Abstract
This paper reports the effect of high-spin Co2+-doped CuCrO2 delafossite-type oxide on the structure and physical properties. X-ray diffraction and Raman spectroscopy show that the structure is maintained for all Co-doped samples for chromium. The incorporation of this element generates anisotropic microstrains in the structure. The temperature dependence of zero field-cooling magnetization was measured. All samples exhibit an AFM transition around 24 K. The high-spin state and the shift due to the exchange splitting of the conduction band suggest strong hybridization between carriers in the Cr 3d t2g band and the t2g states of the high-spin Co2+ to develop other spin orders benefiting to enhance magnetic susceptibility and support the evidence of new FM transition. The coupling between the magnetic order and ferroelectric order is also characterized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The delafossite oxide CuMO2 (M = trivalent cation or mixture of trivalent and bivalent cations) [1, 2] is one of a number of systems possessing an antiferromagnetic triangular sublattice. CuMO2 has a layered structure with a space group of R-3m, which is viewed as the alternate stacking of edge-shared MO6 octahedral ( MO2) layers and Cu layers. Remarkably, all these structural families are based on CdI2-type layers containing first-row transition metals and oxygen. In the delafossite crystal structure, MO2 layers of edge-sharing MO6 octahedra alternate along the c axis with layers of monovalent Cu+ cations with dumbbell O–Cu–O coordination. Interesting thermoelectric performances have been reported in delafossite-type oxides.
The magnetic properties of these layered compounds have attracted much attention, since the geometrical frustration in the magnetic triangular sublattice at the M sites causes intriguing properties, such as field-induced multistep magnetization change [3, 4] and multiferroics [5]. CuCrO2 is reported to exhibit both antiferromagnetic [6] and ferroelectric [7] behaviors below its Néel temperature, T N=25 K. Delafossites have been particularly investigated because of the diversity of their physical properties.
Another reason is the discovery of spin-driven multiferroicity in the layered delafossite compound CuFeO2 [8], which leads us to study the parent oxide CuCrO2, to which only a few studies had been dedicated. The magnetic properties of CuCrO2 were investigated by Doumerc et al. [9] in the two-dimensional (2D) Heisenberg framework. In their pioneer work on the investigation by neutron powder diffraction of the magnetic structure of CuCrO2, Kadowaki et al. [10] proposed several possible magnetic models and reported strong magnetic disorder in the stacking c direction. Very recently, CuCrO2 was reported to show ferroelectric polarization upon spin ordering, [11] suggesting a strong coupling between ferroelectricity and the assumed 120∘ spiral structure. Several points remained unclear however; because of the lack of low-temperature structural data, it was not known whether CuCrO2 exhibits a structural phase transition similar to the one observed in CuFeO2 and which could explain the observed three-dimensional (3D) magnetic ordering in a triangular lattice, whose ground state should otherwise stay degenerated.
In this work, we focus on the effect of magnetic Co2+ substitution on the structural, magnetic, and dielectric properties of CuCrO2 (system CuCr1−xCo x O2).
2 Experimental
Polycrystalline samples of Co2+-doped CuCrO2 for different compositions around 6 % were prepared using the standard solid-state reaction. Stoichiometric mixtures (0.5 g) of Cu2O,Cr2O3, and CoO were ground and pressed in pellets. The samples were fired repeatedly in air at 1, 150 ∘C for 12 h in alumina crucibles with intermediate regrinding and pelletizing. X-ray powder diffraction patterns of the products were collected with a PANalytical diffractometer equipped with a CuKa source ( K α1 and K α2) in the 2 𝜃 range from 10∘ to 90∘ at room temperature.
Strain and size components were extracted from line widths using the Williamson–Hall (WH) analysis [12]. This method uses the fact that the crystallite size and strain contributions to the line widths vary differently with the diffraction angle. The equation used is
where L is the integral width; λ the wavelength; D the size of coherent diffraction domains, which is a near-unity constant; and ε th microstrain term. As a result, a plot of (L ⋅cos 𝜃) as a function of (sin 𝜃) yields D from the constant term and ε from the slope.
Raman spectra were recorded at room temperature with the 514.5-nm excitation from a Spectra Physics krypton ion laser. The compounds were studied with a low laser power (102 mW). One scanning of 60 s has been used for each sample. No damage of the material by the laser has been observed. The beam was focused onto the samples using the macroscopic configuration of the apparatus.
Magnetization measurements vs. temperature were carried out in a Quantum Design superconducting quantum interference device (SQUID) magnetometer in the range 4–300 K.
The typical field used for temperature dependence measurements was 0.1 T. Specific heat was measured in zero magnetic fields by a relaxation method. Dielectric data were collected using an impedance analyzer.
3 Results and Discussion
3.1 Structural Characterization
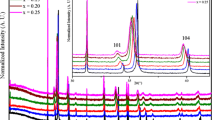
Figure 1 shows the XRD patternse of Co2+-substituted delafossite CuCrO2, in which all the peaks are indexed as the delafossite structure (space group R-3m), except for x = 0.06, where the same picture shows a small amount of impurities, around 2 % of cobalt oxides and spinel. CuCr2O4 was also detected.
The reflections of substituted samples are shifted to lower values of 2 𝜃, indicating a lattice parameter increase with substitution. The lattice parameters a and c of the samples are given in Fig. 2.
Since Co2+ and Cr3+ do not have the same oxidation state, Co2+ substitution for Cr3+ is expected to introduce mainly atomic disorder in the Cr network. In addition, this disorder is probably related to the introduction of strains in the lattice, since Co2+ is a much larger cation than Cr3+ [13]. These results are in good agreement with those reported by Okuda et al. [14]. This increase in ionic radius ( r Cr3+=0.61 Å, r Co2+=0.78 Å) causes the distortion of the network and the increase in lattice parameters. It seems that O–Cu–O bond lengths along the c axis are quite almost not affected by the chromium to cobalt substitution, whereas aparameter increases proportionally to the edge of the MO6 octahedral when x increases. This anisotropy induces a constant increase of the distortion of the MO6 octahedra from chromium to cobalt, which can be correlated to the covalence of the metal-to-oxygen bond [15].
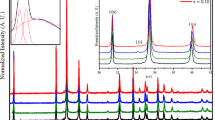
The structure of CuCr1−xCo x O2(0 ≤x ≤ 0.06) closely is delafossite. It consists of close-packed oxygen sheets in which the octahedral sites are occupied by Cr3+/ Co2+ ions. The (Cr/Co)O6 octahedra share six edges to form infinite \(\{\mathrm {(Cr/Co)O}_{2}\}_{\infty }\) layers connected to each other by Cu+ linearly bonded to two oxygen to form (O−C u−O)3− “dumbbell” parallel to the c axis. Each Cu+ is surrounded by six coppers hexagonally arranged in the (a, b) plane. Strain generated by the Co substitution was determined from the Williamson–Hall relationship. Plots of (L ⋅cos 𝜃) as a function of (sin 𝜃) are given in Fig. 3. They show a remarkable difference in angular dependence of the line width for different families of interreticular planes: the h0l planes yield an important contribution of microstrains (high slope), while this effect is almost negligible in 00l planes. This shows that this material behaves rather anisotropically and that strains affect mostly bonding in the basal ab planes.
3.2 Raman Spectroscopic
The delafossite structure belongs to point group C3v and space group R-3m. The four atoms in the primitive cell of the rhombohedral R-3mstructure give rise to 12 optical phonon modes in the zone center (k ∼0): three acoustic and nine optical modes. The latter are Δopt.R3c=A1g+Eg+3A2u+3Eu. Among these, the two phonon modes with A1g and Eg symmetry are Raman active. The former arise from the Cu–O bond vibration along the c axis, whereas the doubly degenerate E modes describe the vibrations along the a axis. The existence of an inversion center in the delafossite structure could classify the normal modes in terms of their parity. The odd modes, denoted with the “u” subscript, are the acoustic modes (A2u+Eu) which are IR active. Since there is only one mode of each symmetry, the exact eigenvector is determined without requiring any lattice dynamical model. Pellicer-Porres et al. [16] have discussed the phonon dispersion at the zone center for CuGaO2 delafossite. They proposed that the inversion center is lost along the direction of Γ(T) direction and the symmetry is reduced from D3d to C3v. According to compatibility relations, A1g and A2u on one hand and Eg and Eu modes on the other transform to A1 and E modes, respectively.
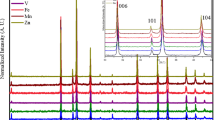
To further study the doping effect on the structure by the replacement of Cr3+ with Co2+ ions, Raman spectroscopy was performed with 514.5-nm excitation light. Figure 4 shows the Raman spectra of CuCr1−xCo x O2 for different cobalt contents. Three Raman peaks were observed for all samples around 453(A g), 531( Eg), and \(707~\text {cm}^{-1}(\mathrm {A}_{\mathrm {1g}})\), which agree well with earlier studies on those compounds with delafossite structure [16, 17]. The A1g mode corresponded to the (Cr/Co)–O stretching of (Cr/Co)O 6 octahedra and the Eg mode to the O–(Cr/Co)–O bending. This assignment was based on the fact that the movement of oxygen atoms attached to the central metal atom viz, (Cr/Co), was responsible for the observed Raman modes. Those results showed that Co substitution did not change the delafossite structure of the material. As shown in Fig. 4, Co-doped samples induce a slight increase in linewidth of the three active Raman modes Ag,A1g, and Eg, indicating an increasing in (Cr/Co)–O bonding, which is consistent with the observed lattice increase along the different axes and the difference in ionic radius between Cr3+ and Co2+.
3.3 Magnetic Properties
Figure 5 shows the temperature dependence of zero field-cooling susceptibilities ( χ–Tcurve) of the CuCr1−xCo x O2 (0 ≤x ≤ 0.06) samples, where a 0.1-T magnetic field was applied. For the first distinguishing, it can be clearly seen that the magnetic susceptibility increases with the increases of the molar concentration in the all compounds. This increase of the magnetic susceptibilities for all samples in the temperature range 2–30 K may be due to interaction between Co2+ and Cr3+ spin systems. In all the curves of susceptibilities, an anomaly appears at 25 K owing to an antiferromagnetic (AFM) transition. The Néel transition ( T N) is almost consistent with the one previously reported [10]. They assume that the samples are not in a phase-separated state as composed of CuCrO2 ( T N=24 K) [10, 18]. At this temperature and above 130 K, all samples are in the paramagnetic state.
Abrupt increases in magnetization appear between 100 and 130 K shown in Fig. 4. It implies a FM transition which should be due to the FM interaction through the Co2+–O– Cr3+ and in CuCr1−xCo x O2(0 ≤x ≤ 0.06) exchange in the first time. All these changes in the χ–Tcurves in the two systems indicate a competition between AFM and FM interactions, and it also indicates that the DE interaction between the Co2+ and Cr3+ ions is very important.
Correlating the increases in lattice parameters a and c in CuCr1−xCo x O2system with increase in magnetic susceptibility and the development of the new FM transition, the increases of the in-plane lattice parameter indicate that the valence state of Co ions was not Co3+ (HS) or Co3+ (L ⋅S) according to the data of the ionic radius of sixfold coordinated Co and Cr ( Co3+ (HS): 0.61 Å, Co3+ (L ⋅S): 0.52 Å, and Cr3+: 0.615 Å) [19] as well as lattice expansion. The lattice parameters a and c would increase if the valence state of the Co ions is 2 + in Co-doped CuCrO2 semiconductors because the ionic radii of Co2+ are larger than that of Cr3+ ( Co2+ (HS): 0.73 Å, and Co2+ (L ⋅S): 0.65 Å) [19]. The development of the new FM transition proves that Co2+ is H ⋅S ( S=3/2), because of its Co2+ L ⋅S ( S=1/2); all samples do not develop a FM transition, and during the coupling spin between Co2+ L ⋅S ( S=1/2) and Cr3+ ( S=3/2), the antiferromagnetic (AF) transition becomes broader and the magnetic susceptibility decreases with increasing of Co content. However, it would remain constant that Co ions is 2 + (HS) since the ionic radius of Co2+ (H ⋅S) is larger than that of Cr3+, whereas the valence state of Co ions cannot be totally confirmed by the changes in the lattice parameters.
The exchange interaction was extensively studied in many works. Li et al. [20] proposed that the Mn3+–O– Cr3+ exchange interaction in CuCr1−xMn x O2 system is superexchange (SE). Ohtsuki et al. [21] supposed that the magnetic interactions between Co2+ and Co2+ ions taking the high-spin configuration should develop weak ferromagnetic interactions; those interactions between Cr3+ and Cr3+ ions in the mother CuCrO2 are antiferromagnetic [22]. The anisotropic magnetic interactions of the A-type structure and induced three-dimensional ferromagnetic interactions in CuCoO 2 compounds show a weak ferromagnetism, by introducing some metal transition in three valences in Co sites. Such a Cr doping on the Co site enhances the ferromagnetic interaction in these compounds.
On the other hand, Li et al. [20] reported that the Mn substitution in Cr sites of the mother compound CuCrO2 induces disorder on the Cr sites and prevents the establishment of conducting paths along the Mn–O–Cr bonds. Conversely, the introduction of trivalent cations does not induce a strong ferromagnetism and the electronic transport is obviously hindered by these foreign cations on the Cr sites. This supposition explains the strong ferromagnetic transition in CuCr1−xMn x O2 which is induced by introducing mixed-valent manganese Mn 3+/Mn 4+, and they induce disorder on the Mn sites. These phenomena explain the variation of the amplitude transition ferromagnetic with the temperature. It is clearly seen that this FM transition in high-spin configuration of Co-doped CuCrO2 in the temperature range around 130 K increases with increasing of Co content. All those results we obtained support the evidence of the FM transition in newly developed Co-doped CuCrO2. The high-spin state and the shift due to the exchange splitting of the conduction band suggest strong hybridization between carriers in the Cr 3d t 2g band and the t 2g states of the high-spin Co2+. These observations support the argument that the temperature of FM transition in high-spin Co2+-doped CuCrO2 is intrinsic. It is clearly seen that this FM transition around 130 K increases with Co content and might be attributed either to the Co2+–O– Cr3+ exchange interaction ( Co2+ HS ( S=3/2)) which help to tune the spin chirality and to develop other spin orders benefiting to enhance magnetic susceptibility.
In the paramagnetic states, χ −1–T (inset of Fig. 5) obeys a Curie–Weiss form χ=C/ (T+Θcw) where C and Θcw (≥0) are the Curie–Weiss constant and magnetic coupling parameter, respectively. Abrupt increases in magnetic susceptibility appear which explain the effect of Co-doped CuCrO2.
The estimated C and Θcw are given in Table 1. Around T N, an anomaly associated with a three-dimensional antiferromagnetic (AFM) order was observed up to well with previous by [23].
All data yield highly negative Θcw values, indicating dominant antiferromagnetic interactions. Therefore, the observed increase of \(\left | {\Theta } \right |\) might be attributed to the effect of Co2+, as the number of magnetic nearest neighbors should decrease as the Cr concentration is more and more accentuated.
An effective moment by formula unit can be extracted using the following formula:
where k B= Boltzmann constant, C = Curie constant, N = Avogadro’s number, and μ B= Bohr magneton. In the spin-only picture usually followed for localized spins of 3d ions, μ eff is given by
where g ≈2.0. The experimental values of μ eff are all slightly lower than the theoretical spin-only value for Cr3+ ( μ eff / μ B=√15 = 3.87). These results are consistent with the behavior commonly observed for 3d cations, where the incomplete quenching of orbital moments yields lower values of μ eff. The effective moments lie between 3.8 and 4.11 μ B, which are closely constant. These results lead to the consideration that Co ions in the delafossite structure are mainly in bivalent high-spin state ( S=3/2) which explains the constancy of the effective moments in substitution of Cr3+ ( S=3/2) by Co2+ HS ( S=3/2), contrary to those in CuCoO2 [24] and AgCoO2 [25].
The interaction of the Cr and Co cations with each other through Cr–O–Co linkages of approximately 180∘ is expected to be the dominant consideration, and this would be antiferromagnetic in nature. Recently, evidence of room temperature ferromagnetism in Co-doped transparent CuAlO2 semiconductor was reported. Specially, the coercivity (Hc) and saturation magnetization are significantly enhanced with Co composition [26]. To further reveal this point, we present the measured χ–Hhysteresis at T=4 K for CuCr1−xCo x O2 (x = 0.00 and 0.06) as shown in Fig. 6. While no loop for CuCrO2 is shown, a remarkable loop for CuCr1−xCo x O2 ( x=0.06) is observed, giving rise to a coercivity of 5,000 Oe and remnant magnetization of ∼4.75 E −4 emu ⋅mol−1, although no magnetization saturation is obtained, probably due to the spin-glasslike essence. Therefore, the substantial ferromagnetic component in the magnetism of CuCr1−xCo x O2 is revealed. The observed magnetic behavior for CuCr1−xCo x O2 is probably due to the disorder of Cr and Co in octahedral sites resulting in short-range Cr–O–Cr and Cr–O–Co interactions between which could give rise to antiferromagnetism coupled with short-range weak ferromagnetism. Thus, the observed ferromagnetism is essentially intrinsic to some extent.
Figure 7 shows the temperature dependence of specific heat (C) for CuCr1−xCo x O2 (x = 0.00 and 0.06). For both compounds, the electric contribution to specific heat is negligible at low temperatures [27]. This figure clearly shows that the specific heat peak at T N becomes broader and that the peak temperature ( T peak) increases with increase in x. Such xdependence of Cis apparently correlated with magnetization (M) [3]. Corresponding to the AFM transition, a sharp peak is observed at about 24 K. In addition to the sharp peak, a broad shoulder is observed around 24 K. Such a broad C (T) peak profile is often observed in frustrated kagome [28] or triangular [29] AFM lattices and indicates high degeneracy due to magnetic frustration which was also observed for all CuCr1−xMg x O2 compounds [30].
3.4 Dielectric Properties
Figure 8a, b—combining also susceptibility curves and dielectric permittivity—reveals a clear relationship between the temperature of the dielectric anomaly and the T N. The strength of the dielectric anomaly increases by increasing the cobalt content in CuCrO2.
The anomaly in dielectric permittivity associated with the noncollinear antiferromagnetic phase has been reported previously for CuCrO2 and Al-doped CuCrO2. Dielectric permittivity anomaly was observed around magnetic transition temperature, indicating magnetoelectric coupling in these samples. The increase of the dielectric permittivity at Neel temperature T N is related to thermally induced enhancement of the hopping conduction [31–33], which is caused by oxygen ion vacancies causing stabilization of the Cr–O couple–oxygen vacancy interaction.
The susceptibility ( χ) curve exhibits a well-defined anomaly with a maximum near 24 K which is also correlated with a dielectric anomaly (Fig. 8b). The broad dielectric anomaly starting at about 24 K with a maximum near 23 K (Fig. 8a) can therefore be associated with the formation of antiferromagnetic phase, from paramagnetic to antiferromagnetic structure. Co doping affects both dielectric permittivity and magnetization curves (Fig. 8a, b). In particular, for CuCr1−xCo x O2, x = 0.04, it was observed that the anomaly is shoulder like and the Neel temperature is shifted towards lower temperature region ( T N= 24 K; Fig. 8b). This anomaly seems also to be an indication of AFM ordering [34, 35]. At almost the same temperature, a clear peak in the dielectric permittivity is induced by Co2+ substitution for Cr3+ (Fig. 8a). Further, doping effect could be more evidenced by measuring polarization, which was not available for us during this study. These anomalies are clear indications of the ME coupling, and the underlying physics is straightforward by considering the fact that the AFM order with the proper spin chirality can be further stabilized by the established ferroelectric order via their coupling.
4 Conclusion
We have prepared polycrystalline CuCr1−xCo x O2 (0 ≤x ≤ 0.06) samples by solid-state reaction. In the present work, we reported the effect of high-spin Co2+-doped CuCrO2 on structural, physical, and spectroscopic properties. The Co incorporation in the delafossite structure generates microstrains showing an anisotropical behavior. The increased strain is likely due to some absence of uniformity of the distribution for incorporated oxygen. Raman spectroscopy showed that Co substitution did not change the delafossite structure of the material. However, the relative intensity of Raman peaks changes rapidly with the content and the peak position also shifts towards shorter wavenumbers, indicating the increase of the lattice distortion with the substitution. It has been revealed that the high-spin Co2+-doping CuCrO2 accentuated the magnetic susceptibility. It is argued that the high-spin state of Co2+ doping destabilizes the antiferromagnetic order of Cr3+ ions, modulates the spin configuration, and suggests a strong hybridization between carriers in the Cr 3d t2g band and the t2g states of the high-spin Co2+ to develop other spin orders benefiting to enhance magnetic susceptibility and support the evidence of new FM transition. Clear hysteresis loops check that FM order exists at 4 K.
References
Elkhouni, T., Colin, C.V., Strobel, P., Ben Salah, A., Amami, M.: J. Supercond. Nov. Magn. 7, 1807 (2012)
Elkhouni, T., Amami, M., Strobel, P., Ben Salah, A.: J. Supercond. Nov. Magn. 7, 2256 (2013)
Okuda, T., Jufuku, N., Hidaka, S., Terada, N.: Phys. Rev. B 72, 144403 (2005)
Terada, N., Nakamura, Y., Katsumata, K., Yamamoto, T., Staub, U., Kindo, K., Hagiwara, M., Tanaka, Y., Kikkawa, A., Toyama, H., Fuyuki, T., Kanmuri, R., Ishikawa, T., Kitamura, H.: Phys. Rev. B 74(R), 180404 (2006)
Kimura, T., Lashley, J.C., Ramirez, A.P.: Phys. Rev. B 73(R), 180404 (2006)
Attili, R.N., Uhrmacher, M., Lieb, K.P., Ziegeler, L., Mekata, M., Schwarzmann, E.: Phys. Rev. B 53, 600 (1996)
Kumar, S., Singh, K., Miclau, M., Simon, C., Martin, C., Maignan, A.: J. Solid State Chem. 4596, 00197–7 (2013)
Kimura, T., Lashley, J.C., Ramirez, A.P.: J. Phys. Rev. B 73, 220401R (2006)
Doumerc, J.P., Wichainchai, A., Ammar, A., Pouchard, M., Hagenmuller, P.: J. Mater. Res. Bull. 21, 45 (1986)
Kadowaki, H., Kikuchi, H., Ajiro, Y.: J. Phys. Condens. Matter. 2, 4485 (1990)
Seki, S., Onose, Y., Tokura, Y.: J. Phys. Rev. Lett. 101, 067204 (2008)
Williamson, G.K., Hall, W.H.: Acta Metall. 1, 22 (1953)
Shannon, R.D., Rogers, D.B., Prewitt, C.T.: Inorg. Chem. 10, 713 (1971)
Okuda, T., Beppu, Y., Fujii, Y., Onoe, T., Terada, N., Miyasaka, S.: Phys. Rev. B 77, 134423 (2008)
Doumerc, J.P., Ammar, A., Wichainchai, A., Pouchard, M., Hagenmuller, P.J.: Phys. Chem. Solids 48, 37–43 (1987)
Pellicer-Poress, J., Martinez-Garcia, D., Segura, A., Rodrigez-hernandez, P., Munoz, A., Chervin, J.C.: Phys. Rev. B 74, 18430 (2006)
Elkhouni, T., Amami, M., Hlil, E.K., Ben Salah, A.: J Supercond. Nov. Magn. 8, 2182 (2013)
Okuda, T., Kishimoto, T., Uto, K., Hokazono, T., Onose, Y., Tokura, Y., Kajimoto, R., Matsuda, M.: J. Phys. Soc. Jpn. 78, 013604 (2009)
Deisenhofer, J., Paraskevopoulos, M., Krug von Nidda, H-A., Loidl, A.: J. Phys. Rev. B 66, 054414 (2000)
Li, D., Fang, X., Dong, W., Deng, Z., Tao, R., Zhou, S., Wang, J., Wang, T., Zhao, Y., Zhu, X.: J. Phys. D: Appl. Phys. 42, 055009 (2009)
Quilty, J.W., Shibata, A., Son, J.Y., Takubo, K., Mizokawa, T., Toyosaki, H., Fukumura, T., Kawasaki, M.: J. Phys. Rev. Lett. 96, 027202 (2006)
Yang, Z., Ye, L., Xie, X.: J. Phys. Condens. Matter. 12, 2737–2747 (2000)
Amami, M., Colin, C.V., Strobel, P., Ben Salah, A.: J. Physica B 406, 2182–2185 (2011)
Beekmana, M., Salvadorb, J., Shic, X., Nolasa, G.S., Yang, J.: J. All. Comp. 489, 336 (2010)
Muguerra, H., Colin, C., Anne, M., Julien, M-H., Strobel, P.: J. Sol. Stat. Chem. 181, 2883 (2008)
Dong, C.J., Yu, W.X., Xu, M., Cao, J.J., Zhang, Y., Chuai, Y.H., Wang, Y.D.: J. All. Comp. 512, 195 (2012)
Okuda, T., Beppu, Y., Fujii, Y., Onoe, T., Terada, N., Miyasaka, S.: Phys. Rev. B 77, 134423 (2008)
Ramirez, A.P., Hessen, B., Winklemann, M.: Phys. Rev. Lett. 84, 2957 (2000)
Nakatsuji, S., Nambu, Y., Tonomura, H., Sakai, O., Jonas, S., Broholm, C., Tsunetsugu, H., Qiu, Y., Maeno, Y.: Science 309, 1697 (2005)
Okuda, T., Beppu, Y., Fujii, Y., Kishimoto, T., Uto, K., Onoe, T., Jufuku, N., Hidaka, S., Terada, N., Miyasaka, S.: J. Phys. Conf. Ser. 150, 042157 (2009)
Huang, F., Lu, X., Lin, W., Wu, X., Kan, Y.: J. Appl. Phys. Lett. 89, 242914 (2006)
Hiroshi, U., Risako, U., Hiroshi, F., Koda, S.: J. Appl. Phys. 100, 014106 (2006)
Mishra, R.K., Pradhan, D.K., Choudhary, R.N.P., Banerjee, A.: J. Phys. Condens. Matter. 20, 045218 (2008)
Luo, S., Li, L., Wang, K.F., Li, S.Z., Dong, X.W., Yan, Z.B., Liu, J.M.: Thin Solid Films 518, e50 (2010)
Kimura, K., Nakamura, H., Ohgushi, K., Kimura, T.: Phys. Rev. B 78, 140401(R) (2008)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Elkhouni, T., Amami, M., Strobel, P. et al. Evidence of Development of New Spin Orders Benefiting to Enhance Magnetic Properties in Co2+-Doped Delafossite-Type Oxide CuCrO2 . J Supercond Nov Magn 28, 1–8 (2015). https://doi.org/10.1007/s10948-014-2842-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-014-2842-3