Abstract
As a chitin- and glucosamine-derived polysaccharide, chitosan is biodegradable, biocompatible, and non-toxic. Among the latest high-value engineering materials, chitosan hydrogels own outstanding features such as biodegradability, biocompatibility, non-toxicity, swelling reversibility, flexible adaption to external triggers, and the capability of drug loading. Recently, applying chitosan hydrogels to facilitate human biological processes like inflammation has become an interesting topic demonstrated by many publications about their potential in wound dressing, tissue engineering, and treatment of cancer, Parkinson’s disease, and gastric ulcer. This paper reviews the most recent procedures for synthesizing chitosan hydrogels as a smart anti-inflammatory drug delivery system. The characteristics of chitosan hydrogel, including pH sensitivity, temperature sensitivity, electric sensitivity, and magnetic strength, for flexible anti-inflammation, are all discussed to provide a comprehensive overview of anti-inflammatory chitosan hydrogel.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
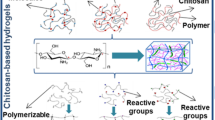
Hydrogels are networks of hydrophilic polymer chains that can absorb and retain a large amount of water in the substitutional spaces between these chains due to the three-dimensional cross-linking structure [1]. This structure is obtained after the hydrophilic domains and groups are hydrated in an aqueous medium and present in a polymeric network [2]. To extend the application and effectiveness of hydrogels, the synthesis of smart hydrogels has been carried out. Synthetic hydrogel networks are applied in many practical use-cases where a material compatible with aqueous solvents is necessary, but with the condition that this material will not dissolve. Their water-insoluble ability is enabled by the network structure of chemical or physical cross-links, which also guarantees physical integrity to the system. Such hydrophilicity and elasticity help to encapsulate drugs for controlled release as well as prolong their lifetime [3]. Many properties of synthetic hydrogels such as biocompatibility and biodegradability make them one of the best materials to apply for contact with human tissues. Only the surrounding tissue is minimally damaged due to its viscoelastic nature [4]. Absorbing and retaining aqueous media help hydrogels to be permeable to small molecules such as metabolites, oxygen, and nutrients as well as strongly resemble with living tissue. Indeed, the possibility of creating a biocompatible synthetic material with a poly-based hydrogel (2- hydroxyethyl methacrylate) (PHEMA) was first proposed by Wichterle and Lim [5]. This seminal work has opened a wide range of investigations on hydrogels for biomedical use-cases drug delivery, wound dressing, scaffolds for tissue engineering, actuators, biosensors, and the production of contact lenses. Since hydrogels have been formulated with different methods using a wide range of polymers. In particular, polymers with properly controlled properties can be used to modify or enhance the features of hydrogels such as pore size. Especially, hydrogels have become a better alternative than other drug delivery systems due to such pore size change, which allows monitoring of the speed of drug diffusion through hydrogels’ network [6].
Among the investigations of hydrogel fabrication, chitosan is one of the most popular biocompatible polymers. It is a cationic polysaccharide that is produced through the process of alkaline deacetylation of chitin, a primary exoskeletal ingredient in crustaceans [7]. Chitosan is a biodegradable, biocompatible, and non-toxic biopolymer with diverse research and application benefits in many industries. Its special properties include antibacterial, antioxidant, regenerating/repairing, film forming, lipid granulation, absorption, binding, etc. Thus allowing chitosan to be used in many industries, including agriculture, food, cosmetics/skin care, medical, pharmaceutical, etc. With strong bioactivity and diverse applications, chitosan and its derivatives have great potential to become the leading ‘green & sustainable’ solution for many industries around the world.
Inflammation is a primary cause of many inveterate diseases such as heart disease, inflammatory bowel disease, and autoimmune disorder, making the treatment of chronic diseases challenging [8]. The current limitation of treatment is the poor selectivity of the drug between normal and inflammation tissue. The side effects of drug toxicity are often observed due to a high dosage of drugs used by traditional therapeutic treatments. During the treatment, drug dosage is limited and drug resistance is increased as a result of these effects, including chronic neurotoxicity, marrow failure, and kidney failure [9]. Therefore, to overcome the aforementioned limitations, some research carry out creating drug carriers, that utilize the targeted area of affected tissues intending to increase the accumulation of drug inflammation cells, release drugs in a controlled manner, and limit drug concentration in healthy tissues [10]. As a result, it will reduce the side effects of the drug and enhance the effectiveness of the treatment.
Many in vitro studies have shown that chitosan hydrogel when loaded with anti-inflammatory drug, has a considerable anti-inflammatory effect. RAW 264.7 macrophages were often employed for incubation and determining inhibitory activity on the production of many pro-inflammatory mediators and cytokines [11]. Furthermore, some in vivo tests were conducted by inducing inflammation on rat paws. The paw thickness was measured along with the reduction in pro-inflammatory cytokines for evaluation of anti-inflammatory activity [12]. As an outcome, the drug-loaded hydrogel was discovered to promote infected wound healing via sequential hemostasis as well as antibacterial and anti-inflammatory mechanisms. Most investigations found that hydrogel as a drug carrier, possessed anti-inflammatory properties by suppressing the formation of NO, PGE2, TNF-, and IL-6 (Pro-inflammatory cytokines which plays the main role in inflammatory response). As a result, when combined with the outstanding properties as sustained release, biocompatibility, biodegradability, etc. , hydrogel might be a promising therapeutic carrier for anti-inflammatory applications [11, 12].
2 Chitosan hydrogels characteristics in anti-inflammatory
With the recent advances in polymer chemistry and drug delivery, chitosan hydrogels have attracted many studies that use them for drug release in a temporal, spatial, and dosage-controlled manner. Release control is needed to systematically control the frequency of drug administration, which in turn avoids side effects caused by high drug concentrations. Drug release is controlled by the porosity of hydrogel, which is dictated by the cross-linking density of the polymer network [3]. To enable localized drug delivery, recent works have considered injectable hydrogels, a.k.a. environment stimulus-responsive hydrogels, as a potential solution [13, 14]. To this end, a wide range of external stimuli such as pH [15, 16], temperature [13], electrical field [14], and magnetic field [17] have been studied, which lead to different types of hydrogels.
2.1 pH-sensitive chitosan hydrogels
Chitosan hydrogels are cationic. They are un-ionized above and ionized below the pKa of the polymeric network. The presence of ions creates a large osmotic swelling force that makes the hydrogel swell at a pH below the polymer pKa [18]. Such pH-dependent deswelling/swelling behavior of the hydrogel can be guided by electrostatic repulsive forces, thereby controlling the release of drugs [19]. This behavior can be controlled by the properties of the polymer as well such as hydrophilicity, hydrophobicity, concentration, charge, ionization degree, ionizable group pKa, and cross-link density. Other controlling factors include the nature of the buffering species and characteristics of the swelling medium such as valency, counterion, ionic strength, and pH [19]. The degradation of pH-responsive hydrogels can be enabled by acidic pH that interrupts the cross-linking between the chitosan and supporting polymers, which results in larger pore size of the hydrogels under low pH. Larger pores will provide larger space for the payload diffusion and provide a larger swelling ratio [14]. Moreover, in such an acidic environment, the chitosan amino groups will be positively charged and protonated, leading to enhanced hydrophilicity and intermolecular electrostatic repulsion which in turn increases the swelling of the hydrogel [14] (Fig. 1).
In practice, pH-responsive chitosan hydrogels are synthesized by Chaves et al. [15] to control the release of DAP, which is a bactericidal agent used in the treatment of leprosy. DAP has low bioavailability and microbial resistance due to its low solubility in water, although it has high therapeutic potential. With the acidic environment in the hydrogel, the Schiff base bonds between NH2 and CHO from the chitosan and other polymers become weaker and decompose over time [14], increasing the swelling and responsiveness of hydrogels in acid pH.
Another practice is creating and using chitosan composite hydrogels [16] to release potent nonsteroidal anti-inflammatory drugs such as piroxicam, which is used in the prevention and treatment of colon cancers and colonic inflammatory conditions with analgesic activities [20], due to their pH-sensitive drug delivery capability. Moreover, it is noteworthy that the swelling of chitosan composite hydrogels gets better under weak alkaline conditions, making them a candidate for carrying drugs in colon-specific drug delivery systems. Being colon-specific, the system can help to release drugs in the upper gastrointestinal tract, thus avoiding side effects including ulceration and gastrointestinal irritation in long-term therapy [21, 22].
The swelling of the chitosan composite hydrogels in aqueous solutions of different pHs can be controlled by different mass ratios between the polyanions [16]. On the one hand, the equilibrium swelling ratio of hydrogels is lower at lower pHs. it is higher with higher pHs but then decreases again after peaking at pH 8.0.
2.2 Thermosensitive chitosan hydrogels
Although chitosan is not a thermoresponsive polymer, such a thermoresponsive behavior can be achieved by adding a particular hydroxyl or amphiphilic thermoresponsive polymers inside the chitosan network [23, 24]. The thermo-responsive property is originated from hydrophobic groups such as propyl, ethyl, and methyl and is evidenced by the interaction of water molecule with these hydrophic groups in various temperature levels [25]. Hydrophobic and hydrophilic components co-exist inside the structure of a thermosensitive hydrogel. These components play the main role in the thermal response mechanism. When there is a shift in temperature, a change in the sol-gel transition behavior is observed due to the interaction between hydrophobic and hydrophilic segments, thus altering the solubility of the crosslinked network [26, 27].
There are three types of thermo-responsive polymers. The first one is the hydrophilic and hydrophobic type that does not need any gelling agents (Copolymer) and repeat units on their backbone. The second type includes a separate, functional, hydrophilic and hydrophobic group that requires a gelling agent. For example, chitosan has a hydrophilic group and a hydrophobic group via glucosamine and acetyle glucosamine repeat unit respectively; and these groups can exhibit a thermo-responsive property via polyol such as beta glycerophosphate. The third type has hydrophilic and hydrophobic groups on a single repeat unit simultaneously, which does not require a gelling agent [28]. In all cases we need to conjugate gelling agent by covalent interactions or intermolecular interactions like polyols and amine group of chitosan, there are some other inherent thermoresponsive polymers like PNIPAAM [28]
To be eligible for biomedical applications, a polymer solution requires low viscosity at room temperature and forming a gel above LCST. This is because it will be injected into a human body as a liquid and when the body temperature is above LCST, it will form a gel in situ. Moreover, these materials can be applied for other biomedical and pharmaceutical applications as carrier matrices [29], by forming a gel in situ at body temperature using thermosensitive polymers [13].
Injectable thermosensitive hydrogels were first synthesized for the treatment of osteoarthritis by mixing chitosan and Pluronic-F127 loaded dexamethasone by Garcia-Couce et al. [13]. Before that, the treatments mainly focused on mitigating the main symptomatology with anti-inflammatory drugs via topical and oral vials [30]. However, administering these drugs frequently via oral vial would cause serious gastrointestinal side effects, whereas topical applications also have low effectiveness. In general, thermosensitive chitosan hydrogels exhibit a sol-gel transition when the temperature changes. Then they form a deposit that allows the controlled release of the drug and prolongs its permanence within the joint [31]. Thanks to the presence of positive charges in its structure, chitosan has excellent bioadhesive, non-toxicity, biodegradability, and biocompatibility properties [32].
Another application of injectable thermosensitive hydrogels is to prevent local tumor recurrence. They are developed in situ by combining neutralized chitosan with b-glycerophosphate-loaded paclitaxel by Ruel-Gariepy et al. [33]. The latter becomes liquid at room temperature but becomes a gel when heated to the body temperature. Such in situ gelling enables a sustained release of paclitaxel at tumor resection sites as well as controlled delivery over 1 month. This application emerges as a therapeutic approach to combat solid tumors as well as prevent metastasis and tumor re-growth. After the tumor is surgically removed, a biodegradable device loaded with an anti-neoplastic agent will be implanted in the resulting cavity. Such an approach allows for high local drug concentration, and thus efficiently removes surviving malignant cells as well as avoids the typical side effects of chemotherapy associated with its intravenous administration. Ye Wang et al. also developed thermosensitive hydrogels by combining chitosan and glycerol-loaded meloxicam as well [34].
2.3 Electro-sensitive hydrogels
Electro-sensitive hydrogels can be developed by combining chitosan with an electro-sensitive polymer. Electro-sensitive polymers are a type of specialized polymers that respond to an external electric field, under which they will make the hydrogel swell or dwell.
Jin Qu et al. [14] synthesized such hydrogels by mixing chitosan-graft-polyaniline and oxide dextran under physiological conditions. Besides the pH sensitivity and electro-responsiveness, hydrogels created this way are also conducive to the release of hydrophilic drugs such as ibuprofen and amoxicillin. The conductivity of hydrogels can be controlled with polyaniline [35]. For example, increasing the applied voltage would increase the cumulative release of drugs significantly. This electric-driven phenomenon of releasing drug molecules from conductive hydrogels can be explained by two factors: (1) the difference in the overall net charge within the polymer upon oxidation or reduction and (2) the migration of the charged molecules under the electric field [36, 37]. Applying a voltage on the hydrogels results in a kind of “on-off” pulse release, reducing the conducting polymers and then releasing the negatively charged drugs. In other words, hydrogels with a voltage release a higher amount of drugs than those without a voltage.
2.4 Magnetic-sensitive hydrogels
Magnetic-sensitive hydrogels, especially magnetic chitosan hydrogel (MCH), help to release the drug from a passive manner to a pulsatile manner from a long distance under a low frequency passing through the magnetic field. They can be synthesized by combining hydrogels with iron oxide nanoparticles with paramagnetic properties.
Mahdavinia et al. [17] synthesized pH-sensitive and magnetic beads by using carboxymethyl chitosan and carrageenan. These beads are used to deliver diclofenac sodium, a water-soluble, anti-inflammatory, and non-steroidal drug. Using in situ method on a mixture of biopolymers can synthesize magnetic \(\mathrm {Fe}_{3}\mathrm {O}_{4}\) nanoparticles. Iron oxide (\(\mathrm {Fe}_{3}\mathrm {O}_{4}\)) is a good candidate to prepare magnetic hydrogels due to its magnetite type with excellent biocompatible properties and saturation magnetization [38]. Controlling the drug release from magnetic hydrogels can be achieved with an external magnetic field.
Indeed, an external alternative-magnetic-field (AMF) can increase the amount of drug released from all samples via magnetic hydrogel beads. Such increase can be attributed to the alignment of the applied AMF with magnetic nanoparticles. While having a constant motion, the magnetic nanoparticles can be agitated by the fluctuation of the applied AMF. This agitation helps to relax the polymer backbones, resulting in an increment of released drug [39]. Dual sensitive hydrogels that simultaneously respond to the pH change and the AMF are also desirable. Such hydrogels can be obtained by using superparamagnetic iron oxide nanoparticles (SPIONs) on top of pH-sensitive hydrogels [40]. This condition can be achieved by applying an external magnetic field on the drug-targeting carrier-based SPIONs that are being directly conducted at the target tissue or cell. This effect will reduce the circulation time, hence leading to a decreased drug dosage and limited side effects [41].
2.5 The drug release mechanism of chitosan hydrogels
Rapid and sustained drug release processes are the two types of drug release mechanisms. Drugs can bind to hydrogels non-covalently, allowing the drug to move freely within the network. When the hydrogel is implanted in the body, the drug is explosively released at the beginning due to the concentration gradient generated between itself and the surrounding environment [42]. Such increased drug concentration, on the other hand, inhibits drug release from the hydrogel over time. Fast drug release might limit therapeutic effectiveness or even cause hazardous adverse effects in some patients. Most medications are either covalently or physically linked to the polymer before gelation to achieve this effect. As a result, the movement of drug molecules can be effectively governed by the hydrogel within its network; limiting the conditions of drug release to the only destruction of the hydrogel network [42]. Polymers that respond to a variety of stimuli can be utilized to regulate drug release rate and timing. Nonetheless, because research on drug release from hydrogels is still in its early stages, it is critical to examine this mechanism [43]. Selective treatment requires a fine-tuned control when hydrogel particles are administered intravenously or orally. This control can be achieved through biological and chemical release. As a result, it allows hydrogel particles to encounter the diseased target site property after interesting with a significant amount of healthy tissues.
3 Synthesis of chitosan hydrogels for drug delivery
3.1 Physical cross linking
Agents and reagents for chemical crosslinking are in immense shortage nowadays, hence, physical crosslinked gels have recently sparked a lot of attention. Many chemical crosslinking agents are hazardous substances that may be easily isolated or detached from produced gels prior to being used. Additionally, they can, as well, alter the state of entrapped molecules such as proteins, medicines, and organisms [44]. Physical gels have several disadvantages, including instability (uncontrolled dissolving), reduced resistance to mechanical action, and hard control of pore size [45]. In physical gels, unbound chain terminals or loops can also appear as temporary entanglement of faults [46]. The most frequent physical cross-linking procedures for the manufacture of chitosan hydrogels are explained below.
Hydrophobic interaction. Hydrophobic polymers crosslink in aqueous settings in the process known as reverse thermal gelation. The process is commonly denoted as ‘sol-gel’ chemical reaction. To make a polymer amphiphile, the hydrophobic segment is connected to a hydrophilic polymer segment via post-polymerization grafting or by directly synthesizing a block copolymer. At low temperatures, the amphiphiles quickly dissolve in water. However, when the rises in temperature occur, hydrophobic domains agglomerate to reduce quantity of structured water covering the hydrophobic domains, increasing the solvent entropy and minimizing the hydrophobic region contacting the bulk water [44].
For tissue regeneration and drug administration, a recommendation for in situ thermo-sensitive chitosan-\(\beta \)-glycerophosphate (CeGP) gel injectable has been approved [44]. The competition between various intermolecular interactions can be summed up as the sol/gel transition. The electrostatic repulsion between positively charged chitosan molecules, the repulsion detection effect caused by this negatively charged \(\beta \)-glycerophosphate, the attractive interaction between chitosan molecules, hydrophobicity, and hydrogen bonding are all examples of these interactions [47].
Polyelectrolyte complexation (PEC). PECs basically refers networks of biocompatible molecules with intriguing expansion properties. PEC gels, which are generated in aqueous solution by electrostatic attraction between two polyelectrolytes with opposite charges, are typical of possessing distinct chemical and physical characteristics. The electrostatically charged contacts in PEC gels, for example, are highly stable as compared to secondary binding interactions. The major interaction that leads to the creation of PECs is the electrostatic pulls between the cationic amino class of chitosan and the anionic categorization other polyelectrolyte [44].
Freeze-Thaw processing. Hydrogels made by freezing and thawing polyvinyl alcohol (PVA) solutions have received a lot of attention. Non-toxicity, non-carcinogenicity, biocompatibility of the resulting polymer, and the lack of crosslinking or initiating chemicals in the synthesis of are some of the advantages of the physical crosslinking of hydrocarbons obtained by freezing/thawing. At freezing point, liquid-liquid disintegration phase occurs through the process of crystallization in the polymer-depleted phase. The hydrogen bonds of PVA and the formation of microcrystals are caused chains full of polymer in the polymerich stage. In addition, liquifaction enhances binding between polymer residues and the formation of crystalline domains to form a hydrogel network [48, 49].
3.2 Chemical cross linking
Chemical cross-linking is a school of methods that polymer chains’ covalent bonding for hydrogel network production. In particular, a wide range of linkage chemistries, such as amine-carboxylic acid bonding and Schiff base formation, can be constructed with the following cross-linkers and -NH2 and -OH chemical handles to prepare cross-linked chitosan networks [50,51,52].
Chemical cross-linkers. These are molecules that have more than two reactive functional groups permitting polymeric chains to connect together. Genipin and glutaraldehyde are the highly used cross-linkers applied to make chitosan hydrogels so far [53].
Photo crosslinking. Researchers have developed polymer mixes capable of producing hydrogels in situ utilizing highly functional photosensitive groups, similar to polymer-polymer crosslinking. When the highly reactive moieties are added to chitosan, the polymer is then capable of generating cross-linkages when exposed to UV light.
The degree of crosslinking reaction is proportional to the amount of UV irradiation time and increases as exposure time increases. As a result, a hydrogel with improved mechanical characteristics and a decreased swelling ratio was developed. Other significant advantages of this technology over traditional chemical procedures such as ease of formation and control low cost, safety and speed. It also includes the absence of multiple reactive species, initiators, or catalysts [44].
Enzymatic cross-linking. Polymers that are highly photosensitive are a great potential family of building blocks for in situ hydrogel formation, however they have limitations. Photo-cross-linking, for example, may necessitate the use of a photosensitizer and extended irradiation, which consequently increases normall temperature, causing damage to surrounding cells and tissue [54]. Enzyme-catalyzed cross-linking reactions provide a new, gentle way to in-situ hydrogel production. The engineering of tissue and drug devovery or protein delivery are two biological applications for which this approach has shown tremendous promise [54]. Cross-linking reactions have been catalyzed by horseradish peroxidase (HRP), a single-chain \(\beta \)-type hemoprotein that catalyzes the coupling of phenol or aniline derivatives via the breakdown of hydrogen peroxide [54, 55].
Graft polymerization. They are a segmented copolymer with a straight structure of a single polymer and unevenly distributed branches of the other polymer. Two types of reactive groups can be grafted onto chitosan. The free amino group and hydroxyl group of the acetate in the distillation unit or the C3 and C6 carbon atoms in the distillation unit increase the water solubility, antibacterial and antioxidant properties.
-
Chemical grafting: Chemical grafting begins with the generation of free radicals on the chitosan chain, which then react with polymerizable monomers to form the grafted chain. There can be a single of numerous free radicals being grafted on the chain. Recent years have witnessed numerous initiators for grafting copolymerization. This art of polymerization has been developed, and incorporates numerous radicals such as Ammonium PerSulfate (APS), Ferrous Ammonium Sulfate (FAS), Potassium PerSulfate (PPS), and Ceric Ammonium Nitrate (CAN) [56].
-
Radiation grafting: Radiation crosslinking, unlike other methods, does not require any additional processes to start reacting the reagents, therefore the final eventual results are often inclusive of polymer. Furthermore, the process of ionizing radiation may often combine together with synthesis and sterilization of polymeric substances in one scientific procedure, resulting in lower costs and faster production. After including chitosan into the PVA hydrogels, the mechanical characteristics and degree of swelling significantly enhanced. Chitosan, on the other hand, is degraded by \(\gamma \)-radiation. Chitosan sterilization by \(\gamma \)-radiation causes discolouration, loss of amino groups, and a reduction in molecular weight.
The following Table 1 shows the summarization of different paths to synthesize chitosan hydrogels:
There are other relevant routes of chitosan crosslinking. In particular, Olmo et al. [62, 63] described a simple experiment procedure can be done to achieve the stability, swelling, tunable rheology, and self-repair ability for chitosan by crosslinking it with a synthesized polyethylene glycol diacid (PEG-diacid). Such achievement can be explained by the high H-bonding tendency of the resulting CHI-PEG hydrogel formulations. On the one hand, the enhancement of anti-inflammatory and antibacterial activities of hydrogels can be achieving by a sustained release of sustainedly acetylsalicylic acid (ASA) anti-inflammatory agent, tetracycline (TCN) and amoxicillin (AMX) antibiotics, and loaded cefuroxime (CFX). Moreover, AMX, TCN, CFX, and ASA can be loaded with biocompatible hydrogels that can be obtained by crosslinking chitosan with genipin (GP). These hydrogels also have and a potential antibacterial efficacy and an almost 100% bacteria reduction against Escherichia coli and Staphylococcus aureus. Such a healing effect combined with an increase in histological analysis, elastin quantities (\(5.82 \pm 0.73\,{\upmu \hbox {g}}\) elastin and \(1.48 \pm 0.07\,{\upmu \hbox {g}}\) collagen per mg dermal tissue), and metabolic activity (\(95.58 \pm 4.40\%\)) can treat ulcerated wounds. On the other hand, improving histological analysis, collagen and elastin quantities (\(4.97 \pm 0.61\,{\upmu \hbox {g}}\) elastin and \(2.12 \pm 0.63\,{\upmu \hbox {g}}\) collagen per mg dermal tissue), and metabolic activity (\(94.51 \pm 4.38\%\)) is a potential solution for treating ulcerated wounds [64].
Mussel-inspired catecholic chitosan hydrogels were developed by He at al. [65] to avoid postsurgical tumor recurrence. Thanks to their excellent tumor inhibition and anti-inflammatory abilities, these hydrogels act as a multifunctional surgical tissue adhesiveto. Surgery is still the idiosyncratic clinical solution to remove metastatic or primary tumors [66,67,68,69]. However, such solution has some risks of surgical traumas and incomplete tumor removal, which could lead to the cascades of systemic/local inflammation. These events can in turn retain cancer cells, preserve their survival, and boost their metastic growth [70, 71]. Augmenting hydrogels with firm adhesive capacity could boost the absorption of pathogenic bacteria, which is the cause of immune responses such as inflammation and infection [72, 73]. More precisely, the tumo-resected cavity is filled with CSG/\(\mathrm{Fe}^{3+}\) hydrogels that is the resulting combination of \(\mathrm{Fe}^{3+}\) and chitosan with conjugated gallic acid (CSG) molecules. These hydrogels have anti-inflammatory and considerable wet-adhesion abilities. Moreover, the surgery-induced acceleration of tumor growth can be reduced essentially by the diminished inflammation on top of the quick healing of irregular injuries.
In Yuan et al. [74], chitosan hydrogels can be augmented with a better mechanical ability by in-situ self-assembly of PUE that enables interpenetrating networks. The outcome of this augmentation is called CS/PUE18 composite hydrogels. More precisely, PUE, as a small molecule in herbal, acts as an activator of self-assembly, leading to anti-inflammatory and antibacterial capacities simultaneously, as well as a pH responsiveness for chitosan hydrogels.
Mohamed et al. [75] synthesized another type of anti-inflammatory chitosan hydrogels that are equipped with thiourea and phthalimido moieties as inhibitors to filter COX-2. More precisely, chitosan crosslinking is achieved by characterizing and synthesizing four various quantities of dibenzoyl isothiocyanate and benzophenone tetracarboxylimide thiourea moiety, with the help of analytical elements such as SEM, XRD, 1H NMR, and FTIR. The resulting hydrogel become an anti-inflammatory agent that enables cyclooxygenase enzymes COX-1 and COX-2. This capability also makes it become an anti-H. pylori agent.
4 Techniques for drug loading on chitosan hydrogels
The effective of a hydrogel as a drug delivery system is determined by the gel’s physical and chemical qualities, including the therapeutic characteristics. Certainly, drug loading, network conformation, and hydrogen substance process ought to be enjoined to enrich the drug features resulting from the formation such as hydrophilicity, charge, and mechanism of action. The mechanisms of action include a combination of sustained drug release in comparison to rapid, high exposure [76]. Diffusion, trapping, and tethering are three basic routes to drug loading. Each approach has its own set of benefits and drawbacks and should be chosen after considering the hydrogel network employed and the type of drugs [54]. The most efficient drug loading approach entails dissolving maximally produced hydrogen in a saturated solution containing the treatment [77]. The drug will dissolve gently into the gel and this process will depend on the porous nature of the hydrogel, the drug quantity, and the composition of each chemical solution. When the drug is placed in vivo, it gently diffuses back out of the hydrogel and spreads around the surrounding tissues. This method works well for loading tiny compounds, but bigger medicines, such as peptides and proteins, are unable to travel through the hydrogel’s microscopic pores. Furthermore, it is important to realize that the drug loading process can consume a lengthy period to complete the entire process.
The payload must be entrapped during the gelation process to have the final product contain bigger medicines and bioligands. The medicine is combined with the polymer solution, and a cross-linking agent is then introduced into the solution at this particular juncture. To avoid undesirable cross-linking or deactivation of the therapy during gelation, it is advisable to refer back to the process of drug molecule formation as indicated in previous reviews regarding cross-linking. The reviews have discussed a variety of encapsulated pharmacological methods [78, 79]. Diffusion and trapping both allow the treatment to travel freely within the hydrogel network. As a result of concentration differences created between the gel and its environs, the results can cause the bursting of the solution released after the hydrogel is implanted in vivo. Drugs can be covalently or physically bonded together with the polymer chains before gelation as this approach will significantly lower down wastage of therapeutic reserve thereby reducing the dangers of hazardous exposure. This form of tethering restricts the exposure of tissues to hydrogel or molecular tether breakage [54]. To manage the speed and timing of the release, environmental enzymes have been utilized to link the medication to a polymer that is susceptible to them.
The drug loading process is further compromised by molecules with the same charge or opposite hydrophilicity as the constituent polymer. For example, before the hydrogel and payload can completely mix within the solution, hydrophobic compounds like paclitaxel require being complexed with amphiphilic additions [80, 81]. Before hydrogel loading, the medication was bound to albumin or mixed in an aqueous citric acid/glyceryl monooleate solution [82]. Before initiating the process of hydrogel encapsulation, therapeutics are converted into tiny secondary release mediums such as liposomes, micro-particles, microgels, and micelles [58, 83]. The hydrogel polymer can gain function through tiny binding domains in addition to altering the medication before encapsulation. In a chitosan hydrogel that is fixed inside hydrophobic drug denbufylline, combining minimal hydrophobic moieties to the polymer before the loading and gelation process verified this approach [84].
5 Smart anti-inflammatory chitosan hydrogels
5.1 Inflammation and why is it important
The basic purpose of the skin is to provide a protective barrier against the environment. Wound healing is critical in repairing the skin’s integrity from damage, whether induced by an accident or a targeted approach. Wound healing is a physiological process that is mostly made up of four steps. The second of four overlapping steps of that intricate process is inflammation. The manifestations of inflammation commonly seen at the site are swelling, heat, redness, and pain. There are also behaviors of inflammation inside the body that cannot be seen by the naked eye to counter inflammatory agents. Acute inflammation and chronic inflammation are two types of inflammation in the body. Acute inflammation occurs when an agent attacks quickly and for a short time, whereas chronic inflammation occurs when an agent attacks silently for a long time [85,86,87].
5.1.1 Acute inflammation
Acute inflammation occurs when the inflammatory agent affects the tissue for a short time, causing a short and not long-lasting inflammatory response. It lasts from a few hours to a few days. Acute inflammation is distinguished by the exudation of fluid and plasma proteins and the emigration of leukocytes. Neutrophils and other motile white cells travel from blood arteries to perivascular tissues and the site of injury. Temperature rise, discomfort, soreness, redness, local edema, and reduced function are symptoms of acute inflammation. Besides, symptoms of an acute inflammatory reaction included fever, elevated blood leukocyte counts, and the development of acute phase proteins such as fibrinogen and C-reactive protein in plasma. The exudation of fluid and plasma proteins and the emigration of leukocytes are indicative of acute inflammation. Neutrophils and other motile white cells travel from blood arteries to perivascular tissues and wound sites. Symptoms of acute inflammation are local edema, redness, soreness, discomfort, as well as temperature rise, and function inhibition to name a few. In addition, acute inflammatory bodily reactions comprised of fever elevated blood leukocyte counts, and the development of acute phase proteins such as fibrinogen and C-reactive protein in plasma.
If the initial inflammatory reaction affects veins, intravascular thrombosis may occur, limiting venous blood return. Acute inflammation can turn into chronic inflammation. The main reaction in acute inflammation is the oozing reaction, in addition, there is often a destructive reaction, if mild, the lesions are degenerative, but in severe cases, they may be necrotic lesions of cells and tissues [88, 89].
5.1.2 Chronic inflammation
The inflammatory process in chronic inflammation can emerge even if there is no damage, and it does not stop when it should. It’s not always clear why the inflammation persists. Bacterial, viral, and parasite infections, chemical irritants, and non-digestible particles are all known to induce chronic inflammation. The longer the inflammation lasts, the greater the chance of carcinogenesis developing and DNA might be damaged. Illnesses such as arthritis, lupus, allergies, asthma, IBS, Crohn’s disease, cardiovascular disease, cancer, etc., are all linked to chronic inflammation [90].
5.2 Biomechanism of inflammation
Inflammatory agents affect organs and organizations causing: cell damage, the release of chemical mediators, and disturbances in circulation and metabolism; this same disorder also produces chemical mediators and aggravates inflammation. These changes are expressed in three types of reactions: degradation reactions, leakage reactions, and proliferation reactions. These reactions do not occur separately but often combine when one reaction is more obvious, sometimes the other [91]. In many pieces of research, hydrogels have been proved to be an interesting candidate for the bioactivity and sustained release of anti-inflammatory drugs. Besides drug delivery, hydrogels can be used for the immobilization of peptides, biological compounds, and proteins due to their water-swelling characteristics affected by some biological conditions. Thanks to such high-water nature, hydrogels can resemble living cells more than any other synthetic biological material [92]. Regarding drug delivery and immobilization of proteins, peptides, and other biological compounds; hydrogels are an ideal material thanks to their propensity to swell in liquid under biological conditions. Their high water content bears a strong resemblance to natural living cells more than any other synthetic biological materials.
These networks have an insoluble cross-linking structure that allows efficient immobilization of active agents or biomolecules and allows for their specific release, leading to hydrogels having the potential of inhibiting the vasodilatory response of the inflammation process.
5.3 Nonsteroidal anti-inflammatory drugs (NSAIDs)
Nonsteroidal anti-inflammatory drugs (NSAIDs) are medications containing analgesic and anti-inflammatory properties, as well as antipyretic and steroid properties (different from the corticosteroid anti-inflammatory group, which has the side effect of salt and water retention). Nonsteroidal anti-inflammatory medications (NSAIDs) have analgesic, anti-inflammatory, antipyretic, and antiplatelet properties. Depending on the drug, the degree of these effects will manifest more or less. As a result, NSAIDs are frequently used to alleviate inflammation. NSAIDs include Diclofenac, Flurbiprofen, Ibuprofen, Aspirin, and Indomethacin, among others. The fundamental mechanism of NSAIDs is the suppression of the enzyme cyclooxygenase (COX). Oral pills are the most prevalent form of NSAIDs [93, 94]. The advantages and disadvantages of some of the NSAIDs are shown in Table 2.
5.4 Anti-inflammatory drugs delivery
According to the Biopharmaceutical classification system of the US Food and Drug Administration (FDA), over 75% of current drug research active ingredients have poor solubility in physiological fluid environments, and over 25% of drugs have low permeability through the gastrointestinal wall, limiting the ability to absorb and put drugs into the blood circulation. Hence, drug delivery has altered considerably during the last several decades, and even more, dramatic developments are expected shortly. Biomedical engineers have made significant contributions to our knowledge of the physiological impediments to effective drug administration. Medications can be administered in a variety of methods, including ingesting, inhaling, absorption via the skin, and intravenous injection. Each approach has benefits and drawbacks. Table 3 shows some examples of drug delivery systems.
Among the biomedical materials, hydrogel stands out as an efficient drug delivery system. A large amount of study has been conducted on the sustainable and controlled release of drugs. The potential of hydrogels, which have a high water content, to provide local and delayed delivery has previously been established for a wide range of medicinal substances and polymer forms. Hydrogel has become one of the most efficient methods of delivering medicinal chemicals due to its numerous exceptional qualities, including high swellability, biocompatibility, mucoadhesiveness, and so on. These materials are already used to make contact lenses, hygiene items, medication capsules, intravenous injections, and wound dressings.
Inflammation is the second among the four overlapping phases of a complicated process called wound healing [85, 87]. Though the human body can provide the necessary means for countering inflammation in normal wounds, providing appropriate pH, moisture, and oxygen pressure, and preventing microbial invasion could significantly improve the process and hence, accelerate wound healing [86, 87, 100]. Because of its unique properties, such as biocompatibility, biodegradability, hemostatic and anti-infection activity, and the ability to hasten wound healing, chitosan has been widely used in the biomedical field [60]. In many studies shown in Table 4, chitosan-based hydrogels have been proved to be an interesting candidate for the bioactivity and sustained release of anti-inflammatory drugs.
The most common method for loading drugs is covalent bonding. Before gelation, the medications are covalently or physically bonded to the polymer chains to reduce the loss of therapeutic reserve and the danger of hazardous exposure, this method is best suited for the entrapment of hydrophilic drugs [107]. The reason for this is the capability of loading larger drugs than the other methods. Besides, this loading method could significantly lower the degree of burst release and prolong the release duration. For instance, the hydrogels synthesized in the studies of Zhang et al., Kim et al., and Hemmingsen et al. exhibited a constant release duration of 85.3% in 12 days up to 30% in 14 days [101, 108, 109]. However, drug release from chitosan-based hydrogels is also influenced by the particulate system’s morphology, size, density, and extent of crosslinking, as well as the drug’s physicochemical properties and polymer properties, such as whether the polymer is hydrophilic or hydrophobic, has gel formation potentials, swelling capacity, mucous bio-adhesive properties, and the presence of other excipients present in the dosage form [107]. In addition, compared to the other methods, covalent bonding exhibits no toxic material leaching, which is more suitable for usage. However, it remains the drawback of a higher chance of drug deactivation during polymer bonding than in the permeation method. Some other studies on loading hesperidin, epicatechin, and resveratrol, however, used the method of permeation and entrapment [103, 104, 110]. To better enhance the loading and delivery efficiency, liposomes are synthesized to support drug loading in this method before being incorporated into the hydrogels. Although not many researchers conduct the entrapment efficiency experiment, the works of Gull et al., Zhang et al., and Joraholmen et al. demonstrated that the efficiency stays at around 70-80% regardless of the loading method [8, 103, 104, 109]. The therapeutic characteristics of nonsteroidal anti-inflammatory drugs (NSAIDS) is enabled by the suppression of cyclooxygenase (COX) (Prostaglandins are also made by this enzyme) [111]. Based on the specific application and features of each drug, the administration route for chitosan-based hydrogel varies, namely oral, transdermal, parental, vaginal, etc. For drugs like benzydamine hydrochloride, which is an NSAID used for the treatment of common diseases like mouth ulcers and sore throats are commonly administered through an oral route in the form of a capsule for its convenience in improving patient compliance and comfort, and to reduce the cost compared to other methods. However, the bioavailability is greatly governed by the initial metabolism and the amount of drug absorbed across the intestinal epithelium [112]. Another disadvantage is that the oral route is limited to micro-molecular drugs. Whereas, hydrogels are candidate materials for oral delivery of macro-molecular drugs and small hydrophilic molecules ( 400 Da to 30 kDa) [113].
Considering the application of anti-inflammatories in wound healing, hydrogels are commonly synthesized for the transdermal administration route. This type of hydrogel is applied directly on the skin of the affected region in the form of a patch and is commonly used for an external wound. After touching the skin, the drug infiltrates the dead stratum corneum and then arrives at the blood vessels, the dermis, and the viable epidermis [114]. By delivering the drug directly to the wound site with the mechanism of transepithelial drug delivery, the risk of systemic side effects can be avoided. For this reason, many chitosan-based hydrogels are synthesized in the form of a patch, such as hydrogels loaded with chlorhexidine, hesperidin, bee venom, and oyster peptide [101, 12, 110, 115]. However, the drawback is that there is a possibility of resulting in skin irritation.
For the application in treating sexually transmitted infections (STIs), drugs of natural origin must be used for safety and chitosan-based hydrogel will act as the delivery system for the vaginal therapy. The vagina is a highly effective site for medication administration, especially in women’s health. Because it allows for smaller dosages, consistent drug levels, and less frequent administration than the oral route, the vaginal route is frequently used for drug delivery. Absorption is unaffected by gastrointestinal problems, there is no first-pass impact, and usage is discreet with vaginal medication delivery [116]. In the study of Joraholmen et al., resveratrol (RES) and epicatechin (EPI) are introduced as anti-inflammatory agents of natural origin [103, 104].
Another widely applied administration route for chitosan-based hydrogels is through injection (parenteral route). The parental route acts more quickly and reaches the highest bioavailability among other methods. For this type of delivery, hydrogels are made into 3 specific types-in situ-gelling hydrogels, shear-thinning hydrogels, and macroporous hydrogels [117]:
-
For the in situ-gelling hydrogels, we can inject them in a liquid form but when inside the human body, they move to a sol-gel transition state. The resultant hydrogels will adapt to the shape of the available space at the injection site, and several techniques can be used to achieve the sol-gel transition. Any drug-loaded hydrogels formed using the method of covalent bonding are possible to form in situ-gelling hydrogels. However, proper consideration must be taken before applying as catalysts or monomers may be required some pre-gel solutions, which in turn are toxic to cells and tissues.
-
For the shear-thinning hydrogels, hydrogels can be pre-gelled outside the body before being administered using shear stress. Under shear tension, these shear-thinning hydrogels flow like low-viscosity fluids during the injection but quickly regain their original stiffness once the shear force is removed from the body. Any hydrogels formed using the physical cross-linking method can apply this mechanism.
-
For macroporous hydrogels, water is pushed out of the pores when the hydrogel is supplied through injection using a needle and syringe, causing the hydrogel to collapse and pass through the needle. The hydrogel may almost quickly regain its original form in the body once it has exited the needle and the mechanical constraint provided by the needle walls has been eliminated. Though having many benefits, interconnected pores may minimize drug release diffusion length and polymer volume fraction, potentially resulting in too quick drug release and reduced drug loading capacity.
To better demonstrate the formation of parenteral-administered hydrogels, benzydamine hydrochloride, melatonin, and epigallocatechin gallate is loaded into chitosan-based hydrogels in the studies conducted by Rossi et al., Chen et al., and Kim et al. respectively [105, 108, 118].
6 Conclusion
Chitosan hydrogels have a lot of potential for use in biological applications. The aforementioned experiments showed that hydrogel can be formed into various drug delivery systems depending on the route of administration or the drug molecules. Due to its exceptional qualities, hydrogel provided an effective and novel anti-inflammation approach. Because of their high porosity, biocompatibility, biodegradability, and flexibility, hydrogels are an excellent choice for drug delivery applications. Additionally, in many particular circumstances, such as in diabetic patients, inflammation is more severe, which is when hydrogel might benefit greatly from serving as a medication delivery system. As a result, an additional study should be done to perfect hydrogel as a delivery mechanism and make it widely used.
References
N.H. Do, Q.T. Truong, P.K. Le, A.C. Ha, Recent developments in chitosan hydrogels carrying natural bioactive compounds. Carbohydr Polym 294, 119726 (2022)
S.K. Gulrez, S. Al-Assaf, G.O. Phillips, Hydrogels: Methods of preparation, characterisation and applications, in Progress in Molecular and Environmental Bioengineering, ed. by A. Carpi (IntechOpen), pp. 117–150 (2011)
M. Kim, C. Cha, Modulation of functional pendant chains within poly (ethylene glycol) hydrogels for refined control of protein release. Sci. Rep. 8(1), 1–12 (2018)
H.Y. Zhou, L.J. Jiang, P.P. Cao, J.B. Li, X.G. Chen, Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohyd. Polym. 117, 524–536 (2015)
R.K. Bose, K.K. Lau, Initiated cvd of poly (2-hydroxyethyl methacrylate) hydrogels: Synthesis, characterization and in-vitro biocompatibility. Chem. Vap. Depos. 15(4–6), 150–155 (2009)
C.-C. Lin, K.S. Anseth, Glucagon-like peptide-1 functionalized peg hydrogels promote survival and function of encapsulated pancreatic \(\beta \)-cells. Biomacromol 10(9), 2460–2467 (2009)
P.S. Bakshi, D. Selvakumar, K. Kadirvelu, N. Kumar, Chitosan as an environment friendly biomaterial—a review on recent modifications and applications. Int. J. Biol. Macromol. 150, 1072–1083 (2020)
N. Gull et al., Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 162, 175–187 (2020)
R. Oun, Y.E. Moussa, N.J. Wheate, The side effects of platinum-based chemotherapy drugs: a review for chemists. Dalton Trans. 47(19), 6645–6653 (2018)
S. Zhang et al., An inflammation-targeting hydrogel for local drug delivery in inflammatory bowel disease. Sci. Transl. Med. 7(300), 300ra128-300ra128 (2015)
Y. Wang et al., Inflammation-responsive drug-loaded hydrogels with sequential hemostasis, antibacterial, and anti-inflammatory behavior for chronically infected diabetic wound treatment. ACS Appl. Mater. Interfaces 13(28), 33584–33599 (2021)
M.A. Amin, I.T. Abdel-Raheem, Accelerated wound healing and anti-inflammatory effects of physically cross linked polyvinyl alcohol-chitosan hydrogel containing honey bee venom in diabetic rats. Arch. Pharmacal Res. 37(8), 1016–1031 (2014)
J. García-Couce et al., Chitosan/pluronic f127 thermosensitive hydrogel as an injectable dexamethasone delivery carrier. Gels 8(1), 44 (2022)
J. Qu, X. Zhao, P.X. Ma, B. Guo, Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized smart drug release. Acta Biomater. 72, 55–69 (2018)
L.L. Chaves et al., pH-responsive chitosan based hydrogels affect the release of dapsone: design, set-up, and physicochemical characterization. Int. J. Biol. Macromol. 133, 1268–1279 (2019)
Y. Chen et al., Preparation of the chitosan/poly (glutamic acid)/alginate polyelectrolyte complexing hydrogel and study on its drug releasing property. Carbohyd. Polym. 191, 8–16 (2018)
G.R. Mahdavinia, H. Etemadi, F. Soleymani, Magnetic/ph-responsive beads based on caboxymethyl chitosan and \(\kappa \)-carrageenan and controlled drug release. Carbohyd. Polym. 128, 112–121 (2015)
P.R. Ninawe, S.J. Parulekar, Drug delivery using stimuli-responsive polymer gel spheres. Ind. Eng. Chem. Res. 51(4), 1741–1755 (2012)
P. Gupta, K. Vermani, S. Garg, Hydrogels: from controlled release to pH-responsive drug delivery. Drug Discov. Today 7(10), 569–579 (2002)
S.N. Murthy, Y.-L. Zhao, A. Sen, S.W. Hui, Cyclodextrin enhanced transdermal delivery of piroxicam and carboxyfluorescein by electroporation. J. Control. Release 99(3), 393–402 (2004)
N. Wilkosz et al., Effect of piroxicam on lipid membranes: drug encapsulation and gastric toxicity aspects. Eur. J. Pharm. Sci. 100, 116–125 (2017)
A.K. Singh, K. Pathak, Colon specific codes based piroxicam tablet for colon targeting: statistical optimization, in vivo roentgenography and stability assessment. Pharm. Dev. Technol. 20(2), 237–245 (2015)
Y. Eguchi, T. Kato, T. Tanaka, T. Maruyama, A DNA-gold nanoparticle hybrid hydrogel network prepared by enzymatic reaction. Chem. Commun. 53(43), 5802–5805 (2017)
C. Krömmelbein et al., Impact of high-energy electron irradiation on mechanical, structural and chemical properties of agarose hydrogels. Carbohyd. Polym. 263, 117970 (2021)
H.P. James, R. John, A. Alex, K. Anoop, Smart polymers for the controlled delivery of drugs-a concise overview. Acta Pharm. Sin. B 4(2), 120–127 (2014)
H. Huang, X. Qi, Y. Chen, Z. Wu, Thermo-sensitive hydrogels for delivering biotherapeutic molecules: a review. Saudi Pharm. J. 27(7), 990–999 (2019)
S. Hussain, C. Keary, D. Craig, A thermorheological investigation into the gelation and phase separation of hydroxypropyl methylcellulose aqueous systems. Polymer 43(21), 5623–5628 (2002)
H. Hamedi, S. Moradi, S.M. Hudson, A.E. Tonelli, M.W. King, Chitosan based bioadhesives for biomedical applications: a review. Carbohydr. Polym. 119100 (2022)
B. Jeong, S.W. Kim, Y.H. Bae, Thermosensitive sol-gel reversible hydrogels. Adv. Drug Deliv. Rev. 64, 154–162 (2012)
Z. Zhang et al., Intra-articular injection of cross-linked hyaluronic acid-dexamethasone hydrogel attenuates osteoarthritis: an experimental study in a rat model of osteoarthritis. Int. J. Mol. Sci. 17(4), 411 (2016)
J. Li et al., Advances of injectable hydrogel-based scaffolds for cartilage regeneration. Regen. Biomater. 6(3), 129–140 (2019)
A.H. Salama, A.A. Abdelkhalek, N.A. Elkasabgy, Etoricoxib-loaded bio-adhesive hybridized polylactic acid-based nanoparticles as an intra-articular injection for the treatment of osteoarthritis. Int. J. Pharm. 578, 119081 (2020)
E. Ruel-Gariépy et al., A thermosensitive chitosan-based hydrogel for the local delivery of paclitaxel. Eur. J. Pharm. Biopharm. 57(1), 53–63 (2004)
Y. Wang, M. Chen, X. Li, Y. Huang, W. Liang, A hybrid thermo-sensitive chitosan gel for sustained release of meloxicam. J. Biomater. Sci. Polym. Ed. 19(9), 1239–1247 (2008)
R. Dong, X. Zhao, B. Guo, P.X. Ma, Self-healing conductive injectable hydrogels with antibacterial activity as cell delivery carrier for cardiac cell therapy. ACS Appl. Mater. Interfaces 8(27), 17138–17150 (2016)
X. Zhao, R. Dong, B. Guo, P.X. Ma, Dopamine-incorporated dual bioactive electroactive shape memory polyurethane elastomers with physiological shape recovery temperature, high stretchability, and enhanced c2c12 myogenic differentiation. ACS Appl. Mater. Interfaces 9(35), 29595–29611 (2017)
C. Pérez-Martínez et al., Electroconductive nanocomposite hydrogel for pulsatile drug release. React. Funct. Polym. 100, 12–17 (2016)
T.-Y. Liu, S.-H. Hu, T.-Y. Liu, D.-M. Liu, S.-Y. Chen, Magnetic-sensitive behavior of intelligent ferrogels for controlled release of drug. Langmuir 22(14), 5974–5978 (2006)
R. Gupta, A. Bajpai, Magnetically guided release of ciprofloxacin from superparamagnetic polymer nanocomposites. J. Biomater. Sci. Polym. Ed. 22(7), 893–918 (2011)
O. Philippova, A. Barabanova, V. Molchanov, A. Khokhlov, Magnetic polymer beads: Recent trends and developments in synthetic design and applications. Eur. Polymer J. 47(4), 542–559 (2011)
S. Kayal, R. Ramanujan, Doxorubicin loaded pva coated iron oxide nanoparticles for targeted drug delivery. Mater. Sci. Eng., C 30(3), 484–490 (2010)
B. Tian, S. Hua, Y. Tian, J. Liu, Chemical and physical chitosan hydrogels as prospective carriers for drug delivery: A review. J. Mater. Chem. B 8(44), 10050–10064 (2020)
F. Lotfipour, M. Alami-Milani, S. Salatin, A. Hadavi, M. Jelvehgari, Freeze-thaw-induced cross-linked pva/chitosan for oxytetracycline-loaded wound dressing: the experimental design and optimization. Res. Pharm. Sci. 14(2), 175 (2019)
L. Yahia et al., History and applications of hydrogels. J. Biomed. Sci. 4(2), 0 (2015)
C.M. Kirschner, K.S. Anseth, Hydrogels in healthcare: from static to dynamic material microenvironments. Acta Mater. 61(3), 931–944 (2013)
A. Danno, Gel formation of aqueous solution of polyvinyl alcohol irradiated by gamma rays from cobalt-60. J. Phys. Soc. Jpn. 13(7), 722–727 (1958)
W. Otto, L. Drahoslav, Hydrophilic gels in biologic use. Nature 185, 117–118 (1960)
J. Kopeček, J. Vacik, D. Lim, Permeability of membranes containing ionogenic groups. J. Polym. Sci. Part A-1: Polym. Chem. 9(10), 2801–2815 (1971)
I. Yannas, E. Lee, D.P. Orgill, E. Skrabut, G.F. Murphy, Synthesis and characterization of a model extracellular matrix that induces partial regeneration of adult mammalian skin. Proc. Natl. Acad. Sci. 86(3), 933–937 (1989)
T.R. Hoare, D.S. Kohane, Hydrogels in drug delivery: progress and challenges. Polymer 49(8), 1993–2007 (2008)
J. Berger et al., Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur. J. Pharm. Biopharm. 57(1), 19–34 (2004)
W.E. Hennink, C.F. van Nostrum, Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 64, 223–236 (2012)
F. Ullah, M.B.H. Othman, F. Javed, Z. Ahmad, H.M. Akil, Classification, processing and application of hydrogels: a review. J. Mater. Sci. C Eng. 57, 414–433 (2015)
E.M. Ahmed, Hydrogel: preparation, characterization, and applications: a review. J. Adv. Res. 6(2), 105–121 (2015)
M. Rizwan et al., pH sensitive hydrogels in drug delivery: Brief history, properties, swelling, and release mechanism, material selection and applications. Polymers 9(4), 137 (2017)
F. Lira, J. Rosa Neto, B. Antunes, R. Fernandes, The relationship between inflammation, dyslipidemia and physical exercise: from the epidemiological to molecular approach. Curr. Diabetes Rev. 10(6), 391–396 (2014)
Z. Jiang, A. Bhaskaran, H.M. Aitken, I.C. Shackleford, L.A. Connal, Using synergistic multiple dynamic bonds to construct polymers with engineered properties. Macromol. Rapid Commun. 40(10), 1900038 (2019)
N. Bhattarai, J. Gunn, M. Zhang, Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 62(1), 83–99 (2010)
J. Qu, X. Zhao, P.X. Ma, B. Guo, pH-responsive self-healing injectable hydrogel based on n-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 58, 168–180 (2017)
H. Hamedi, S. Moradi, S.M. Hudson, A.E. Tonelli, Chitosan based hydrogels and their applications for drug delivery in wound dressings: a review. Carbohyd. Polym. 199, 445–460 (2018)
R. Parhi, Cross-linked hydrogel for pharmaceutical applications: a review. Adv. Pharm. Bull. 7(4), 515–530 (2017)
J.A. Del Olmo et al., Self-healing, antibacterial and anti-inflammatory chitosan-peg hydrogels for ulcerated skin wound healing and drug delivery. Biomater. Adv. 139, 212992 (2022)
J.A. Del Olmo et al., Wound healing and antibacterial chitosan-genipin hydrogels with controlled drug delivery for synergistic anti-inflammatory activity. Int. J. Biol. Macromol. 203, 679–694 (2022)
S. Peers, A. Montembault, C. Ladavière, Chitosan hydrogels incorporating colloids for sustained drug delivery. Carbohyd. Polym. 275, 118689 (2022)
G. He et al., Anti-inflammatory catecholic chitosan hydrogel for rapid surgical trauma healing and subsequent prevention of tumor recurrence. Chin. Chem. Lett. 31(7), 1807–1811 (2020)
S. Tohme, R.L. Simmons, A. Tsung, Surgery for cancer: a trigger for metastases. Can. Res. 77(7), 1548–1552 (2017)
Q. Chen et al., In situ sprayed bioresponsive immunotherapeutic gel for post-surgical cancer treatment. Nat. Nanotechnol. 14(1), 89–97 (2019)
H. Chen, Y. Ma, X. Wang, Z. Zha, Multifunctional phase-change hollow mesoporous prussian blue nanoparticles as a nir light responsive drug co-delivery system to overcome cancer therapeutic resistance. J. Mater. Chem. B 5(34), 7051–7058 (2017)
J.G. Hiller, N.J. Perry, G. Poulogiannis, B. Riedel, E.K. Sloan, Perioperative events influence cancer recurrence risk after surgery. Nat. Rev. Clin. Oncol. 15(4), 205–218 (2018)
S.M. Murthy et al., The influence of surgical trauma on experimental metastasis. Cancer 64(10), 2035–2044 (1989)
S. Tohme et al., Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stressnets promote liver metastases after surgical stress. Can. Res. 76(6), 1367–1380 (2016)
Y. Huang et al., Near-infrared photothermal release of hydrogen sulfide from nanocomposite hydrogels for anti-inflammation applications. Chin. Chem. Lett. 31(3), 787–791 (2020)
X. Zhao et al., Antibacterial anti-oxidant electroactive injectable hydrogel as self-healing wound dressing with hemostasis and adhesiveness for cutaneous wound healing. Biomaterials 122, 34–47 (2017)
H. Yuan, W. Li, C. Song, R. Huang, An injectable supramolecular nanofiber-reinforced chitosan hydrogel with antibacterial and anti-inflammatory properties as potential carriers for drug delivery. Int. J. Biol. Macromol. 205, 563–573 (2022)
N.A. Mohamed, N.A. Abd El-Ghany, M.M. Abdel-Aziz, Synthesis, characterization, anti-inflammatory and anti-helicobacter pylori activities of novel benzophenone tetracarboxylimide benzoyl thiourea cross-linked chitosan hydrogels. Int. J. Biol. Macromol. 181, 956–965 (2021)
S. Mihai et al., Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J. Immunol. Res. 2018, 2180373 (2018)
I. Zabetakis, R. Lordan, C. Norton, A. Tsoupras, Covid-19: the inflammation link and the role of nutrition in potential mitigation. Nutrients 12(5), 1466 (2020)
R. Milo, A.D. Korczyn, N. Manouchehri, O. Stüve, The temporal and causal relationship between inflammation and neurodegeneration in multiple sclerosis. Mult. Scler. J. 26(8), 876–886 (2020)
T. Jin, Mechanisms underlying the metabolic beneficial effect of curcumin intervention: beyond anti-inflammation and anti-oxidative stress. Obes. Med. 13, 1–5 (2019)
D. Elieh-Ali-Komi, M.R. Hamblin, Chitin and chitosan: production and application of versatile biomedical nanomaterials. Int. J. Adv. Res. 4(3), 411 (2016)
E. Azevedo, Chitosan hydrogels for drug delivery and tissue engineering applications. Int. J. Pharm. Pharm. Sci. 7(12), 8–14 (2015)
F.C. Mooren, A. Berthold, W. Domschke, J. Kreuter, Influence of chitosan microspheres on the transport of prednisolone sodium phosphate across ht-29 cell monolayers. Pharm. Res. 15(1), 58–65 (1998)
N.P. Nirmal, C. Santivarangkna, M.S. Rajput, S. Benjakul, Trends in shrimp processing waste utilization: an industrial prospective. Trends Food Sci. Technol. 103, 20–35 (2020)
P.T.D. Phuong et al., Recovery of protein hydrolysate and chitosan from black tiger shrimp (penaeus monodon) heads: approaching a zero waste process. J. Food Sci. Technol. 54(7), 1850–1856 (2017)
V. Falanga, Wound healing and its impairment in the diabetic foot. The Lancet 366(9498), 1736–1743 (2005)
T. Velnar, T. Bailey, V. Smrkolj, The wound healing process: an overview of the cellular and molecular mechanisms. J. Int. Med. Res. 37(5), 1528–1542 (2009)
R.S. Kirsner, W.H. Eaglstein, The wound healing process. Dermatol. Clin. 11(4), 629–640 (1993)
F..S.. Zetoune, P.. A.. Ward, xPharm: the comprehensive pharmacology reference, in Inflammatory Disorders. ed. by S.. Enna, D.. B.. Bylund (Elsevier, Amsterdam, 2007), pp.1–5
G.. M.. Raghavendra, K.. Varaprasad, T.. Jayaramudu, Biomaterials: Design, Development and Biomedical Applications (Elsevier, Amsterdam, 2015), pp.21–44
E.. Shacter, S..A.. Weitzman, Oncology, Chronic inflammation and cancer. Oncology 16(2), 217–226, 229 (2002)
E.R. Sherwood, T. Toliver-Kinsky, Mechanisms of the inflammatory response. Best Pract. Anaesthesiol. Res. Clin. 18(3), 385–405 (2004)
V.. Manigandan, R.. Karthik, S.. Ramachandran, S.. Rajagopal, Chitosan Applications in Food Industry (Elsevier, Amsterdam, 2018)
R.O. Day, G.G. Graham, Non-steroidal anti-inflammatory drugs (NSAIDs). BMJ 346, f3195 (2013)
J.N. Cashman, The mechanisms of action of NSAIDS in analgesia. Drugs 52(5), 13–23 (1996)
C. Lee, L. Lo, C. Mou, C.J.A.F.M. Yang, Synthesis and characterization of positive-charge functionalized mesoporous silica nanoparticles for oral drug delivery of an anti-inflammatory drug. Adv. Funct. Mater. 18(20), 3283–3292 (2008)
C. Bharti, U. Nagaich, A.K. Pal, N. Gulati, Mesoporous silica nanoparticles in target drug delivery system: A review. International journal of pharmaceutical investigation 5(3), 124 (2015)
R.L. Barkin, Topical nonsteroidal anti-inflammatory drugs: the importance of drug, delivery, and therapeutic outcome. Am. J .Ther. 22(5), 388–407 (2015)
M..L.. Bruschi, Strategies to Modify the Drug Release from Pharmaceutical Systems (Woodhead Publishing, Sawston, 2015)
S.. Peers, A.. Montembault, C.. J.. J.. o. C.. R.. Ladavière, Chitosan hydrogels for sustained drug delivery. J. Control. Release 326, 150–163 (2020)
C.K. Field, M.D. Kerstein, Overview of wound healing in a moist environment. Am. J. Surg. 167(1), S2–S6 (1994)
Z. Bagher et al., Wound healing with alginate/chitosan hydrogel containing hesperidin in rat model. Journal of Drug Delivery Science and Technology 55, 101379 (2020)
Z. Wu et al., Optimization for production of exopolysaccharides with antitumor activity in vitro from paecilomyces hepiali. Carbohyd. Polym. 99, 226–234 (2014)
M.W. Jøraholmen, P. Basnet, M.J. Tostrup, S. Moueffaq, N. Škalko Basnet, Localized therapy of vaginal infections and inflammation: liposomes-in-hydrogel delivery system for polyphenols. Pharmaceutics 11(2), 53 (2019)
M.W. Jøraholmen, N. Škalko Basnet, G. Acharya, P. Basnet, Resveratrol-loaded liposomes for topical treatment of the vaginal inflammation and infections. Eur. J. Pharm. Sci. 79, 112–121 (2015)
S. Rossi et al., Thermally sensitive gels based on chitosan derivatives for the treatment of oral mucositis. Eur. J. Pharm. Biopharm. 74(2), 248–254 (2010)
S. Miyazaki, K. Ishii, T. Nadai, The use of chitin and chitosan as drug carriers. Chem. Bull. Pharm. 29(10), 3067–3069 (1981)
H. Liu et al., A functional chitosan-based hydrogel as a wound dressing and drug delivery system in the treatment of wound healing. RSC Adv. 8(14), 7533–7549 (2018)
B. Kim et al., Enzyme-mediated one-pot synthesis of hydrogel with the polyphenol cross-linker for skin regeneration. Mater. Today Bio 8, 100079 (2020)
D. Zhang et al., Catechol functionalized chitosan/active peptide microsphere hydrogel for skin wound healing. Int. J. Biol. Macromol. 173, 591–606 (2021)
L.M. Hemmingsen et al., Liposomes-in-chitosan hydrogel boosts potential of chlorhexidine in biofilm eradication in vitro. Carbohyd. Polym. 262, 117939 (2021)
J. Vane, R. Botting, Anti-inflammatory drugs and their mechanism of action. Inflamm. Res. 47(2), 78–87 (1998)
M..F.. Hebert, Impact of pregnancy on maternal pharmacokinetics of medications. Clin. Pharmacol. During Pregnancy 20, 17–39 (2013)
D.A. Carr, M. Gómez-Burgaz, M.C. Boudes, N.A. Peppas, Complexation hydrogels for the oral delivery of growth hormone and salmon calcitonin. Ind. Res. Eng. Chem. 49(23), 11991–11995 (2010)
F.. Rodrigues, M.. B.. P.. Oliveira, Cell-Based in vitro Models for Dermal Permeability Studies (Elsevier, Amsterdam, 2016), pp.155–167
C.J. Mate, S. Mishra, Synthesis of borax cross-linked jhingan gum hydrogel for remediation of remazol brilliant blue r (rbbr) dye from water: adsorption isotherm, kinetic, thermodynamic and biodegradation studies. Int. J. Biol. Macromol. 151, 677–690 (2020)
N.J. Alexander et al., Why consider vaginal drug administration? Fertil. Steril. 82(1), 1–12 (2004)
J. Li, D.J. Mooney, Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 1(12), 1–17 (2016)
K. Chen et al., Injectable melatonin-loaded carboxymethyl chitosan (cmcs)-based hydrogel accelerates wound healing by reducing inflammation and promoting angiogenesis and collagen deposition. J. Mater. Sci. Technol. 63, 236–245 (2021)
Acknowledgements
We acknowledge Ho Chi Minh City University of Technology (HCMUT), VNU-HCM for supporting this study.
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
HTTN: Writing—original draft preparation, visualization, resources, formal analysis. NHND: Conceptualization, validation, writing—review and editing. HDL: Formal analysis, data curation, resources. PLNN: Resources. PKL: Supervision, writing—review and editing, validation. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflicts of interest concerning the research, authorship, and/or publication of this review.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nguyen, H.T.T., Do, N.H.N., Lac, H.D. et al. Synthesis, properties, and applications of chitosan hydrogels as anti-inflammatory drug delivery system. J Porous Mater 30, 655–670 (2023). https://doi.org/10.1007/s10934-022-01371-6
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-022-01371-6