.C., Zhu a, J.L., Jin a, C.Q., Uchida, S.: Phys. C 470, S19-S20 (2010)
Sekkina, M.M.A., Elsabawy, K.M.: Phys, C 377, 254–259 (2002)
Gao, W.B., Liu, Q.Q., Yang, L.X., Yu, Y., Li, F.Y., Wang, X.C., Zhu, J.L., Jin, C.Q., Uchida, S.: Phys. C 470, S19–S20 (2010)
Sato, T., Nakane, H., Mori, N., Yoshizawa, S.: Phys. C 344-360, 244–247 (2003)
Manmeet Kaur, R., Srinibasan, G.K., Mehta, D., Kanjilal, R., Pinto, S.B., Ogale, S., Ganesan, M.V.: Phys. C 443, 61–68 (2006)
Lawrence, W.E., Doniach, S. In: Kanda, E. (ed.) : Proceedings of the twelfth international conference on low temperature physics, p 361, Keigaku, Tokyo (1971)
Abu Aly, A.I., Ibrahim, I.H., Awad, R.A., El-Harizy, A.: J. Supercond. Nov. Magn. 23, 1325 (2010)
Rojas Sarmiento, M.P., Uribe Laverde, M.A., Vera Lopez, E., Landinez Tellez, D.A., Roa-Rojas, J.: Phys. B 398, 360 (2007)
Ben Azzouz, F., Zouaoui, M., Annabi, M., Ben Salem, M.: Sol. Stat. Phys. (C) 3, 3048 (2006)
Solovjov, A.L., Dmitriev, V.M., Habermeier, H.-U.: Phys. Rev. B 55, 8551 (1997)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khan, N.A., Zeb, M.H. Charge Transfer Mechanism and Superconductivity in Tl0.5Cu0.5 (Ba1−x Sr x )Ca2Cu3O y (x = 0, 0.15,0.25,0.35) Samples. J Supercond Nov Magn 28, 3267–3273 (2015). https://doi.org/10.1007/s10948-015-3193-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-015-3193-4