Abstract
We prepared three ferrite nanocatalysts: (i) copper ferrite (CuFe2O4) (ii) ferrite where cobalt was substituted by nickel (Ni x Co1−x Fe2O4, with x=0, 0.2, 0.4, 0.6), and (iii) ferrite where nickel was substituted by zinc (Zn y Ni1−y Fe2O4 with y=1, 0.7, 0.5, 0.3), by the sol-gel method. The X-ray diffraction patterns show that the ferrite samples have been crystallized in the cubic spinel structural phase. We obtained the size of grains by field emission scanning electron microscopy images and their magnetic properties by vibrating sample magnetometer. Next, carbon nanotubes were grown on these nanocatalysts by the catalytic chemical vapor deposition method. We show that the catalytic activity of these nanocrystals on the carbon nanotube growth depend on cation distributions in the octahedral and tetrahedral sites, structural isotropy, and catalytic activity due to cations. Our study may have applications in finding a suitable candidate of doped ferrite nanocrystals as catalysts for carbon nanotube growth. More interestingly, the yield of fabrication of carbon nanotubes can be considered as an indirect tool to study catalytic activity of ferrites.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In the spinel ferrites of MFe2O4, the metallic cations M2+ and Fe3+ can occupy octahedral and tetrahedral sites. If the M2+ cations occupy tetrahedral sublattices in the cubic closed-packed O2− lattice, the spinel ferrite is a normal spinel; otherwise, the ferrite is an inverse spinel. If both of the sublattices contain M2+ and Fe3+ cations, the ferrite is a mixed spinel. The occupations of cations at these sites have a significant effect on the properties of spinels, such as magnetic behavior, conductivity, and catalytic activity [1–3]. Mixed nickel ferrites with different magnetization and various cation distributions form an important class of magnetic materials [4].
Ni/Zn ferrites have the mixed spinel structure with the unit cell consisting of eight units of the form [Zn x 2+Fe1−x 3+]tet [Ni1−x 2+Fe1+x 3+]octO4 2−. The Zn2+ cations preferably occupy the tetrahedral sites and the Ni2+ cations always occupy the octahedral sites [5]. In Ni/Co ferrites the cation distribution of Co2+ depends on heat treatment [6, 7]. As an application, spinel ferrites can acts as good catalysts [8]. The catalytic activity of these materials was studied in some chemical reactions such as decomposition of hydrogen peroxide [9], oxidation of carbon monoxide [10], and oxidative dehydrogenation of butane [11]. Chemical composition, crystal structure, electronic, electrochemical, and micro structural factors have been found to contribute to the overall activity of such catalysts [9]. Since the nano-sized catalysts with non-zero magnetic moment are widely used for growth of carbon nanotubes (CNTs), it is of paramount practical and theoretical importance to investigate the catalytic activity of spinel ferrite nanocrystals on the growth of CNTs.
In this paper, we study the catalytic effect of Ni/Co ferrites (Ni x Co1−x Fe2O4 with x=0, 0.2, 0.4, 0.6), Ni/Zn ferrites (Zn y Ni1−y Fe2O4 with y=0.3, 0.5, 0.7, 1), and copper ferrite (CuFe2O4) on the growth of CNTs by the catalytic chemical vapor deposition (CCVD) method. Specifically, we investigate the occupation effect of ferromagnetic ions Fe3+, Co2+, Ni2+ and non-magnetic ions such as Cu2+ and Zn2+ in the tetrahedral and octahedral sites of ferrospinels on their catalytic activity for growth of CNTs.
2 Experiment
2.1 Preparation of Catalyst
The sol-gel method is one of the best procedures for fabricating the ferrite nanocrystals. Thus in our experiment, Ni/Co, Ni/Zn, and Cu ferrites were all prepared by this method. In this way, with the stoichiometric laws, (depending on the combination), we prepared 0.5 M solutions of Fe(NO3)3⋅9H2O (98 %), Co(NO3)2⋅6H2O (98 %), Ni(NO3)2⋅6H2O (99 %), Zn(NO3)2⋅3H2O (99 %) and Cu(NO3)2⋅3H2O (99 %); and added these solutions to 0.5 M solution of citric acid with 1:1 mol ratio for nitrates:citric acid. The pH value of the solution was adjusted to 1 by ethylenediamine in order to make the environment more conducive for generating fine particles. The prepared solution was baked in 70 °C to form a brown gel. The obtained gel was dried in 135 °C during 24 hours and was ground into a fine powder. Finally, the sample was calcined in 450–600 °C range (depending on the doping value of the ferrites) for 4 hours.
2.2 Growth of Carbon Nanotubes
The CNTs were grown by the CCVD method in a quartz reactor with a programmable furnace. The carbon source was acetylene (C2H2) with argon (Ar) as the carrier gas. To synthesize, an alumina boat containing 0.05 g of catalyst was placed in the hot zone of the quartz reactor. A mixture of C2H2 and Ar with 1:8 volume ratios was passed over the catalyst in atmospheric pressure. The temperature was risen with the rate of 5 °C/min. to a specific temperature that depending on the crystallization temperature of each catalyst, and the reaction time was 45 minutes. Since the cation distribution of ferrites depends on heat treatment [6, 7], the growth temperatures were selected equal to the calcination temperatures. The amount of carbon was evaluated by the mass of the fabricated samples. These samples contain amorphous carbons, CNTs, and catalyst. In order to remove the amorphous carbons, we oxidized the samples in the air at 400 ∘C for 1 hour.
2.3 Characterization
X-ray diffraction (XRD) patterns of the synthesized nanocatalysts were obtained using a Philips® PW1800 X-ray diffractometer with Cu Kα radiation (λ=1.54056 Å) operated at 40 kV and 30 mA. The refinement method of Rietveld was applied with the “Material Analysis Using Diffraction” (MAUD) program (v.2.056) for the XRD patterns of nanocatalysts. The average grain size and morphology of the nanocatalysts were observed by a Hitachi® S4160 Field Emission Scanning Electron Microscopy (FE-SEM). The magnetic properties of the samples were measured by Meghnatis Daghigh Kavir Co.® Vibrating Sample Magnetometer (VSM) at the room temperature. The maximum applied field during such measurements was 9 kOe. Also, we characterized the CNTs by these X-ray diffractometer and FE-SEM.
3 Results and Discussion
3.1 Structural Studies of Nanocatalysts
We studied the crystalline structure of the ferrite nanocatalysts with the XRD analysis. Figure 1 shows the XRD pattern of the prepared nanocrystals. These patterns were compared with the Joint Committee on Powder Diffraction Standards (JCPDS). The presence of (220), (311), (400), (422), (511), and (440) major lattice planes revealed the cubic spinel phase with Fd3m space group. In addition, the minor lattice planes of (111) and (222) are present. These results emphasize the presence of only spinel phase without any significant impurities. The XRD pattern of samples was refined by using the MAUD software and Reitveld method for structural analysis, cation distribution, and lattice parameter calculation. Crystallographic properties of the samples were obtained from this calculation and are listed in Table 1. We report the details of the MAUD analysis for Ni/Zn and Cu ferrite nanocatalysts in the other paper. Table 2 shows the before- and after-refinement values indicating high accuracy in synthesizing the nanocatalysts without formation of extra phases. The pattern fitness can be checked for XRD data. There are several parameters for the calculation of pattern fitness. The goodness of fit (S) is described by S=R wp/R exp, where R wp is the weighted residual error and R exp is the expected error. Refinement has been continued until a convergence was reached for a value of S close to 1 which confirms the goodness of refinement. These parameters are listed in Table 3. This study indicates the inverse spinel structure for Ni/Co and copper ferrites. This means that in the Ni/Co ferrites, the Ni2+ and Co2+ cations occupy the octahedral sites, and the Fe3+ cations occupy the octahedral and tetrahedral sites equally; and, in the copper ferrite the Cu2+ cations fill only the half of the octahedral sites. This study shows as well the normal spinel structure for the zinc ferrite and mixed spinel structure for the Ni/Zn ferrites because the Ni2+ cations occupy the octahedral and the Zn2+ cations occupy the tetrahedral sites. Notice that these results are consistent with Ref. [5].
The lattice constant of the samples were obtained from the formula a=d (h 2+k 2+l 2)1/2, where d is the interplane spacing and is calculated from the position of the highest peak in the XRD pattern (here (311) plane) by Bragg’s formula (and refined later by using MAUD software). In Table 1, we observe that the lattice constant increases with the Co content in the Ni/Co ferrites and the Zn content in the Ni/Zn ferrites. This can be attributed to the higher ionic radii of Co2+ (0.79 Å) and Zn2+ (0.82 Å) compared to Ni2+ (0.69 Å).
3.2 Magnetic Study of Nanocatalysts
The magnetic properties of the nanocrystals were measured by VSM at the room temperature (∼25 °C). The coercivity (H c) and the saturation magnetization (M S) of the nanocatalysts are listed in Table 1. H c of Ni/Co ferrites decrease inversely with the nickel content since the coercivity of a magnetic material is a measure of magneto-crystalline anisotropy. This decrease is attributed to the lower magneto-crystalline anisotropy of nickel as compared to cobalt, which in turn leads to a lower coercivity. According to the MAUD analysis, the Ni/Co and Cu ferrites are inverse spinels. The decrease in M S of Ni/Co ferrites with the increase of Ni content is attributed to the smaller magnetic moment of the Ni2+ (2μ B) as compared to the Co2+ (3μ B). In addition, the M S of CoFe2O4 is more than CuFe2O4 as a result of the higher magnetic moment of Co2+ (3μ B) than Cu2+ (1μ B). As we explain in our other work, the Ni/Zn ferrites as a result of near zero H c show superparamagnetic behavior at room temperature. The cation distribution of the zinc ferrite, zero magnetic moment of the Zn2+ cations, and the anti-ferromagnetic interactions between the Fe3+ cations in the octahedral sites cause the magnetic moment (M S) for each ZnFe2O4 formula to vanish. The cation distribution changes by the substitution of Zn2+ by Ni2+ cations and occupation of the octahedral sites with Ni2+ cations; thus this substitution transfer part of the Fe3+ cations to the tetrahedral sites, and accordingly makes the M S of the nanocrystals vary. This is consistent with Néel’s ferrimagnetic theory [12]. This behavior of the Ni/Zn ferrite nanocrystals for the different values of M S is similar to that of bulk samples [5]. It is important to notice that the maximum M S is found in the Zn0.5Ni0.5Fe2O4 nanocrystals.
3.3 Morphological Study of Nanocatalysts
The FE-SEM images of the nanocatalysts are shown in Fig. 2. We observe that the prepared nanocrystals have a spherical morphology and cohesion of particles is due to the magnetic attraction. The average grains diameters of the nanocrystals (D ave) were obtained from the FE-SEM images and are listed in Table 3. In addition, the surfaces/gram ratios of the nanocatalysts (S) were found from dividing the average surface of a particle by its mass.
3.4 XRD and FE-SEM Studies of Carbon Nanotubes
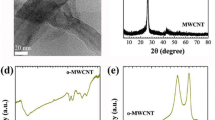
The samples were characterized by XRD after the growth of CNTs. Figure 3 shows these XRD patterns. The (002), (100), (101), (004) and (110) peaks are related to the presence of the CNTs. The presence of the (002) plane between 2θ=26–26.5∘ is due to the presence of multiwall CNTs (MWNTs) [13]. According to Ref. [14], the (110) and (100) peaks are in the (hk0) group peaks and display an asymmetric shape due to the curvature of the CNT. The (004) and (101) reflections are also due to flat graphitic layers, residual carbon particles, and the defect of the CNTs [15, 16].
Figure 4 is shows the FE-SEM images of the CNTs obtained on these nanocatalysts. The particles of the nanocatalysts are observed in the top of the CNTs. The CNTs are not uniformly straight because of catalyst particle movements during the growth process [17]. These movements induce structural defects that were observed in the XRD patterns by the (101) and (004) peaks.
3.5 Catalytic Activity of Nanocatalysts
The catalytic activity of the spinel ferrite nanocatalysts for the growth of the CNTs is evaluated by the rate of production of the CNTs in the surface unit of all nanocatalysts. This parameter was obtained by normalizing the amount of the CNTs to unit time, surface, and mass of the catalysts in the crystallization temperature of each catalyst.
In Table 4, we observe that the catalytic activity of the Ni/Co ferrites increases with the nickel content—as a result of higher catalytic activity of Ni in comparison to Co. Besides, in the Ni/Zn ferrites it is observed that Ni0.5Zn0.5Fe2O4 has the highest catalytic activity while ZnFe2O4 comes next. The grown CNTs on ZnFe2O4 are more pure than CNTs grown on Zn0.5Ni0.5Fe2O4. As mentioned above, in the ZnFe2O4 nanocatalyst, Zn2+ cations occupy the tetrahedral sites and all of these sites are in the same conditions. Thus the octahedral sites are isotropic. This structural isotropy enhances the catalytic activity. With the decrease in the Zn2+ content and entrance of the Ni2+ cations into the structure, the Ni2+ cations occupy the octahedral sites and transfer part of Fe3+ to the tetrahedral sites. These changes cause a decrease in the structural isotropy and the catalytic activity of Ni0.3Zn0.7Fe2O4 and Ni0.7Zn0.3Fe2O4 nanocatalysts. Because of the occupation of the half of tetrahedral sites with Zn2+ and remainder with Fe3+, the Ni0.5Zn0.5Fe2O4 nanocatalyst has a structural isotropy, and thus the catalytic activity increases. This isotropy exists completely in the tetrahedral sites of the inverse spinels. This fact makes the catalytic activity of the copper ferrite nanocrystals increase compared to zinc ferrite—see Ref. [11], where the oxidative dehydrogenation of butenes in the presence of ferrospinel catalysts have been considered. Moreover, the catalytic activity of the Ni/Co ferrites is more than the copper ferrite because the catalytic activity due to Ni2+ and Co2+ in the growth of CNTs is high in comparison to Cu2+. Besides, it notices that the catalytic activity and the magnetic properties (M S, and H c) of the nanocatalysts are not in the same manner—see Tables 1 and 3. The studies in this issue are continued.
4 Conclusion
In summary, we prepared Ni/Co, Ni/Zn, and Cu ferrites by the sol-gel method. XRD showed the cubic spinel structure for all of these ferrites. The MAUD analysis on the XRD patterns confirmed the inverse spinel structure for the Ni/Co and Cu ferrites, the normal spinel for Zn ferrite, and the mixed spinel for the Ni/Zn ferrites. Magnetic properties of these nanocrystals were measured by VSM at the room temperature. M S and H c of the Ni/Co ferrites were shown to decrease with the increase of the nickel content because the magnetic moment of Ni2+ as compared to Co2+ and magneto-crystalline anisotropy of Ni as compared to Co are lower. The behavior of the Ni/Zn ferrite nanocrystals with different values of M S appeared similar to that in bulk samples. The maximum M S was found in the Zn0.5Ni0.5Fe2O4 nanocrystals. The catalytic activities of these nanocatalysts were obtained from the growth of the CNTs on them. We found that the catalytic activity of the spinel ferrites is related to the structural isotropy, the cation distribution, and the catalytic activity of cations. Hence, the catalytic activity of the Ni/Co ferrites increases with the increase in the Ni content due to higher catalytic activity of Ni compared to Co. In the Ni/Zn ferrites, the structural isotropy is an effective factor for their catalytic activity. The catalytic activity of Cu ferrite is higher than the Ni/Zn ferrites since it is inverse spinel, and less than Ni/Co ferrites as a result of the catalytic activity due to cations.
Briefly, we have found that the catalytic activity has the following order: Ni0.6Co0.4Fe2O4>Ni0.4Co0.6Fe2O4>Ni0.2Co0.8Fe2O4>CoFe2O4>CuFe2O4>Zn0.5Ni0.5Fe2O4>ZnFe2O4>Ni0.7Zn0.3Fe2O4>Ni0.3Zn0.7Fe2O4.
References
Bersuker, I.B.: Electronic Structure and Properties of Transition Metal Compounds: Introduction to the Theory. Wiley, New York (1996)
Burns, R.: Mineralogical Applications of Crystal Field Theory, vol. 5. Cambridge University Press, Cambridge (1993)
Borg, R.J., Dienes, G.J.: Physical Chemistry of Solids. Academic Press, San Diego (1992)
El-Sayed, A.M.: Electrical conductivity of nickel–zinc and Cr substituted nickel–zinc ferrites. Mater. Chem. Phys. 82, 583 (2003)
Goldman, A.: Modem Ferrite Technology, 2nd edn., pp. 63–71. Springer, Pittsburgh (1987). ISBN 10: 0-387-29413-9
Ferreira, T.A.S., Waerenborgh, J.C., Mendonca, M.H.R.M., Nunes, M.R., Costa, F.M.: Structural and morphological characterization of FeCo2O4 and CoFe2O4 spinels prepared by a coprecipitation method. Solid State Sci. 5, 383 (2003)
Murray, P.J., Linnette, J.W.: Cation distribution in the spinels Co x Fe3−x O4. J. Phys. Chem. Solids 37, 1041 (1976)
Lahiri, P., Sengupta, S.K.: Spinel ferrites as catalyst: a study on catalytic effect of coprecipitated ferrites on hydrogen peroxide decomposition. Can. J. Chem. 69, 33 (1991)
Goldstein, J.R., Tseung, A.C.C.: The kinetics of hydrogen peroxide decomposition catalyzed by cobalt-iron oxides. J. Catal. 32, 452 (1974)
Krishnamurthy, K.R., Viswanathan, B., Sastri, M.V.C.: Catalytic activity of transition metal spinel type ferrites: structure-activity correlations in the oxidation of CO. J. Res. Inst. Catal, Hokkaido Univ. 24, 219 (1976)
Massoth, F.E., Scarpiello, P.A.: Catalyst characterization studies on the Zn–Cr–Fe oxide system. J. Catal. 21, 294 (1971)
Néel, L.: Magnetic properties of femtes: ferrimagnetism and antiferromagnetism. Ann. Phys. Paris 3, 137 (1948)
Belin, T., Epron, F.: Characterization methods of carbon nanotubes: a review. Mater. Sci. Eng. B, Solid-State Mater. Adv. Technol. 119, 105 (2005)
Lambin, P., Loiseau, A., Culot, C., Biro, L.: Structure of carbon nanotubes probed by local and global probes. Carbon 40, 1635 (2002)
Liu, M., Cowley, J.: Structures of the helical carbon nanotubes. Carbon 32, 393 (1994)
Bernaerts, D., Amelinckx, S., Lambin, P., Lucas, A.: The diffraction space of circular and polygonized multishell nanotubules. Appl. Phys. A 67, 53 (1998)
Figueiredo, J.L., Orfao, J.J.M., Cunha, A.F.: Hydrogen production via methane decomposition on Raney-type catalysts. Int. J. Hydrog. Energy 35, 9795 (2010)
Acknowledgements
The authors acknowledge the Iranian Nano Technology Initiative Council and Vice Chair for research of Alzahra University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hosseini Akbarnejad, R., Daadmehr, V., Rezakhani, A.T. et al. Catalytic Activity of the Spinel Ferrite Nanocrystals on the Growth of Carbon Nanotubes. J Supercond Nov Magn 26, 429–435 (2013). https://doi.org/10.1007/s10948-012-1758-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10948-012-1758-z