Abstract
A highly ordered mesoporous carbon nanocage (OMC) direct synthesis method is introduced in this article. Petroleum pitch is chosen as a hydrophobic carbon precursor, tetrahydrofuran (THF) is used as solvent, the triblock copolymer F127 as structure directing agent, while the liquid crystal templating (LCT) is used as the templating mechanism, and the OMC was obtained via a one-step nanocasting method. Experimental results showed that the introduction of proper amounts of petroleum pitch and THF ratio does not hamper the synthetic process of the structure of OMC, thus a uniform carbon composite is finally formed. It was found that the as-prepared nanoporous carbon has a three-dimensional 7.5 nm-sized carbon nanocage network. Such a uniform mesoporous carbon material exhibits a high Brunauer–Emmett–Teller (BET) surface area (446.9 m2 g− 1) and a total pore volume (0.6 cm3 g− 1).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Nowadays, great deal of efforts has been made to prepare various types of novel carbon nanomaterials, and their nanostructures have also been tailored for meeting some specific applications, such as catalysis, biomedicine, absorption, and energy storage [1,2,3]. Especially, the OMC materials possess additional advantages, such as well-defined pore size, large surface area, excellent thermal stability, chemical inertness and flexible framework composition [4,5,6]. Thus, more and more methods have been developed to prepare OMC. Currently, the most widely used method to prepare OMC is template method, including the hard and the soft-templating approach [7, 8], which can realize the controllable of the structure of OMC effectively [9, 10]. In addition, these two template methods are simple and efficient nanocasting methods that can form the mesoporous structures combined with certain number of surfactants and inorganic precursors [11]. During this process of reaction, silica-contained substances such as tetraethyl tetramethyl orthosilicate (TMOS) and orthosilicate (TEOS) are often chosen as carbon precursors, thus only some highly hydrophilic substances have been reported as carbon precursors, such as cyclodextrin, sucrose [12], resorcinol–formaldehyde [13], resol precursor [14], etc. Meanwhile, the carbon precursors can also largely determine the frame work of the OMC, as well as its physical and chemical properties [15]. The hydrophilic carbon sources mentioned above can generally prepare the OMC with amorphous nature frame work [16,17,18,19]. Since the hydrophobic substances, such as pitch and acenaphthene, possess the graphitic building blocks, thus can easily afford the frame work of OMC with some degree of graphitization [20]. However, OMC prepared from the hydrophobic substances are usually by a two-step templating method, which is tedious, incomplete pore filling, and the carbon source is easly to deposited on the external surface of nanoporous silica template [21]. Therefore, the one-step fabrication or a direct templating method to prepare OMC from hydrophobic precursors which rarely reported is eager to proach [17].
This research is aimed to propose a new direct fabrication process to prepare OMC materials from the hydrophobic precursors. In this article, a novel direct fabrication of OMC from highly hydrophobic petroleum pitch is introduced. During this process, the amphiphilic triblock copolymer Pluronic F127 and pitch are dissolved in the THF, while the liquid crystal templating (LCT) is used as the templating mechanism.
2 Experimental sections
2.1 Raw materials
The AH-90 petroleum pitch used as carbon precursor in this experiment is provided by Sinopec co., Ltd, Guangzhou, China, which softening point is 51 °C, and the elemental contents is 85.56 wt% C, 10.42 wt% H, 0.76 wt% N, and 1.14 wt% S. The amphiphilic triblock copolymer Pluronic F127 was purchased from the Aldrich co., Ltd, which purity is 99%. The reagent and solvent used without further purification were supplied by Tianjin Fuchen chemical reagents factory: ammonium persulfate, ammonium perchlorate, ethyl orthosilicate, hydrochloric acid, sulfuric acid, ethyl alcohol, sodium hydroxide, hydrofluoric acid and THF. The deionized water was from the laboratory made.
2.2 Synthesis of OMC
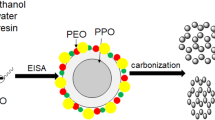
The F127 and petroleum pitch were first dissolved in THF thoroughly, and the pitch/F127/THF solution was successfully obtained, and the ratio of pitch/F127/THF is 8 g /1.6 g/30 ml. This solution was then aged in a polytetrafluoroethylene (PTFE) sealed pot under the temperature of 50 °C for 3 days. The mixture obtained in the previous steps was then transferred into a petri dish under 80 °C for 24 h, thus the solvent of the mixture can be vaporized, and the composites pitch/F127 was obtained. The preoxidation of pitch/F127 composites was reacted at 300 °C in air atmosphere and last for 12 h. These samples were then carbonized at 900 °C for 3 h with N2 flow, and the OMC were finally obtained (as shown in Fig. 1).
2.3 Characterization
The thermogravimetric analysis (TGA) of pitch/silica composite was carried out by a TGA Q50 thermogravimetric analyzer in the oxygen flowing condition at a heating rate of 5 °C /min. The ordered structure of the samples was investigated with a XRD-7000X diffractometer (SHIMADZU Co., Japan) using Cu–Kα radiation with k = 1.54060 Å. The copper anode was operated at a voltage of 40 kV and a current of 40 mA. The microstructure of the OMC samples was investigated by a JEM 2010HR transmission electron microscope (TEM, JEOL Ltd. with an energy dispersive X-ray microanalysis system), and an ASAP 2010 surface area and porosity analyzer (Micromeritics Instrument Corporation). To guarantee precise of the measurement, each sample was evacuated at 250 °C for 8 h under vacuum before the porosity analysis. The Brunauer–Emmett–Teller (BET) surface area was calculated by BET theory based on the adsorption data in the partial pressure (P/P0) range 0.06–0.21, the total volume was determined from the amount of nitrogen adsorbed at P/P0 = 0.99, while the micropore volume was obtained by t-plot method. The pore size distribution was determined by Barret–Joyner–Halendar (BJH) theory according to the adsorption branch data.
2.4 Adsorption experiments for Pb(II) and Cd(II)
The adsorption capacity of Pb(II) and Cd(II) onto the OMC was determined by the following procedure; 0.05 g of the sample were placed in 25 mL glass vials and 10 mL of a 0.3 mM Pb(II) solution were added. This dissolution was prepared by dissolving a salt of lead nitrate [Pb(NO3)2·2H2O] analytic grade in a dissolution of HNO3 at 1% to avoid hydrolysis of the metal. All samples were adjusted at pH 5 and the vials bottles containing the reaction mixture were placed in an incubator shaking at 150 rpm and 25 °C. The pH of the dissolution was adjusted daily with 0.1 N HNO3 for 5 days until there was no change and the equilibrium was reached. The dissolutions were filtrated with a glass membrane with an average porous diameter of 0.22 μm. The final concentrations in the aqueous dissolutions were determined by atomic absorption spectroscopy using a Perkin Elmer Analyst 400 and the adsorbed metal was calculated by a mass balance. The same experimental procedure was conducted for the adsorption of Cd(II). The employed dissolution at 0.3 mM of Cd(II) was prepared from cadmium nitrate [Cd(NO3)2·7H2O] analytic grade.
2.5 Adsorption isotherms
In a 25 mL glass bottle, 0.05 g of the sample were weighed and 10 mL of a dissolution of Pb(II) were added. Concentrations from 0.05 to 0.35 g/L were tested at pH 5 and 25 °C. The same procedure was repeated for Cd(II) ions.
3 Results and discussions
3.1 The thermal properties of pitch/F127 composite
Generally, there are two common direct fabrication methods to prepare OMC materials have been proved to be effectively, one is proposing a proper solvent which can dissolve all kinds of components; and the other one is choosing a kind of solvent that the amphiphilic triblock copolymer Pluronic F127 can achieved the state of micelle. This article is aiming to search for a proper solvent to realize the second method mentioned above. Therefore, the THF was chosen as the solvent to dissolve these raw materials (i.e., petroleum pitch, F127) in the hydrophobic condition according to the reference [17]. It can be observed that the F127 and petroleum pitch can achieve a uniform micelle configuration when added into the THF solvent at the same time.
In addition, the suitable ratio of petroleum pitch and F127 in THF solvent that can achieve the ordered structure is the critical issue to be resolved. The repeatedly experiments showed that when the optimum proportion of raw materials is petroleum pitch 1 g and F127 0.4 g for 7.5 ml of THF, thus a black uniform solution can be finally obtained. The phenomena can provide a possibility to develop a direct preparation of ordered nanoporous carbons from petroleum pitch. To further study the uniformity of the pitch/F127 composite, the TGA is employed, while the three specimens are obtained from the different part (top, middle, bottom) of the petroleum pitch and F127 in THF solution, respectively. It can be observed in Fig. 2 that, the shape of TGA curves of the specimens from the different part of the pitch/F127/THF are very close to each other, and the weight loss at 800 °C of these samples are almost the same. Furthermore, the derivative thermogravimetry (DTG) curves of these samples with the similar tendency and also exhibit one thermolysis stages with their peaks of maximum rate of weight loss at about 480 °C, which indicating that the thermolytic mechanism of these samples are the same. All of the phenomenons above demonstrating that the content of the pitch/F127/THF solution is very homogeneous.
The content of OMC was also determined by TEM-EDX analysis (Fig. 3). The EDX analysis shows that the atomic percentage (at%) of carbon is about 95.8% in the mesoporous carbon. The others content have only a tiny percentage.
3.2 The X-ray diffraction results of OMC
The XRD pattern of the OMC prepared as above is shown in Fig. 4. It can be seen clearly from Fig. 4 that there are three diffraction peaks in the region 2θ = 0.5°–5° indexed as crystal planes (100), (110), and (200), which indicates a well-ordered two-dimensional (2D) hexagonal structure. Furthermore, the intensity of the OMC is very high, which indicating that it has the excellent ordered structure.
3.3 The microstructure of OMC
To observe the micro-morphology of the OMC as-prepared from the experiment, the TEM is employed. It can be clearly seen from the Fig. 5 that the OMC exhibits a unique ordered nanoporous structure, as well as a three dimensional small-sized cagelike nanoporous network with well-distributed large-sized particle-like mesopores. Moreover, these types of mesopores are interconnected very well to each other in all directions. Such mesoporous structure is very similar to that of KIT-5 mesoporous silica obtained by liquid crystal templating (LCT) [22].
It has been reported that when the use amount of F127 is relatively low, the micelle would also be extremely few and then aggregated together with adjacent frameworks during the drying process, thus many petroleum pitch frameworks will be extraordinary thick; and the forming carbon nano-agglomerates will be uniformly distributed in dried pitch/F127 composites and subsequent carbon/F127 composites, which can be attributed to the wet pitch/F127 composite has a uniform mesostructure (as shown in Fig. 1). Therefore, the small cage-like pore systems should result from the F127 micelle framework. Furthermore, it should be noted that the different hydrophilic-hydrophobic property between petroleum pitch (hydrophobic) and F127 (hydrophilic) can also provide favorable conditions to the occurrence of the aggregation phenomenon. This phenomenon suggests that as compared to the reported LCT system containing hydrophobic carbon precursors [23], this novel LCT system should possess a stronger ability to form the ordered nanoporous structure, which is believed to have superior mass transport than the uniform nanoporous structure [9, 23].
3.4 The N2 adsorption–desorption isotherm of OMC
Figure 6 shows N2 adsorption–desorption isotherm and corresponding pore size distribution curve of the as-prepared nanoporous carbon sample with 8 g of pitch. It can be clearly seen that the isotherm is of type-IV and has a clear hysteresis loop, indicating that this carbon sample is a typical mesoporous material. Furthermore, this loop is narrow, demonstrating that its mesopores have a distinct monodispersity in size, which agrees well with TEM observation mentioned above. BJH calculation reveals that there exist two PSD peaks at 7.5 nm (as shown in Fig. 5). Obviously, they should be ascribed to micelle framework-templated cagelike mesopore. BET calculation shows that the BET surface area is up to 446.9 m2 g− 1. Moreover, according to the N2 adsorption amount at P/P0 = 0.99, the total pore volume is as high as 0.60 cm3 g− 1, which is much higher ordered pore than that of other pitch-based porous carbons prepared by the two-step templating method [20]; whereas t-plot calculation shows that the micropore volume is as low as 0.027 cm3 g− 1. This demonstrates that the as-obtained petroleum pitch-based carbon nanomaterials are very highly mesoporous.
3.5 Raman spectrum of OMC
The data was fitted to a linear combination of four Lorentzians, and a third order polynomial for the background. The three parameters of each peak, i.e., the peak position, the height, and the width were allowed to vary. Excellent fits were obtained for the observed data, with the R 2 factor exceeding 0.995 (R 2 = 1 for a perfect fit). The resolved peaks are shown under the overall spectrum (without background) in Fig. 7.
It is clear from these results that the present carbons have structural features quite different from those that have been investigated. It may not be possible to explain this with the model used for graphitic carbons (i.e., highly conducting carbons with almost entirely sp2 bonding), that is, an assembly of graphite-like clusters in which carbon atoms are arranged in hexagonal rings. The clusters may be arranged with varying degrees of order. None of these studies (including the above cited ones) have shown any possibility of a predominantly active Raman mode of around 1174 cm− 1.
Thus we may have to look for a structural model where a stable arrangement of carbon atoms does occur not only in aromatic rings, but also in polymer-like chains with a double and single bond alternation. We make this suggestion, as the Raman peak around 1174 cm− 1 is commonly observed in conjugated polymers, such as petroleum pitch and has been studied extensively. They are attributed to C–C bond stretching and CH in-plane bending mixed modes. The peak at 800 cm− 1 also is observed in these and some other polymers, although very weakly. Its origin is not identified, but is considered to be due to disorder. In fact more than one peak is observed in the neighborhood of these frequencies.
However one problem is that in conjugated polymers the –C=C– bond alternating chain structure is stabilized by the presence of hydrogen. OMC cannot contain that amount of hydrogen, as they are synthesized at as high a temperature as 900 °C. It is known that when the carbonaceous materials are pyrolized, hydrogen escapes almost entirely at such temperatures. Thus, the presence of –C=C– chains has to be stabilized with some sort of networking with aromatic ring domains.
3.6 Pb(II) and Cd(II) equilibrium adsorption isotherms
Langmuir model is related to the number of layers of adsorbate in the surface of the adsorbent which depends on the concentration of adsorbate in solution. The homogenous adsorption on the surface of the material, the idealization that the molecules travel though the adsorbent surface and no interaction among adsorbed molecules and adsorbate occurs, are other of its characteristics [24,25,26]. The following equation describes this model:
where “qe”, is the quantity of adsorbate adsorbed on the substrate, “Ce”, is the concentration of adsorbate in the solution at equilibrium, “a”, the maximum quantity of adsorbate adsorbed and “b”, is the Langmuir constant. Figure 8 shows the experimental data and the fit for the sample OMC isotherms, the constants for both cases are presented. The material exhibits a behavior that match with the model [27]. The maximum adsorption capacity was determined for Cd(II) (20 mg g−1) and for Pb(II) (46 mg g−1) at the experimental conditions described above.
4 Conclusion
In this article, a petroleum pitch-based OMC has been fabricated successfully by LCT technique, the THF have been proven to be the appropriate solvent. Moreover, the use amount of petroleum pitch is a key factor to obtain a uniform pitch/F127 composite. The as-prepared carbon material has a uniform mesoporous structure with 7.5 nm diameter mesopores. This uniform mesoporous obtained here is highly mesoporous: its total pore volume is as high as 0.60 cm3 g− 1, whereas its micropore volume is as low as 0.027 cm3 g− 1. In addition, its BET surface area is up to 446.9 m2 g− 1. The sample of CMK-3 modified with nitric acid at 70 °C and 12 h of reactions time had the greater capacity to remove Cd(II) (20.4 mg g−1) and Pb(II)(46 mg/g) at pH 5 and 25 °C. The adsorption isotherms for both metals follow the Langmuir model and show that the adsorption of Pb(II) is greater than that for Cd(II) at pH 5 and 25 °C, in agreement with the classical adsorption theory which states that the more electronegativity ion have the higher adsorption capacity. Considering that many hydrophobic and hydrophilic carbon precursors can be dissolved in the THF medium, we hope that the simple and efficient method introduced in this study can be further extended to prepare other nanoporous carbon materials.
References
J. Lee, J. Kim, T. Hyeon, Recent progress in the synthesis of porous carbon materials. Adv. Mater. 18, 2073–2094 (2006)
D.C. Wu, R.W. Fu, M.S. Dresselhaus, G. Dresselhaus, Fabrication and nano-structure control of carbon aerogels via a microemulsion-templated sol–gel polymerization method. Carbon 44, 675–681 (2006)
D.W. Wang, F. Li, M. Liu, G.Q. Lu, H.M. Cheng, A 3D aperiodic hierarchical porous graphitic carbon for high rate electrochemical capacitive energy storage. Angew Chem. Int. Ed. 47, 373–376 (2008)
S.H. Joo, S.J. Choi, I. Oh, J. Kwak, Z. Liu, O. Terasaki et al., Ordered nanoporous arrays of carbon supporting high dispersions of platinum nanoparticles. Nature 412, 169–172 (2001)
Y. Wan, Y.F. Shi, D.Y. Zhao, Supramolecular aggregates as templates: ordered mesoporous polymers and carbons. Chem. Mater. 20, 932–945 (2008)
H.D. Du, L. Gan, B.H. Li, P. Wu, Y.L. Qiu, F.Y. Kang et al., Influences of mesopore size on oxygen reduction reaction catalysis of Pt/carbon aerogels. J. Phys. Chem. C 111, 2040–2043 (2007)
M. Jaroniec, J. Gorka, J. Choma, A. Zawislak, Synthesis and properties of mesoporous carbons with high loadings of inorganic species. Carbon 47, 3034–3040 (2009)
R.L. Liu, Y.F. Shi, Y. Wan, Y. Meng, F.Q. Zhang, D. Gu et al., Triconstituent co-assembly to ordered mesostructured polymer-silica and carbon-silica nanocomposites and largepore mesoporous carbons with high surface areas. J. Am. Chem. Soc. 128, 11652–11662 (2006)
J.B. Pang, Q.Y. Hu, Z.W. Wu, J.E. Hampsey, J.B. He, Y.F. Lu, Direct synthesis of unimodal and bimodal nanoporous carbon. Microporous Mesoporous Mater. 74, 73–78 (2004)
D. Kawashima, T. Aihara, Y. Kobayashi, T. Kyotani, A. Tomita, Preparation of mesoporous carbon from organic polymer/silica nanocomposite. Chem. Mater. 12, 3397–3401 (2000)
J.B. Pang, X. Li, D.H. Wang, Z.W. Wu, V.T. John, Z.Z. Yang et al., Silica-templated continuous mesoporous carbon flims by a spin-coating technique. Adv. Mater. 16, 884–886 (2004)
S.J. Han, M. Kim, T. Hyeon, Direct fabrication of mesoporous carbons using in-situ polymerized silica gel networks as a template. Carbon 41, 1525–1532 (2003)
B.H. Han, W.Z. Zhou, A. Sayari, Direct preparation of nanoporous carbon by nanocasting. J. Am. Chem. Soc. 125, 3444–3445 (2003)
D.C. Wu, Z.H. Li, Y.R. Liang, X.Q. Yang, X.H. Zeng, R.W. Fu, Pore size control of wormholelike mesoporous carbons. Carbon 47, 916–918 (2009)
K.P. Gierszal, M. Jaroniec, C.D. Liang, S. Dai, Electron microscopy and nitrogen adsorption studies of film-type carbon replicas with large pore volume synthesized by using colloidal silica and SBA-15 as templates. Carbon 45, 2171–2177 (2007)
K.P. Gierszal, M. Jaroniec, Large pore volume carbons with uniform mesopores and macropores: synthesis, characterization, and relations between adsorption parameters of silica templates and their inverse carbon replicas. J. Phys. Chem. C 111, 9742–9748 (2007)
S.B. Yoon, G.S. Chai, S.K. Kang, J.S. Yu, K.P. Gierszai, M. Jaroniec, Graphitized pitch-based carbons with ordered nanoporous synthesized by using colloidal crystals as templates. J. Am. Chem. Soc. 127, 4188–4189 (2005)
Z.J. Li, M. Jaroniec, Silica gel-templated mesoporous carbons prepared from mesophase pitch and polyacylonitrile. Carbon 39, 2080–2082 (2001)
Z.J. Li, M. Jaroniec, Colloidal imprinting: a novel approach to the synthesis of mesoporous carbons. J. Am. Chem. Soc. 123, 9208–9209 (2001)
S.Y. Li, Y.R. Liang, D.C. Wu, R.W. Fu, Fabrication of bimodal mesoporous carbons from petroleum pitch by a one-step nanocasting method. Carbon 48, 839–843 (2010)
S. Polarz, B. Smarsly, L. Bronstein, M. Antonietti, From cyclodextrin assemblies to porous materials by silica templating. Angew Chem. Int. Ed. 40, 4417–4421 (2001)
A. Vinu, M. Miyahara, V. Sivamurugan, T. Mori, K. Ariga, Large pore cage type mesoporous carbon, carbon nanocage: a superior adsorbent for biomaterials. J. Mater. Chem. 15, 5122–5127 (2005)
D.C. Wu, Y.R. Liang, X.Q. Yang, Z.H. Li, C. Zou, X.H. Zeng et al., Direct fabrication of bimodal mesoporous carbon by nanocasting. Microporous Mesoporous Mater. 116, 91–94 (2008)
A. Sayari, Y. Yang, SBA-15 templated mesoporous carbon: new insights into the SBA-15 pore structure. Chem. Mater. 17, 6108–6113 (2005)
H.G. Park, T.W. Kim, M.Y. Chae, I.K. Yoo, Activated carbon-containing alginateadsorbent for the simultaneous removal of heavy metals and toxic organics. Process Biochem. 42, 1371–1377 (2007)
M. Machida, R. Yamazaki, M. Aikawa, H. Tatsumoto, Role of minerals in carbona-ceous adsorbents for removal of Pb(II) ions from aqueous solution. Sep. Purif. Technol. 46, 88–94 (2005)
Y. Liu, L. Shen, From Langmuir kinetics to first- and second-order rate equationsfor adsorption. Langmuir 24, 11625–11630 (2008)
Acknowledgements
This work was financially supported by the National Science Foundation of China (21541009), the Industria Research Project of Science and Technology, Department of Shaanxi Province (No. 2017GY-126) and the Science & Technology Project Foundation Xi’an (CXY1531WL13).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Jia, Y., Wang, W. et al. Fabrication of new ordered mesoporous carbons from petroleum pitch by triblock copolymer direct templating method. J Porous Mater 25, 771–777 (2018). https://doi.org/10.1007/s10934-017-0490-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10934-017-0490-2