Abstract
The geochemistry of lake (Renstradträsket) and estuarine (Pieni Pernajanlahti Bay) sediment was investigated in a medium sized watershed draining to the Gulf of Finland, Baltic Sea. Catchment land-use types were compared and found similar. Sediment cores were dated using 210Pb- and 137Cs-chronologies and analyzed for Al, K, Cu, Zn, Fe, Mn, phosphorus fractions, TN, TC and biogenic silica (BSi). Differences between the sediment cores were studied by using linear regression analysis and principal components analysis (PCA). Despite similarities in catchment land-use and history, the sediment geochemical profiles of the sites varied significantly. Some of the differences could be related to differences in chemical sedimentation environment (lacustrine versus estuarine). TP concentration was found to be positively correlated with sediment iron content in estuarine sediment but negatively correlated with Fe in lake sediment. In the estuarine core sedimentary iron was not correlated to lithogenic potassium and aluminum but in the lake core the iron seemed to be lithogenic in origin, as suggested by the strong positive correlations (r 2 = 0.95–0.96) between these three variables. Most similarities among the cores were found in Al concentrations. Estuarine nutrient profiles appeared relatively monotonous compared to the lake core. This is probably due to more vigorous mixing of the sediments that may ensure more rapid and complete consumption of the organic matter deposited on the bottom of the estuary. Therefore the lake sediment appeared to preserve the historical record of eutrophication better. Biologically less active and more particle-bound materials like the trace metals Cu and Zn seemed to retain good records of anthropogenic impact also in the estuarine core. The study highlights the need to take the sedimentation environment into account when interpreting geochemical record.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In a physical sense, lake environments differ from marine areas in several significant ways. Viewed in a long-term perspective, marine areas are much more permanent features on the earth’s surface than lakes. Lake levels tend to vary more rapidly than sea level, and lakes are short-lived, transient features in the landscape that generally disappear in a relatively short period of geologic time: they accumulate organic and inorganic materials on the their bottom, becoming shallower and eventually filled with sediment (Korhola 1995). These differences strongly influence also the expression of depositional sequences.
Coastal embayments such as estuaries, gulfs and bays form a habitat intermediate between these two depositional environments; they are closely linked to sediment supply from the catchment area but at the same time are interacting with larger marine systems (Day et al. 1989; Ridgway and Shimmield 2002). Physical processes, such as wind, waves, tides, surges, etc., are usually very effective in coastal environments, for which reasons they are more energetic and dynamic systems than lake environments. Estuaries and other coastal inlets receive sediment inputs from many different sources, including allochthonous terrestrial materials transported from land by rivers and groundwater, allochthonous marine materials brought in through tidal action from the open sea and autochthonous production of algae and higher vegetation (Goñi et al. 2003). The sediment stratigraphic sequence in such a hydrologically and morphologically energetic environment rarely results from simple particle settling and accumulation. Rather, sediments typically undergo many phases of deposition and erosion and the sediment layers, which are finally preserved, can reflect these phases, as well as the consequential exposure to bioturbation, resuspension and seabed diagenesis (Canfield 1989; Thomas and Bendell-Young 1999; Billon et al. 2002; San Miguel et al. 2003). Although the processes causing scavenging and release of elements from the sediment are basically the same in lacustrine and marine environments (Stumm 1992), it is by reason of this complexity, why lakes are generally considered much more suitable for paleolimnological investigations than estuaries due to more stable short-term sedimentological conditions and hence less post-depositional disturbances in them. Estuarine areas are much more challenging since it is not always easy to determine the origin of the sedimented matter due to the flow of fresh water through the system on one hand and the currents and inflows of saline water from the pelagic area on the other hand.
The estuaries and various inlets of the Baltic Sea are exceptional in this context in that they are far less energetic than the World’s marine coastal environments in general. Tidal forces in the Baltic are practically negligible (tidal ranges less than 0.2 m), and the coastal areas are usually well sheltered against heavy wave action and currents by surrounding islands, in particular along the Swedish and Finnish coastlines. Hence, the shallow embayments and estuaries of the Finnish coast resemble in many respects lake-like habitats and are assumed to behave more like lakes also from the sedimentological point of view.
There are nevertheless some additional differences in the marine chemical environment that may cause changes in sedimentary products when compared with lakes. These are predominantly related to salinity and presence of sulfates in marine waters. Increasing salinity increases the aggregation of dissolved iron and phosphorus (Forsgren et al. 1996). Sulfates form more or less insoluble components with divalent metals. The major sulfide component found in marine sediments is iron sulfide, due to the usually high abundance of iron oxyhydroxides and their ease of reduction to Fe(II) in anoxic conditions. Amorphous iron sulfide (FeS) is a relatively soluble metal sulfide, and therefore other metal cations can displace Fe to form more stable sulfides (Cooper and Morse 1999). Therefore, through a complicated binding system the presence of many elements in estuarine systems are somehow related to sulfides and iron.
Past studies have also revealed that marine or brackish-water cores are much more difficult to date using radioactive isotopes than lake cores (Tikkanen et al. 1997; Plater and Appleby 2004). This is linked to more energetic conditions in marine areas. Especially estuarine systems receive both direct atmospheric radionuclide deposition and also delayed river-derived inputs from catchment soil erosion (Oldfield et al.1989). Hence, the radionuclide chronologies require careful integration of 210Pb activities and at least one independent tracer, such as 137Cs, 239 + 240Pu or 241Am (Abril 2003). Also the presence of radon in embayments that are located in tectonic fault lines, as is often the case, for instance, in Baltic Sea estuaries might affect 210Pb chronologies. Rn is a member of the same radioactive decay series as 210Pb and therefore it might disturb the normal decay-profiles. However, very good 210Pb-chronologies have recently been obtained from sheltered lake-like embayments on Finland’s coastline (Vaalgamaa 2004; Weckström 2006) and reasonably good chronologies also from some Danish estuaries (Adser 1999; Clarke et al. 2003).
Although the processes affecting the settling and release of elements in different types of sedimentation environments have been widely studied, no study has compared the paleopreservation across different sedimentation environments. Too often the same analytical methodology and the ways to interpret the data are applied to new study sites, without much consideration on the possible fundamental differences between the new site and the environment for what the methods originally were developed. Especially geochemical data need to be interpreted with caution in paleoenvironmental studies, since sediment diagenesis can alter the geochemical signature significantly depending on the sedimentation environment.
For this study we selected a medium-sized Finnish estuary in the Gulf of Finland and an adjacent coastal lake from the estuary’s catchment for comparison. Our primary objective was to compare sediment profiles in a lake and an estuary from both sedimentological and paleoenvironmental points of view in a situation where catchment-driven differences were minimized. It is an important and challenging task to compare lake and estuarine sedimentary environments as repositories of geochemical record and pollution history. The geochemical and/or mineralogical study of such sediments can provide valuable insight into the regional hydrodynamics including patterns of sediment transport and deposition. In addition, conservation and management of aquatic systems require detailed information of the processes that affect their functioning and development. Understanding sediment dynamics is important in view of the need to determine past changes, monitor ongoing and ultimately predict future trends.
Study sites
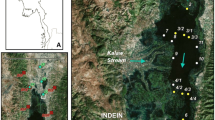
Pieni Pernajanlahti Bay (60°23′ N; 25°54′) is a medium-sized estuary of the Gulf of Finland, approx. 75 km east of Helsinki with a surface area of 9 km2 and catchment area of 356 km2 (Fig. 1). The bedrock of Pieni Pernajanlahti’s drainage basin consists mainly of granite, granodiorite and other Svecokarelian rocks. Quartz-feldspar schist and gneiss form a small area north of Lake Renstrandträsket. Geochemically these rocks are not easily soluble. In the river valley the dominating surface soils are clay and silt (Atlas of Finland 1992). The catchment area consists mainly of forests (64%) and agricultural areas (26%). Industrial and residential areas cover 6% of the catchment (Table 1). Pieni Pernajanlahti Bay is located on a fault line valley and the maximum depth of the bay is approximately 15 m. River Ilolanjoki drains into the Pieni Pernajanlahti Bay with a mean annual discharge of 2.9 m3 s−1 (Weckström et al. 2004). It is connected to the open sea through a scattered archipelago, which limits the water exchange in the bay. The salinity is approx. 4.5 per mil.
Lake Renstrandträsket is located in Pieni Pernajanlahti Bay’s catchment area. It is a fairly small (28 ha) and shallow (1.2 m) lake with a catchment area of 489 hectares. Water chemistry data from the lake are sparse covering only some winter values from a few years. However, it is known that the lake is eutrophic and occasionally anoxic during winter ice-cover. Land-use patterns are similar to Pieni Pernajanlahti Bay’s catchment: Lake Renstrandträsket has slightly more fields (30%) and forests (68%) in its catchment area than Pieni Pernajanlahti Bay, but less dwellings and industrial areas (2%).
Methods
Sediment sampling and determination of loss-on-ignition
The sediment master cores PP3 (estuarine core, 87 cm) and Rm (lake core, 79 cm) and additional cores PP1–2, PP4–5 and R1–4 were collected in summer 1998 using a mini Mackereth-corer (Mackereth 1969) (Fig. 1). The cores were sliced in the laboratory at 1 cm intervals and stored in cold (4°C). The water content and loss-on-ignition (LOI) of the sediment was determined after SFS 3008, which compares well with the method given in Heiri et al. (2001).
Particle size measurements and data normalization
Particle size distribution of minerogenic matter was analyzed from the master cores in 2 to 5 cm sample intervals using a particle size analyzer (Coulter LS-200 laser diffractometer; Agrawal et al. 1991). Before analysis, the organic matter was removed with 30% solution of hydrogen peroxide. An ultrasonic disintegrator was used to deflocculate the clay fraction. The mean value of two measurements was used.
There is usually a close relationship between metal concentrations and grain size both in estuarine and lake sediments since metals tend to associate with finer-grained particles. In order to compare samples it is therefore often necessary to diminish the grain-size effect by applying a correction factor; otherwise finer-grained samples may show relatively high metal concentrations. The grain size effect can be compensated for by rationing metal concentrations to grain size for each sample, but this has the disadvantage of being laborious. Accordingly, it has become common to normalize metal concentrations to some element found dominantly in the clay-size fraction. Aluminum has been frequently used as a grain-size proxy in estuarine sediments. To judge whether Al was suitable for use as the proxy in this study, grain size from every other sample was analyzed and plotted against Al. However, regression analysis showed poor correlations between the grain size and Al in both of the sites (r 2 = 0.11 in the estuary and r 2 = 0.22 in the lake site) suggesting that Al cannot be used for the correction purpose. In addition to this, Van der Wejden (2002) discussed problems with comparison of normalized trace element data in marine sediments. According to him, from the statistical perspective, normalization can lead to spurious self-correlations between uncorrelated variable. Hence, we decided not to normalize concentration data for this survey. This decision was further supported by the fact that there is rather little variation in the sediment grain-size in both cores (Fig. 2), for which reason grain-size impact is thought to be negligible for the correct interpretation of the element distributions.
Chemical analyses
For chemical analyses the samples were dried in plastic bags at 80°C (Tanner and Leong 1995), and subsequently homogenized in an Ika A10 mill with a W-Co blade. A sub-sample was digested by autoclaving the sample in 7 M Nitric acid at 125°C for 30 min for the analysis of Al, Ca, K, Mg, Na, Cu, Fe, Mn, Zn and total P (SFS 3044). Concentrations of metals were measured using a Varian SpectrAA 10+ atomic absorption spectrophotometer. Cs at a concentration of 2,000 μg/ml was used as an ionization suppressor in the Al, Na, and K analyses and La at a concentration of 1,000 μg/ml was used as a releasing agent in the Ca and Mg analyses (Aasarød and Storaas 1996). The total phosphorus (TP) concentration was measured using the ammonium molybdate method with ascorbic acid reduction (SFS-EN 1189). Organic phosphorus (OP) was obtained by subtracting the inorganic fraction of phosphorus from TP. Inorganic phosphorus was measured by digesting samples in 1 M HCl for 18 h (Aspila et al. 1976) and then analyzed according to SFS-EN 1189. The total nitrogen (TN) and total carbon (TC) content of the sediment was measured using a Leco-analyzer. The concentration of biogenic silica (BSi) was measured using a modification of DeMaster (1981). For further details regarding the Bsi determination, see Vaalgamaa (2004).
The repeatability and precision of the procedures used for digestion and metal and nutrient determinations were verified using certified reference materials (VKI CMR Municipal sludge A, NIST 1646 Estuarine sediment). Relative standard deviations (RSD%) from replication were <5% for all metal and TP analyses. For TN and TC analyses RSD% were 1.9 and 2.3, respectively. For BSi and IP analyses the variations within replicates were <10%. The average recoveries from the standard reference materials were 91% for TP, 96% for Zn, 98% for Cu and for 89% for K (others not certified). Quality control of BSi analysis was carried out by using the same reference samples used in an inter-laboratory comparison (Conley 1998). Due to the absence of certified BSi standards it was not possible to estimate the absolute accuracy of BSi-measurements, but BSi recoveries were nearly identical to the recoveries achieved with similar techniques in the inter-laboratory comparison.
Sediment dating
In order to achieve chronologies for the study sites the samples were analyzed for 210Pb, 226Ra, and 137Cs by direct gamma assay at the Liverpool University Environmental Radioactivity Laboratory, using Ortec HPGe GWL series well-type coaxical low background intrinsic germanium detectors (Appleby et al. 1986).
Data analyses
Regression analyses were performed by using SPSS in order to point out how changes in different indicators (possible carrier particles) influence the change in other indicators in different sedimentation environments. Principal components analysis (PCA) was used to summarize the variation within the data and to determine correlations among the variables. PCA involves a mathematical procedure that transforms a number of (possibly) correlated variables into a (smaller) number of uncorrelated variables called principal components. The first principal component accounts for as much of the variability in the data as possible, and each succeeding component accounts for as much of the remaining variability as possible (Prentice 1986). PCA-analysis with scaling focused on inter-species correlations was performed using CANOCO for Windows version 4.0 (Ter Braak and Smilauer 1998).
Results and discussion
Sediment dating
In Pieni Pernajanlahti Bay equilibrium between total 210Pb and the supporting 226 Ra was reached at a depth of 60 cm (Fig. 3a). Since the maximum unsupported 210Pb activity was only 116 Bq kg−1, it is likely that this represents a period of not more than about 100 years. The 137Cs activity had two subsurface peaks, at 4–5 cm and 16–17 cm. The high concentrations and large total inventory (69,360 Bq m−2) suggests that both peaks derived from the Chernobyl fallout. The deeper peak is the most likely indicator of the 1986 horizon. The more recent peak can be caused by shifts in the pattern of sedimentation or delayed washout from the watershed. A small but significant shoulder on the 137Cs profile at 31–32 cm may record the 1963 fallout maximum from the atmospheric testing of nuclear weapons. 210Pb dates were calculated using the CRS model, together with the 1986 and 1963 depths indicated by the 137Cs stratigraphy. Use of the CIC model was precluded by the non-monotonic variations in 210Pb activity. The 210Pb and 137Cs results are in quite good agreement. 210Pb chronology and sedimentation rates are given in detail in Table 2. The mean sedimentation rate up to 1940 was calculated to be c. 0.3 cm year−1. Since then sediment accumulation rates have increased generally towards the present, with peaks in the early 1950s, c. 1980 and during the past few years. The mean post-1963 sedimentation rate was 1.2 cm year−1.
Lake Renstrandträsket retains a fairly poor 210Pb-record. In all of the samples assayed, total 210Pb activity was barely above equilibrium with the supporting 226Ra (Fig. 3b). The 210Pb inventory corresponds to a flux of 68 Bq m−2 year−1 and is comparable to the atmospheric fallout value. The most likely explanation for the low concentrations is dilution of the atmospheric flux by relatively rapid sediment accumulation. Anyhow these sediment accumulation rates are lower than in the top parts of the Pieni Pernajanlahti Bay because the Renstrandträsket Lake is flat-bottomed and lacking clear sedimentation focus. The 137Cs activity versus depth profile has a well-resolved peak at 8.5 cm, demonstrating that the core retains a good record. Since the total 137Cs inventory (9,100 Bq m−2) is three times higher than typical weapons fallout values in Finnish lakes, the 137Cs peak almost certainly records fallout from the 1986 Chernobyl accident. This inference is also supported by the high 137Cs/210Pb inventory ratio (4.2). No clear evidence of the 1963 weapons fallout maximum was detected. Due to the poor 210Pb record the dates calculated using the CRS model had large uncertainties. The 1986 137Cs date gives a mean sedimentation rate during the past 13 years of 0.15 ± 0.004 g cm−2 year−1, and is comparable to the mean sedimentation rate calculated using the CRS model of c. 0.13 g cm−2 year−1. In the absence of more reliable evidence the safest procedure is to calculate a sediment chronology based on the average sediment accumulation rate of 0.14 g cm−2 year−1.
The estuarine site exhibits a major increase in sediment accumulation rate during the past few years, a phenomenon that has been observed also from other estuarine sites from the Baltic Sea (Vaalgamaa 2004; Weckström 2006). This is caused by flattening of the unsupported 210Pb activity versus depth profile in the top sediment layers. This feature can be explained by either incomplete vertical mixing within the top sediment zone (Abril 2003) or by acceleration of the sedimentation rate in recent years. Due to uncertainties of the cause of the phenomenon and the huge impacts it has on the recent sediment accumulation rates, sediment geochemical data were chosen to be presented in concentrations rather than accumulation profiles.
Lithology and particle size distribution
There were no major changes in the visual characteristics of the marine sediment core. The top 1 cm of the master core PP3 was oxidized brownish sediment and below that the color of the sediment was dark gray with FeS-patches. The organic content of the sediment, measured as loss on ignition (LOI%, dry weight), was around 12% at the sediment surface and from there it gradually declined to c. 8% at approx. 20 cm depth (Fig. 2). From 20 cm downwards the organic content of the sediment fluctuated around 8%. There was no great variation in the median grain size within this sediment core, the grain size varying from 4 to 6 microns (μm).
In the lake core there was one clear visual change in sediment lithology. From the sediment surface downwards to 34 cm depth the color of the sediment was grayish. At 34 cm the sediment color changed to dark brown. At the same depth also the sediment LOI% increased. The LOI% of the top layer varied around 12–14% (Master core RM) and from 34 cm downwards the organic content ranged from 16 to 25%. There was slightly more variation in the sediment grain size in the lake core in comparison to the estuarine core. The grain size retained the same peak horizon than the organic content with highest values from 40 to 60 cm and lower values in the top part of the core. The median grain size varied from 3 to 8 microns (μm).
Because geochemical analyses were done from the single cores only (per site), additional sediment cores were taken from both study sites in order to compare the sedimentation conditions in different parts of the basins (Fig. 1). The percentages and shapes of the LOI curves were found very similar in different parts of the marine site, suggesting rather stable sedimentation conditions in this estuary (Fig. 2a). Slightly more variation was observed in the LOI profiles of the lacustrine cores (Fig. 2b). Four of the lake cores had lower LOI values in the top parts and distinct peaks around 30 to 60 cm (in R1 from 45 cm downwards). However, core R3 had more complicated stratigraphy with more variation in the top part and no clear peak between 30 and 60 cm. Nevertheless, the data suggest also rather stable sedimentation conditions in the lake basin. Overall, the data suggest that the two master cores seem to represent the general sedimentation conditions in the two basins reasonably well.
The median particle size varied between 4–6 in the estuarine core and between 3 and 8 in the lake core. Despite the small variation observed in the grain size data, there are nevertheless some distinct trends in particle size distribution, in particular in the lake core, with coarser grain size in the bottom parts of the core and finer in the top. Sediment grain size seems to vary in the lake core approximately in the same manner as nutrient elements. This is in contradiction with the common assumption of increasing concentrations with decreasing grain size (Thorne and Nickless 1981, Duquesne et al. 2006). Possible explanation for this anomaly could be related to the abundance of diatom remains in the lake core. BSi concentration, which is supposed to reflect diatom abundance in the sediment (see Conley 1988), is five times higher in the lacustrine core than in the estuarine core (Fig. 4b). Average diatoms are commonly 10–200 microns in length which is a much more that the median grain size in this core. When diatom remains are present in large abundance it is directly reflected in the sediment by increased median grain size. It is therefore likely that diatoms disturb the grain size distribution in this particular case so that that the possible changes in mineral fraction are masked.
(a) Metal and nutrient concentrations, Fe:Mn- and C:N-ratios of Pieni Pernajanlahti master core (P3) plotted against time. Corresponding depths in centimeters are given on the right side of the figures. TC: total carbon, TN: total nitrogen, TP: total phosphorus, OP: organic phosphorus BSi: biogenic silica. (b) Metal and nutrient concentrations, Fe:Mn- and C:N-ratios of Lake Renstrandträsket master core (RM) plotted against time. Corresponding depths in centimeters are given on the right side of the figures. TC: total carbon, TN: total nitrogen, TP: total phosphorus, OP: organic phosphorus BSi: biogenic silica
Sediment geochemistry
In both sites, erosion indicators (K and Al) had high concentrations around the turn of the 19th century suggesting increased catchment erosion (Fig. 4a, b). In the 1920s and 1930s the values stabilized for a while and then rose again in the mid 1940s referring to more peaceful erosion conditions for a few decades followed by increased erosion activity in both watersheds. In the lake core the erosion indicators remained stable from the 1940s onwards with a slight peak in the late 1960s. There was much more variation within the estuarine core with a local maximum in the late 1960s and a significant increase in the early 1980s. Mg and Ca concentrations followed the same trends with Al and K in both sites, although Ca showed more variation. Sedimentary Na concentrations were stable in the estuarine core except for the enrichment in the top sediment. This enrichment is most likely due to higher water content of the top sediment. In the lacustrine core there is a marked decline in Na concentrations from the bottom parts of the sediment to the top (app. from 800 μg g−1 to 440 μg g−1).
The estuarine iron profile is fairly stable in the lower part of the core. From the 1960s to the 1970s there is a slight decline in concentrations and in the top part of the core there is a slight increase starting from 1990. The manganese profile retains a broad peak from the 1930s to 1970. There is also a clear increase in the top part of the core beginning from year 1990. The redox indicator (Fe/Mn-ratio) remained high from 1900 to 1930, then decreased clearly and remained low until the early 1970s when the most significant increase occurred.
In addition to source changes there are several factors influencing the precipitation and accumulation of Fe and Mn into the sediments (ie. the ionic composition of the water, redox conditions and microbial activity). Therefore the interpretation of Fe, Mn and Fe/Mn profiles needs to be done with caution. Despite the restrictions of the method, the clear peak in Fe/Mn ratio from 1970 to 1990 could point to less oxic conditions in the sediment, since the peak coincides with an increase in planktonic diatom taxa, which indicates more eutrophic conditions in the embayment (Weckström 2006).
Lacustrine Fe and Mn profiles resemble each other markedly. They both display a peak around 1890 and a steady increase from the 1940s towards the top. In Fe/Mn-ratio there is a slight increase from the 1860s to the late 1940s followed by a more rapid increase from 1940s onwards. Fe and Mn profiles, and also Fe/Mn ratio to some extent, resemble lithogenic indicator profiles suggesting that supply is controlling the presence of these elements in the sediment.
Copper and zinc profiles in the estuarine core showed increases in their concentrations from the 1850s to the 1970s and decreases in the most recent sediments (Fig. 4a). The copper profile in the lake core showed the biggest increase from the 1870s to 1930s. Zn concentrations were highest from the 1860s to approx. 1935 with four successive peaks between 1915 and 1930. The overall Cu-concentrations were higher in the estuarine core than in the lake core. Zinc concentrations were clearly higher in the lacustrine core between 1850 and 1935, but slightly higher in the estuarine core from ca. 1935 onwards. The zinc concentrations are well above crustal average which points out to pollutant contribution in both of the sites. The sediment cores were not long enough to reach pre-industrial reference levels, and therefore it is difficult to define any background values. However Cu-profiles in both of the sites show constant increases towards the top which could be related to atmospheric pollution.
TC, TN and especially TP profiles in the estuarine core were quite monotonous showing increasing trends towards the top of the core (Fig. 4a). However, there was a clear local minimum in TC and TN profiles in the mid 1940s. In most marine sediment cores there is a typical trend of increasing nutrient concentrations toward the top section of the core, regardless of the changes in nutrient loading or water nutrient concentrations (Cornwell et al. 1996; Vaalgamaa 2004). This is due to preservational artifacts or so called steady-state input-decomposition balance, in which the recent sediments tend to have more TN, TC and TP because less of the material has degraded (Cornwell et al. 1996). This is a complex process, which involves a certain amount of mixing in the top sediment layers (Kelly and Nixon 1984). The Renstrandträsket core retained an organic matter (LOI %) and nutrient peak from 1900–1945. Preservational artifacts were not seen in Lake Renstrandträsket’s TC and TN profiles.
The overall TC concentrations were found four times higher in the lake sediment core than in the estuarine core. The pre-1950 TP concentrations were about in the same level in both the estuarine and the lake sites. After 1950, TP concentrations in the estuarine core were higher than in the lake core. Also the estuarine sediment seemed to retain more OP compared to the lake sediment.
The C/N-ratio in the marine core was throughout the past century around 9 indicating that most of the carbon is derived from marine phytoplankton. In the top sediment layers the C:N ratio decreased gradually towards the Redfield-ratio (6.6) and reached 7.5 in the top sediment indicating stable decay of plankton-derived organic matter. The lake core differed significantly from the marine core with regard to C:N ratio. The C:N ratio in the lower part of the lake core was around 13, decreased markedly in 1880–1900 and reached a relatively stable level of around 9 in the top part of the core. This pattern indicates a change from predominantly terrestrially derived organic material to authigenic organic material (e.g. Kaushal and Binford 1999).
BSi fluctuated in the marine core reaching highest concentrations in approx. 1930, 1970 and 1996. In the freshwater core there were quite high BSi concentrations in the lower parts of the core, a weak peak between 1900–1945 and lower concentrations in the top part of the core. In the lake core there was much more BSi throughout the core compared to the marine sediment even though the BSi concentrations decreased significantly in the former in the recent sediments.
Elemental correlations
In order to study metal (Cu and Zn) and nutrient (TP, OP, TN and BSi) relations to active sorbents (i.e., organic matter, Fe- and Mn-oxides) and lithogenic indicator Al, a regression analysis was used. The relations of sediment Fe to Na and Al concentrations were also studied. In both of the cores the strongest positive relationships were obtained between sediment nutrients and LOI (Estuary: TP/LOI, r 2 = 0.7; TN/LOI, r 2 = 0.86; OP/LOI, r 2 = 0.45; Lake: TP/LOI, r 2 = 0.77; TN/LOI, r 2 = 0.87; OP/LOI, r 2 = 0.6; and BSi/LOI, r 2 = 0.51). The strongest negative correlations were found for lake sediment nutrients in relation to Fe concentrations (TP/Fe, r 2 = −0.49; OP/Fe, r 2 = −0.33; TN/Fe, r 2 = −0.5; and BSi/Fe, r 2 = −0.71). Mn correlations to nutrients were negative in the lake core. A strong negative correlation was also found for BSi and Al in the lake core (r 2 = −0.57). Cu and Zn were not strongly correlated to other indicators: best correlations for Cu and Zn were obtained to Al in the marine core (r 2 = 0.38 and 0.24, respectively). In the lake core Cu was correlated most strongly to Al (r 2 = 0.29) and Zn to LOI (r 2 = 0.38). In the lake core there was a negative correlation between Zn and Fe (r 2 = −0.27). When different elemental correlations between the sites were compared, the most important differences were related to sedimentary iron. The presence of iron in the sediment, and on the other hand, the binding of nutrient elements (TP, OP, BSi) to iron seems to be drastically different in estuarine versus lacustrine sedimentation environments as shown as linear regressions in Fig. 5.
PCA
For the PCA, the measured geochemical and lithological data from both sites were combined in order to compare the study sites and the chemical composition of the samples. Since the first two principal components (based on correlation matrix) together captured 77.7% (59.5 and 18.2%, respectively) of the total variation in the data, the PCA may be said to describe the structure of the variance in the material reasonably well. The variables were spread out in two main groups along the PCA axis 1. Nutrient variables (TN, LOI, TC and BSi) formed a tight group that scored high on PCA axis 1. Other variables with positive values on axis 1 were Zn, C/N-ratio and Ca. Variables such as Fe, K, Mg and Mn formed another fairly tight cluster with clearly negative values on PCA axis 1. The first PCA axis divided the variables into lithogenic and nutrient aggregates and can therefore be interpreted to reflect mostly the changes in sediment organic and nutrient content. OP and TP both scored high on axis 2 while Fe:Mn-ratio scored lowest. Since it is known that TP is a redox-sensitive parameter and Fe:Mn-ratio is a commonly used indicator of redox conditions PCA axis 2 could represent, at least partly, a redox gradient.
The lake core samples are spread out widely along both the first and second PCA axis. The main temporal trend along the primary axis was that the older samples were more dominated by nutrient elements while the more recent samples were located around the mid-section of the ordination space. In relation to the second PCA axis the lake samples achieved highest scores in samples that were formed around 1915 and lowest scores in samples from around 1967. The estuarine samples did not form any clear time-related trajectory along the first axis. In comparison to lake samples the estuarine samples were positioned on the negative side of axis 1 while the lake samples were mainly on the positive side. The biggest change in the estuarine samples took place along the second axis with increasing scores with the increasing age of the sample.
General discussion
Differences in elemental composition of lake and estuarine cores
The studied sites differ from each other significantly in size, depth and energy levels and therefore it was not expected that the sites would retain same elemental concentrations and geochemical profiles. Instead the main focus was to study the differences in elemental relations between the sites. As mentioned before, the most important differences were related to sedimentary iron. Roughly, the differences in sites can be divided into two categories: the presence of iron in the sediment, and on the other hand, the binding of nutrient elements (TP, OP, BSi) to iron.
Firstly, in the marine core there was no correlation between Fe and the lithogenic indicator K (r 2 = −0.05) (Fig. 5). In contrast, sediment Fe was very closely linked to lithogenic K (r 2 = 0.95) in the lake core. Both iron and potassium are lithogenic in origin but Fe(III) oxides have an important role as electron acceptors in organic matter oxidation in both marine and lacustrine microbial mineralization processes (Froelich et al. 1979). Data from the lake core show that Fe was most likely derived from the catchment and that its sedimentation was governed by the same processes as the sedimentation of other lithogenic metals: Fe-curve is notable similar to Al and K curves in the lake core, also in the lower parts of the core, and therefore we see that it is more likely that there are similarities in supply, capture and preservation of these elements at least in this lacustrine site. In the estuarine core, in contrast, the sedimentation and remobilization of iron was seemingly unrelated to catchment erosion. Instead, it seemed to be more related to internal processes such as oxidation and reduction reactions. In the marine areas, in contrast to most lake systems, the diffusion of Fe may be inactivated by FeS formation (Trudinger 1979), which could be the governing factor for the iron distribution in the estuarine sediment core.
Also the nutrient–iron relations were found different in studied cores. In the estuarine core TP and OP were positively related to sediment iron content, whereas in the lake core an inverse correlation between these variables was found. Several explanations for the observed phenomenon are possible. Firstly, sediment supply from the catchment could be different, even though the catchments are similar and overlapping. Nevertheless, the overall catchment of the estuary is much larger, as is also the estuary itself compared to the size of the lake. Secondly, there are differences in sedimentation of these two different physical environments due to particle size sorting via focusing, flocculation and resuspension. Thirdly, there are differences in Fe retention of these sites due to different oxygen dynamics: anoxia in freshwater systems can lead to quite poor retention of labile Fe, leading to dominance of Fe by the more resistant lithogenic component (Eusterhues et al. 2005). Fourthly, there can be differences in P cycling between these two different sedimentation environments, related to the area, volume and water retention time of the basins.
In addition to these possible causes, there are also marked differences in the chemical environment of estuarine and lacustrine systems. It is widely known that sedimentary P cycling is linked mainly to the Fe cycle in both lake (Caraco et al. 1990; Wang et al. 2005) and marine (Jensen et al. 1995) systems. In addition to this, marine P and Fe cycles are tightly coupled with S-cycle (Jensen et al. 1995), which could lead to differences in P cycling and deposition in SO 2−4 poor and SO 2−4 rich systems. Experimental evidence shows that increased salinity can increase sedimentation of iron and phosphorus aggregates (Carignan and Flett 1981; Forsgren et al. 1996). Also clay particles, that are very abundant in estuarine waters, absorb phosphorus aggregates and increase net sedimentation. Therefore, P sedimentation seems to be governed by multiple factors in estuarine systems. In a freshwater environment, phosphorus sedimentation appears to be more straightforward with a more direct linkage to organic matter sedimentation, as shown also by the PCA (Fig. 6).
As compared to nutrient elements, regression analysis didn’t reveal very strong correlations for Cu and Zn to LOI% or Fe. In the marine core Cu and Zn were strongly correlated with each other, but in the lake core there was no significant correlation between these variables. This could suggest that there is a local point source of either one in the lake catchment area. Based on the shapes and concentrations of the downcore Cu and Zn profiles (Fig. 4b) it is more likely that there has been a point source of Zn in the lake catchment area, possibly a fertilizer, since there are no exclusion areas surrounding the lake. The zinc concentrations are generally in the same level or higher (around 170–210 μg g−1) that has been reported from the contaminated surface sediments in Bothnian Bay, Bothnian Sea and Gulf of Finland (Leivuori 1998) but not as high as in badly polluted urban estuaries such as Laajalahti Bay (300 μg g−1) (Vaalgamaa 2004). The lacustrine levels are even higher. Copper concentrations are a bit higher in the estuary than in the lacustrine site except for the surface enrichment in the latter core. These surface trace element enrichments are most likely linked to trace element cycling in lakes which need not have much impact on the longer term sediment record (Boyle 2001). Despite of possible surface enrichments, it seems that in relatively uncontaminated, mainly oxic sediments as these, the trace metal concentrations may be more controlled by local changes in deposition rates and local geochemical background, rather than other active sorbents such as iron and manganese oxides and organic matter. Therefore they seem to be indicators that work similarly in both lacustrine and estuarine environments.
Interpreting sediment geochemical data as a palaeoproxy: lake versus estuary
The discussion above shows that there are certain critical issues related to elemental preservation in sediment cores, which could be a significant problem when using sediment geochemistry as a palaeoenvironmental proxy. There are also significant differences in the chemical sedimentation environment between lake and estuary that must be taken into consideration when interpreting sediment cores. For example, in the complex estuarine environments a multitude of factors are affecting the presence of iron compounds and other chemical substances in the sediments and therefore it is unlikely that clear temporal trends could be seen in the sediment elemental profiles.
For this study our aim was to choose two sites that were as similar as possible from the perspective of their catchment land use history in order to focus on the differences that are caused by the chemically different sedimentation environments. Unfortunately, it was not possible to totally rule out the differences in physical environment (e.g. size, depth, energy levels). The only similarities found in the geochemical signature between the studied cores were among erosion indicators (K and Al). These records indicated increased catchment erosion in the 1870s, while in the 1920s and 1930s the erosion conditions in the watershed stabilized again. Even though the curves of the lithophilic elements are not identical, similar trends are fairly easily observed. The differences can be explained by watershed scale: large watershed may mask the effects of individual events, while in the smaller lake catchment even minor changes will be recorded in the sediment.
In general, our study suggests that lakes can be considered more suitable for palaeoenvironmental studies than marine estuaries and embayments, which is in line with previous investigations. Even very closed, shallow and lake-like marine inlets are much more challenging environments for palaeoecological studies because of more complex chemical cycles. The preservation of nutrient elements in the sediment strata seems to be governed by many factors including normal sediment diagenesis but also cycling of other metals. This study revealed similar, rather monotonous nutrient profiles in the estuarine core as observed in many previous studies (Cornwell et al. 1996; Ruttenberg and Goñi 1997; Vaalgamaa 2004). This probably has something to do with the more vigorous mixing of the sediment by more abundant macrofauna and by faster currents that may ensure more rapid and complete consumption of the organic matter deposited on the bottom (Yingst and Rhoads 1980). In contrast to lake sediments, which often appear to preserve a historical record of increasing eutrophication and organic deposition from the overlying waters, the coastal marine sediments tend to be enriched in C, N, and P only in the surface 5–10 cm, which could be the zone of active mixing. Below this zone, nutrient concentrations in many cases fall to background levels. This seems to be case with the cores studied here: the lake site retains signals of historical nutrient enrichment but the estuarine site follows the monotonous patters typical of sediment diagenesis (Anderson and Odgaard 1994).
The behavior of the above-mentioned biologically active elements contrasts with that of the less active particle-bound elements like Cu and Zn. Lake cores commonly retain fairly good records of anthropogenic Cu and Zn inputs, which was also the case here. Pieni Pernajanlahti Bay, like many other estuaries in the world (Nixon 1988; Cornwell et al. 1996; Vaalgamaa 2004), shows increasing concentrations of Cu at a much greater depth in the sediment than where C, N, or P began to increase. Independent datings show that Cu profiles all around the world show reasonable reflections of increasing anthropogenic inputs up to the 1970s. Since urban development preceded and then increased with industrialization and sewer system development from the late 1800s onwards, the impact of C, N, and P enrichment is not clearly recorded in the sediment, but anthropogenic activity in general, seems to be recorded as increases in human-derived metal profiles.
An important consequence of the differences in chemical and physical sedimentation environments in freshwater and marine sediments is that while lakes seem to act as sinks for both metals and nutrients, estuaries and embayments may retain and accumulate a smaller portion of the nutrients that flow into them from rivers and anthropogenic sources. At the same time, a relatively large fraction of metals may be retained in coastal sediments. For example, it has been shown that the retention of Cu in coastal sediment is as high as 70–95% (Nixon et al. 1986).
From the paleoenvironmental point of view strong cycling of nutrients is problematic, since it makes the interpretation of sediment geochemical data quite challenging. There are not very many robust chemical paleoindicators for studying eutrophication in coastal environments. From the basic geochemical indicators studied the most reliable seems to be OP, which is much less mobile than TP. BSi seems to retain independent trends from other nutrient indicators, but it has not yet been proved that the observed changes in BSi-profiles reflect changes in the productivity of the basin, since a great deal of BSi can be transported from terrestrial sources (Conley 2002).
Even though the interpretation of sediment geochemical data from the paleoenvironmental point of view is frequently challenging it is known that even the most mobile pollutants leave certain marks in the sediment composition that can be interpreted later. Therefore, it is important to study a broad range of different geochemical indicators since they can support each other in the interpretation of the land-use and pollution history. Sedimentary pigments, C and N stable isotopes along with determination of phosphorus and nitrogen fractions with nuclear magnetic resonance spectroscopy could additionally provide valuable information on eutrophication history. However, this study shows that the differences in sedimentation environments, i.e. marine or lacustrine, must be taken into consideration when interpreting the geochemical records. Paleolimnological methods commonly used to study lake histories cannot be directly applied to brackish water or marine environments.
References
Anderson NJ, Odgaard BV (1994) Recent palaeolimnology of three shallow Danish lakes. Hydrobiologia 275/276:411–422
Aasarød K, Storaas LB (1996) EMEP manual for sampling and chemical analysis. Norwegian Institute for Air Research
Abril JM (2003) Difficulties in interpreting fast mixing in the radiometric dating of sediments using 210Pb and 137Cs. J Paleolimnol 30:407–414
Adser F (1999) Paleoecological Studies of Sediment from Roskilde Fjord With Reference to the Tracing Eutrophication and Other Anthropogenic Impacts. Unpublished Masters Thesis, University of Odense, Department of Biology, Denmark
Agrawal YC, McCave IN, Riley JB (1991) Laser diffraction size analysis. In: Syvitski JPM (ed) Principles, methods and application of particle size analysis. Cambridge University Press, Cambridge, pp 119–128
Appleby PG, Nolan PJ, Gifford DW, Godfrey MJ, Oldfield F, Anderson NJ, Battarbee RW (1986) 210Pb dating by low background gamma counting. Hydrobiologia 141:21–27
Aspila KI, Agemian H, Chay ASY (1976) A semi-automated method for the determination of inorganic, organic and total phosphate in sediments. Analyst 101:187–197
Atlas of Finland (1992) Appendix 123–126 Geology. National Board of Survey, Geographical Society of Finland
Billon G, Ouddane B, Recourt P, Boughriet A (2002) Depth variability and some geochemical characteristics of Fe, Mn, Ca, Mg, Sr, S, P, Cd and Zn in anoxic sediments from Authie Bay (Northern France). Estuar Coast Shelf S 55:167–181
Boyle JF (2001) Inorganic geochemical methods in paleolimnology. In: Last WM, Smol JP (eds) Tracking environmental change using lake sediments, vol 2, physical and geochemical methods. Kluwer Academic Publishers, The Netherlands
Canfield DE (1989) Reactive iron in marine sediments. Geochim Cosmochim Acta 53:619–632
Caraco N, Cole J, Likens GE (1990). A comparison of phosphorus immobilization in sediments of freshwater and coastal marine systems. Biogeochemistry 9:277–290
Carignan R, Flett RJ (1981) Postdepositional mobility of phosphorus in lake sediments. Limnol Oceanogr 26:361–366
Clarke A, Juggins S, Conley DJ (2003) A 150-year reconstruction of the history of coastal eutrophication in Roskilde Fjord, Denmark. Mar Pollut Bull 46:1614–1617
Conley DJ (1988) Biogenic silica as an estimate of siliceous microfossil abundance in Great Lake sediments. Biogeochemistry 6:161–179
Conley DJ (1998) An interlaboratory comparison for the measurement of biogenic silica in sediments. Mar Chem 63:39–48
Conley DJ (2002) Terrestrial ecosystems and the global biogeochemical silica cycle. Global Biogeochem Cycles 16:1121, doi:10.1029/2002GB001894
Cooper DC, Morse JW (1999) Selective extraction chemistry of toxic metal sulfides from sediments. Aquat Geochem 5:87–97
Cornwell JF, Conley DJ, Owens M, Stevenson JC (1996) A sediment chronology of the eutrophication of Chesapeake Bay. Estuaries 2B:488–499
Day JW, Hall AS, Kemp WM, Yanez-Arancibia A (1989) Estuarine ecology. Wiley, New York, 558 pp
DeMaster DJ (1981) Measuring biogenic silica in marine sediments and suspended matter. In: Hurd DC, Spencer DW (eds) Marine particles: analysis and characterization. American Geophysical Union, pp 363–368
Duquesne S, Newton LC, Giusti L, Marriott SB, Stärk H-J, Bird DJ (2006) Evidence for declining levels of heavy-metals in the Severn Estuary and Bristol Channel, U.K. and their spatial distribution in sediments. Environ Pollut 143:187–196
Eusterhues K, Heinrichs H, Schneider J (2005) Geochemical response on redox fluctuations in Holocene lake sediments, Lake Steisslingen, Southern Germany. Chem Geol 222:1–22
Forsgren G, Jansson M, Nilsson P (1996) Aggregation and sedimentation of iron, phosphorus and organic carbon in experimental mixtures of freshwater and estuarine water. Estuar Coast Shelf S 43:259–268
Froelich PN, Klinkhammer GP, Bender ML, Luedtke NA, Heath GR., Cullen D, Dauphin P, Hammond D, Hartman B (1979) Early oxidation of organic matter in pelagic sediments of the eastern equatorial Atlantic: suboxic diagenesis. Geochimica et Cosmochimica Acta 43:1075–1090
Goñi MA, Teixeira MJ, Perkey DW (2003) Sources and distribution of organic matter in a river-dominated estuary (Winyah Bay, SC, USA). Estuar Coast Shelf S 57:1023–1048
Heiri O, Lotter AF, Lemcke G (2001) Loss on ignition as a method for estimating organic and carbonate content in sediments: reproducibility and comparability of results. J Paleolim 25:101–110
Jensen HS, Mortensen PB, Andersen FØ, Rasmussen E, Jensen A (1995) Phosphorus cycling in a coastal marine sediment, Aarhus Bay, Denmark. Limnol Oceanogr 5:908–917
Kaushal S, Binford MW (1999) Relationship between C:N ratios of lake sediments, organic matter sources, and historical deforestation in Lake Pleasant, Massachusetts, USA. J Paleolimnol 22:439–442
Kelly JR, Nixon SW (1984) Experimental studies of the effect of organic deposition on the metabolism of a coastal marine bottom community. Mar Ecol Prog Ser 17:157–160
Korhola A (1995) Lake terrestrialization as a mode of mire formation: a regional review. Publ Wat Env Admin A207:11–21
Leivuori M (1998) Heavy metal contamination in surface sediments in the Gulf of Finland and comparison with the Gulf of Bothnia. Chemosphere 36:43–59
Mackereth FJH (1969) A short core sampler for subaqueous deposits. Limnol Ocenanogr 14:145–151
Nixon SW (1988) Physical energy inputs and the comparative ecology of lake and marine ecosystems. Limnol Oceanogr 33:1005–1025
Nixon SW, Hunt CD, Nowicki BL (1986) The retention of nutrients (C, N, P), heavy metals (Mn, Cd, Pb, Cu), and petroleum hydrocarbons in Narragansett Bay. In: Lasserre P, Martin J-M (eds) Biogeochemical processes at the land-sea boundary. Elsevier, pp 99–122
Oldfield F, Maher BA, Appleby PG (1989) Sediment source variations and 210Pb inventories in recent Potomac Estuary sediment cores. J Quaternary Sci 4:198–200
Plater AH, Appleby PG (2004) Tidal sedimentation in the Tees estuary during the 20th century: radionuclide and magnetic evidence of pollution and sedimentary response. Estuar Coast Shelf S 60:179–192
Prentice IC (1986) Multidimensional scaling as a research tool in Quaternary palynology: a review of theory and methods. Rev Palaeobot Palynol 31:71–104
Ridgway J, Shimmield G (2002) Estuaries as repositories of historical contamination and their impact on shelf seas. Estuar Coast Shelf S 55:903–928
Ruttenberg KC, Goñi MA (1997) Depth trends in phosphorus distribution and C:N:P ratios of organic matter in Amazon Fan sediments: indices of organic matter source and burial history. Proceedings in the Ocean Drilling Program, Scientific Results 155
San Miguel EG, Bolívar JP, García-Tenorio R (2003) Mixing, sediment accumulation and focusing using 210Pb and 137Cs. J Paleolimnol 29:1–11
SFS 3008 (1990) Determination of total residue and total fixed residue in water, sludge and sediment. SFS standards, Finland
SFS 3044 (1980) Metal content of water, sludge and sediment determined by atomic absorption spectroscopy, atomization in flame. General principles and guidelines. SFS standards, Finland
SFS-EN 1189 (1997) Water quality. Determination of phosphorus. Ammonium molybdate spectrometric method. SFS standards, Finland
Stumm W (1992) Chemistry of the solid–water interface. John Wiley & Sons, New York, 428 pp
Tanner PA, Leong LS (1995) The effects of different drying methods for marine sediment upon moisture content and metal determination. Mar Pollut Bull 31:325–329
Ter Braak CJF, Smilauer P (1998) CANOCO reference manual and user’s guide to Canoco for Windows: software for canonical community ordination (version 4). Microcomputer Power, Ithaca, New York, USA
Thomas CA, Bendell-Young LI (1999) The significance of diagenesis versus riverine input in contributing to the sediment geochemical matrix of iron and manganese in an intertidal region. Estuar Coast Shelf S 48:635–647
Thorne LT, Nickless G (1981) The relation between heavy-metals and particle size fractions within the Severn Estuary (UK) intertidal sediments. Sci Total Environ 19:207–213
Tikkanen M, Korhola A, Seppä H, Virkanen J (1997) A long-term record of human impacts on an urban ecosystem in the sediments of Töölönlahti Bay in Helsinki, Finland. Environ Conserv 24:326–337
Trudinger PA (1979) The biological sulphur cycle. In: Trudinger PA, Swaine DJ (eds) Biogeochemical cycling of mineral-forming elements. Elsevier, pp 293–313
Vaalgamaa S (2004) The effect of urbanisation on Laajalahti Bay, Helsinki City, as reflected by sediment geochemistry. Mar Pollut Bull 48:650–662
Van der Weijden CH (2002) Pitfalls of normalization of marine geochemical data using a common divisor. Mar Geol 185:167–187
Wang S, Jin Y, Pang Y, Zhao H, Zhou X, Wu F (2005) Phosphorus fractions and phosphate sorption characteristics in relation to the sediment compositions of shallow lakes in the middle and lower reaches of Yangtze River region. China J Colloid Interface Sci 289:339–346
Weckström K (2006) Assessing recent eutrophication in coastal waters of the Gulf of Finland (Baltic Sea) using subfossil diatoms. J Paleolimnol 35:571–592
Weckström K, Vaalgamaa S, Kangas J, Shemeikka P (2004) The eutrophication history of coastal embayments in Uusimaa – an assessment using palaeolimnological methods: Pieni Pernajanlahti, Fasarbyviken, Varlaxviken, Nikuviken, “Insjön” and Sipoonlahti. Report to the Uusimaa Regional Environment Centre and the cities of Porvoo, Pernaja and Sipoo, 24 pp (in Finnish)
Yingst JY, Rhoads DC (1980) The role of bioturbation in the enhancement of bacterial growth rate in marine sediments. In: Tenore KR, Coull BC (eds) Marine benthic dynamics. Univ. S. Carolina, pp 407–421
Acknowledgements
This study was funded by EC Energy, Environment and Sustainable Development Programme (Contract EVK3-CT-2000-00031 – MOLTEN) and The Academy of Finland – BIREME. We thank Kaarina Weckström and Finn Adser for the teamwork in collecting the sediment cores. Juhani Virkanen and Seija Kultti are greatly acknowledged for their guidance in analytical work. Peter Appleby carried out the radiochemical dating of the cores. We also thank Cathy Jenks for linguistic improvements and Richard Telford for valuable comments and suggestions on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vaalgamaa, S., Korhola, A. Geochemical signatures of two different coastal depositional environments within the same catchment. J Paleolimnol 38, 241–260 (2007). https://doi.org/10.1007/s10933-006-9071-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10933-006-9071-0