Abstract
Previously we reported that site-specific modification of the human granulocyte-macrophage colony-stimulating factor (GM-CSF) A3C analog with polyethylene glycol (PEG) dramatically improved the pharmacokinetic properties of the protein in rats. However, we could not evaluate the hematological properties of the PEG-A3C protein in rats because human GM-CSF is inactive in rodents. To study the biological effects of PEGylated GM-CSF analogs in rodents we created a homologous site-specific PEGylated murine (mu) GM-CSF (T3C) protein. muGM-CSF and the T3C protein were expressed in Escherichia coli and purified by column chromatography. The purified T3C protein was covalently modified with a linear 20 kDa- or a branched 40 kDa-maleimide-PEG, and the monoPEGylated proteins purified by column chromatography. muGM-CSF, T3C and the two PEG-T3C proteins had comparable in vitro biological activities, as measured by stimulation of proliferation of the murine FDC-P1 cell line. The PEG-T3C proteins had 10- to 25-fold longer circulating half-lives than muGM-CSF and stimulated greater and longer lasting increases in neutrophils and white blood cells than muGM-CSF following a single intravenous or subcutaneous administration to rats. Treatment of rats made neutropenic with cyclophosphamide with the PEG-T3C proteins shortened the time for recovery of neutrophils to normal levels from 9 or 10 days to 5 or 6 days, whereas muGM-CSF showed no benefit versus vehicle solution. Acceleration of neutrophil recovery in cyclophosphamide-treated rats required a minimum of three PEG-T3C treatments over five days. The PEG-T3C proteins should prove useful for evaluating the potential therapeutic benefits of GM-CSF and long-acting GM-CSF proteins in rodent disease models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) is a 14 kilodalton (kDa) cytokine that serves as a growth and differentiation factor for a variety of white blood cell types, including macrophages, neutrophils, dendritic cells, and microglia [1, 2]. Clinical uses of recombinant human (hu) GM-CSF include accelerating neutrophil recovery following chemotherapy in cancer patients, accelerating hematopoietic recovery in bone marrow transplant recipients, and stimulating release of hematopoietic progenitor cells from the bone marrow into the peripheral circulation where they can be collected for transplantation [1, 2]. GM-CSF is being investigated as a therapy or adjuvant for various cancers and cancer vaccines [3,4,5,6], and as a treatment for neurological disorders [7,8,9], Crohn’s disease [10], autoimmune diseases [11,12,13,14], and to promote wound-healing [15, 16]. Most patients receive huGM-CSF by daily injection due to the protein’s short in vivo half-life [1, 2]. A longer-acting form of GM-CSF that could be administered less frequently without sacrificing efficacy would be attractive to patients and potentially expand the range of clinical uses for the protein, particularly for treating chronic diseases.
In a previous report we described the construction of huGM-CSF analogs with improved pharmacokinetic properties by targeted attachment of a maleimide-polyethylene glycol (PEG) to engineered cysteine analogs of huGM-CSF [17]. We identified several sites in huGM-CSF where a cysteine residue could be introduced and modified with PEG (PEGylated) without appreciably affecting in vitro biological activity of the protein [17]. One of the most promising analogs had a cysteine residue substituted for alanine at position-3 of huGM-CSF (referred to as A3C). PEGylated A3C proteins had up to a 47-fold longer half-life than huGM-CSF in rats, with the half-life increasing with increasing size of the attached PEG molecule. huGM-CSF is not active in rodents, which limits the number of disease models available for evaluating the in vivo effects of the PEG-A3C proteins. The first 6 amino acids of huGM-CSF (APARSP; one letter amino acid code) and murine (mu) GM-CSF (APTRSP) are identical except for amino acid position 3, which is an alanine in huGM-CSF and a threonine in muGM-CSF [18, 19]. Here we describe the construction of a muGM-CSF analog in which threonine at position 3 is changed to a cysteine (T3C). The muGM-CSF (T3C) protein was expressed in Escherichia coli and modified with different size PEGs. We characterize the in vitro bioactivities of the proteins and their in vivo effects in normal and neutropenic rats.
2 Materials and Methods
2.1 Cloning of muGM-CSF and muGM-CSF (T3C)
Total RNA isolated from the mouse EL4.IL-2 cell line (American Type Culture Collection, Manassas, VA; catalog number TIB-181) was used in a reverse transcriptase-polymerase chain reaction (RT-PCR) [20] to prepare a cDNA encoding the mature muGM-CSF protein [18]. The cells were treated with 1 μg/mL phytohemagglutinin-L (Sigma-Aldrich Chemical Company, St. Louis, MO) and 10 ng/mL phorbol 12-myristate 13-acetate (Sigma-Aldrich Chemical Company) for 6 h at 37 °C prior to RNA isolation. RNA was isolated using an RNeasy Mini RNA isolation kit (Qiagen, Inc., Santa Clarita, CA). Single-stranded cDNA was synthesized from the RNA using a 1st Strand cDNA Synthesis Kit for RT-PCR (AMV) from Boehringer Mannheim Corp (Penzberg, Germany) and random hexamers as the primer. The single-stranded cDNA was used as the template in a PCR reaction using forward primer 5′-GCGACGCGTACGCAGCACCCACCCGCTCACCCATCACT-3′ and reverse primer 5′-GCGGAATTC TTATTTTTGGACTGGTTTTTTGCATTCAAAGGG-3′. The forward primer anneals to the 24 nucleotides encoding the first eight amino acids of mature muGM-CSF, and adds, immediately 5′ to this sequence, nucleotides that overlap DNA encoding the carboxyterminal 4 amino acids of the E. coli STII signal sequence [21]. The forward primer includes an Mlu I restriction site (underlined) for cloning purposes. The reverse primer anneals to the nucleotides encoding the carboxy-terminal 10 amino acids of muGM-CSF and adds a TAA translational stop codon and an Eco RI restriction site (underlined) immediately following the coding sequence. The 400 bp PCR product was digested with Mlu I and Eco RI, and cloned into a similarly digested pUC18 plasmid that contains the remainder of the STII signal sequence [22], creating pBBT435. After confirming the DNA sequence of the STII-GM-CSF gene, an Nde I—Eco RI fragment encoding STII-GM-CSF was subcloned from pBBT435 into expression vector pBBT257, which contains a tac promoter and a tetracycline resistance gene. A pBBT257 plasmid encoding the T3C mutein was constructed in an identical manner except that the forward primer used in the PCR reaction was 5′-GCGACGCGTACGCAGCACCCTGCCGCTCACCCATCACT-3″. The resulting plasmids were introduced into E. coli strain W3110 for protein expression studies.
2.2 Preparation of the muGM-CSF and PEGylated muGM-CSF (T3C) Proteins
The muGM-CSF and muGM-CSF (T3C) proteins were expressed in E. coli, refolded, and purified by ion-exchange and hydrophobic interaction (phenyl) column chromatography essentially as described for huGM-CSF cysteine analogs [17]. The purified muGM-CSF (T3C) protein was modified with a linear 20 kDa-maleimide PEG (Nektar, Inc, Huntsville, AL) by diluting the protein to 0.1 mg/mL with 0.1 M Tris, pH 8 and incubating the protein with a 20-fold molar excess of the PEG reagent and a 15-fold molar excess of Tris[2-carboxyethylphosphine]hydrochloride (TCEP) for 4 h at room temperature. The reaction mixture was then diluted with 50 mM sodium acetate pH 4.8 the and loaded on to an S-Sepharose HiTrap column (Amersham Pharmacia) equilibrated in 50 mM sodium acetate, pH 4.8 (Buffer A). The bound proteins were eluted with a linear salt gradient from 0 to 35% Buffer B (1 M NaCl, 50 mM sodium acetate, pH 4.8). Column fractions were analyzed by non-reducing sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and column fractions enriched in the monoPEGylated protein pooled, aliquoted and stored at − 70 °C until use. The same procedure was used to prepare the protein modified with a branched 40 kDa-maleimide PEG (Nektar, Inc.). Protein concentrations were determined using a Micro BCA kit (Thermo Fisher Scientific, Inc.), utilizing bovine serum albumin as the protein standard.
2.3 In Vitro Bioactivity Assay
muGM-CSF stimulates proliferation of the mouse FDCP-1 cell line (American Type Culture Collection, catalog number CRL-12103) and this assay was used to measure in vitro bioactivities of the muGM-CSF proteins. The FDCP-1 cells were routinely passaged in RPMI 1640 media containing 10% fetal bovine serum (FBS), 50 μg/mL streptomycin, 50 units/mL penicillin, 50 μM beta-mercaptoethanol and 10–20 units/mL recombinant muIL-3 (R&D Systems, Inc., Minneapolis, MN). The proliferation assays were performed in the same media minus muIL-3 essentially as described [17] using serial dilutions of the protein samples and 5,000 cells per test well of a 96 well tissue plate. Protein samples were assayed in triplicate wells. CellTiter 96® AQueous One Solution (Promega Corporation, Madison, WI) was added to each well after 3 days and absorbance of the wells, which is proportional to cell number, was read at 490 nm using a microplate reader. A recombinant muGM-CSF standard (E. coli-expressed; R&D Systems, Inc.) was analyzed in parallel on the same plates.
2.4 Animal Experiments
The Institutional Animal Care and Use Committee at the University of Colorado, Boulder approved all animal studies. Experimentally naïve Sprague–Dawley male rats were obtained from Harlan Sprague Dawley, Inc. (Indianapolis, IN) and acclimated for at least 7 days prior to study initiation. Rats were housed 2 to 3 per cage and given ad libitum access to rat chow and tap water. The animal facility was maintained at a temperature of 67–76 °C, 30–70% relative humidity, and provided 12 h of light and dark each day. All but the terminal blood draws were collected from the tail vein under isoflurane anesthesia using EDTA as the anticoagulant. Terminal blood samples were obtained from the descending aorta (normal rat studies) or by cardiac puncture (neutropenic rat studies) using EDTA as the anticoagulant. muGM-CSF and the PEG-T3C proteins used for these studies were manufactured by Bolder BioTechnology, as described above.
Pharmacokinetic (PK) properties of the muGM-CSF proteins were measured using three rats per group. The rats were 9 to 11 weeks of age, weighed approximately 342 g (range of 319–364 g), and the proteins were dosed at 100 μg protein/kg. Only the protein portion of the PEG-proteins was used to calculate the PEG-protein doses. The proteins were diluted in phosphate buffered saline prior to administration to the rats by intravenous (iv) or subcutaneous (sc) injection. The iv and sc PK studies were performed separately, approximately two months apart. Blood samples (0.4 mL) were obtained from the rats prior to dosing and at various times post-dosing and used to prepare plasma and for complete blood cell (CBC) analyses, which were performed by Antech Diagnostics (Irvine, CA). Plasma was prepared by centrifuging EDTA blood samples in a microfuge for 10 min at 1500×g at 4 °C and transferring the plasma into separate tubes which were stored frozen in a − 70 °C freezer until used for ELISA analyses. Plasma levels of the proteins were quantitated using muGM-CSF enzyme-linked immunosorbent assay (ELISA) kits (R & D Systems, Inc.; detection range of 7.8–500 pg/mL). Typically, serial threefold dilutions of thawed plasma samples covering all time points from one rat per test group were prepared in RPMI media containing 10% fetal bovine serum and analyzed in the ELISA to identify dilutions that fell within the linear range of the ELISA. Plasma samples from other rats within that group were then diluted to these optimum dilutions in the same way and tested in the ELISAs. ELISA testing of the plasma samples occurred over the course of several weeks, typically analyzing plasma samples from 1 or 2 rats per day. Stability of the muGM-CSF proteins in frozen plasma was not validated, but plasma levels of the proteins from different rats within a test group gave similar results in the ELISAs even when the analyses were performed on different days, suggesting that the muGM-CSF proteins were stable at − 70 °C during the storage period. The WinNonlin software program (Pharsight, Inc, Palo Alto, CA) was used to calculate pharmacokinetic parameters using non-compartmental methods.
The neutropenia studies were performed using 6–8 week old rats (5/group). Rats were made neutropenic by intraperitoneal injection of 100 mg/kg cyclophosphamide (CPA; Sigma-Aldrich, Inc., St. Louis, MO) on day 0. One day later (day 1), rats received sc injections of muGM-CSF, 20 kDa-PEG-T3C or 40 kDa-PEG-T3C using various doses and dosing schedules. Body weights averaged 216 g (range of 194–230 g) for the first day 1–5 dosing study, 183 g (range of 167–199 g) for the second single dose study, and 206 g (range of 190–227 g) for the third dose variation study. Protein samples were formulated in phosphate buffered saline. One group of CPA-treated control rats was injected with phosphate buffered saline (vehicle solution). Another group of control rats did not receive CPA, but did receive injections of vehicle solution. Blood samples were collected from the rats prior to CPA administration and at various times post drug administration for CBC analyses. CBC analyses for the day 1–5 dosing and single dose studies were performed by Antech Diagnostics and for the dose variation study by Premier Laboratory (Longmont, CO).
2.5 Statistical analyses
Statistical comparisons per groups were compared using two-tailed t-tests, with p ≤ 0.05 considered significant.
3 Results
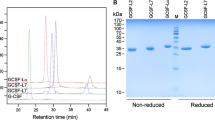
muGM-CSF and the muGM-CSF (T3C) analog (cysteine substituted for threonine-3) were expressed in E. coli as periplasmically secreted proteins using the E. coli STII signal sequence. Similar to what was observed for huGM-CSF proteins expressed in E. coli using the STII signal sequence [17], the muGM-CSF proteins were largely insoluble and the signal sequence appeared to be correctly removed, as judged by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). When the cells were lysed and centrifuged, the muGM-CSF proteins were recovered predominantly in the pellet fraction. The insoluble proteins were refolded and purified by sequential Q-sepharose and phenyl-sepharose column chromatography steps. Yields were about 3 mg per 400 mL shake flask culture. The purified muGM-CSF and T3C proteins comigrated by reducing and non-reducing SDS-PAGE, with apparent molecular masses of about 14 kDa (Fig. 1). Both proteins stimulated proliferation of the murine FDC-P1 cell line and had similar EC50s of about 70 pg/mL (Table 1 and Fig. 2). Potencies of the proteins were comparable to that of a commercial muGM-CSF standard in this assay (Table 1). The T3C protein was modified with a linear 20 kDa maleimide-PEG and a 40 kDa branched maleimide- PEG. The PEG reactions yielded almost exclusively monoPEGylated proteins (Fig. 3), which were purified from unreacted T3C protein and T3C disulfide dimers that form during the PEG reaction by S-sepharose column chromatography (Fig. 3). Recoveries of the PEG-T3C proteins from the PEGylation reaction and column purification step were in the range of 30–50%. The apparent molecular masses of the purified 20 kDa- and 40 kDa-PEG-T3C proteins were 55 kDa and 92 kDa, respectively, by non-reducing SDS-PAGE (Fig. 1). EC50s of the PEG-T3C proteins were comparable to (within twofold) EC50s of muGM-CSF and the T3C protein in the FDC-P1 bioassay (Table 1 and Fig. 2).
In vitro bioactivities of muGM-CSF, T3C and PEG-T3C proteins. Serial dilutions of the purified proteins were incubated with murine FDCP-1 cells and incubated at 37 °C. Cell number was quantitated 3 days later using CellTiter 96® AQueous One dye solution. Absorbance on the Y-axis is proportional to cell number. a Compares GM-CSF (R&D Systems) and T3C. b Compares T3C and T3C modified with 20 kDa- and 40 kDa-PEGs. Data are means ± standard deviation for triplicate wells
PEGylation and purification of PEG-T3C. The purified T3C protein was incubated with a 20 kDa-PEG in the presence of the reducing agent TCEP for 4 h at room temperature as described in Sect. 2, and the reaction mixture then fractionated by S-Sepharose column chromatography. The column chromatogram is shown in a The PEG-T3C protein elutes between fractions 23–29, while unreacted T3C protein and T3C disulfide dimers that form during the PEGylation reaction elute later around fraction 38. Non-reducing SDS-PAGE analysis of the PEGylation reaction (lane Rx) and fractions 23 to 31 of the main PEG-T3C peak and fraction 38 are shown in b. Fractions 27 and 28 containing the main PEG-T3C protein were pooled. MW, molecular weight markers; Rx, the PEGylation reaction before column fractionation; 23–38, fractions across the main PEG protein peak and the later eluting peak. The position of PEG-T3C, unmodified T3C, and T3C dimers are indicated on the right. Molecular weights of the marker proteins are indicated in kDa on the left
3.1 Pharmacokinetic and Hematopoietic Properties of muGM-CSF and PEG-T3C Proteins in Rats
Pharmacokinetic (PK) and hematopoietic properties of the 20 kDa- and 40 kDa-PEG-T3C proteins were compared to each other and to muGM-CSF following intravenous (iv) and subcutaneous (sc) administration of 100 μg protein/kg to rats. Rats were used for these studies because their large size (compared to mice) allowed blood sampling for PK and CBC analyses to be obtained from the same animals over time. All rats survived to study termination and no obvious adverse effects of the proteins were noted.
3.2 IV Dosing Study
3.2.1 PK Results (Fig. 4a)
Plasma levels and hematopoietic properties of muGM-CSF and PEG-T3C proteins following iv administration of 100 μg/kg of the proteins to rats. a Shows plasma levels of the proteins over time, as measured by ELISA. b and c Show changes in circulating neutrophil and white blood cells, respectively, over time following iv administration of the proteins. Data are means ± SD (a) or SEM (b and c) for 3 rats per group
Following iv administration, plasma levels of muGM-CSF were highest at the first sampling time point, 15 min post-dosing, (134 ± 72 ng/mL) and then decreased rapidly (nearly 10,000-fold within 10 h) and could not be detected 24 h post-administration. The terminal half-life of muGM-CSF was 1 h. By comparison, at 15 min post-dosing, peak plasma levels of the 20 kDa- and 40 kDa PEG-T3C proteins were 789 ± 133 ng/mL and 322 ± 89 ng/mL respectively. The PEG-T3C proteins were cleared more slowly than muGM-CSF and were detectable in plasma for 120 to 144 h post-administration (Fig. 4a). Plasma levels of the 20 kDa-and 40 kDa-PEG-T3C proteins were significantly (p ≤ 0.05) higher than plasma levels of muGM-CSF from 0.25 to 120 h post-dosing (40 kDa-PEG-T3C until 144 h post-dosing). Plasma levels of the 40 kDa-PEG protein were higher than plasma levels of the 20 kDa-PEG protein at most time points examined. The terminal half-life of the 20 kDa- PEG T3C protein was 25.1 h (measured from 72 to 120 h); the half-life was shorter when earlier time points were included in the calculation, e.g., the half-life was 13.5 h when measured from 24 to 120 h. The terminal half-life of the 40 kDa-PEG T3C protein was 22.9 h (measured between 96 to 144 h); the half-life was shorter when earlier time points were included in the calculation, e.g., the half-life was 16.5 h when measured from 48 to 144 h.
3.2.2 Neutrophil Effects (Fig. 4b)
Neutrophils showed rapid and significant (p ≤ 0.05) increases following iv administration of muGM-CSF or the PEG-T3C proteins. Neutrophil levels increased from 294 ± 155 cells/µL at baseline to 9349 ± 2222 cells/µL at peak at 4 h post-administration of muGM-CSF, and returned to baseline values by 24 h post-administration. By comparison, neutrophil levels peaked 10 h post-administration of the 20 kDa-PEG-T3C protein, increasing from 579 ± 125 cells/µL at baseline to 9080 ± 1034 cells/µL at peak, and did not return to baseline levels until 72 h post-administration. Neutrophil levels did not peak until 48 h following administration of the 40 kDa-PEG protein, increasing from 472 ± 95 cells/µL at baseline to 9980 ± 1794 cells/µL at 48 h, and were still elevated above baseline levels 120 h post-administration of the protein. Neutrophils were significantly (≤ 0.05) higher in rats treated with 20 kDa-PEG T3C compared to muGM-CSF at 24 h post-dosing, and significantly higher in rats treated with 40 kDa-PEG T3C compared to muGM-CSF at 24 h, 48 h, and 96 h post-dosing.
3.2.3 WBC Effects (Fig. 4C)
WBCs also showed rapid and significant (p ≤ 0.05) increases following iv administration of muGM-CSF or the PEG-T3C proteins. WBC levels increased from 3.8 ± 1.1 × 103 cells/µL at baseline to 13.0 ± 2.0 × 103 cells/µL at peak at 4 h post administration of muGM-CSF, and returned to baseline values by 24 h post-administration. WBC levels peaked later at 10 h post-administration of the 20 kDa-PEG-T3C protein, increasing from 6.6 ± 1.2 × 103 cells/µL at baseline to 13.7 ± 0.7 × 103 cells/µL at 10 h, and did not return to baseline levels until 72 h post-administration. WBC levels did not peak until 48 h following administration of the 40 kDa-PEG protein, increasing from 4.6 ± 1.0 × 103 cells/µL at baseline to 19.4 ± 4.4 × 103 cells/µL at 48 h, and were still elevated above baseline levels 120 h post-administration of the protein. WBCs were significantly (≤ 0.05) higher in rats treated with 20 kDa-PEG T3C compared to muGM-CSF at 24 h, 96 h and 144 h post-dosing, and significantly higher in rats treated with 40 kDa-PEG T3C compared to muGM-CSF at 48 h and 96 h post-dosing.
3.2.4 Other Blood Cell Effects
No significant effects on platelet or red blood cell levels were observed following iv administration of any of the proteins (data not shown). Monocytes showed few consistent changes over time, and were elevated (about twofold) in rats treated with the PEG-T3C proteins compared to muGM-CSF only at 48 h (data not shown).
3.3 SC Dosing Study
3.3.1 PK Results (Fig. 5a)
Plasma levels and hematopoietic properties of muGM-CSF and PEG-T3C proteins following sc administration of 100 μg/kg of the proteins to rats. a Shows plasma levels of the proteins over time, as measured by ELISA. b and c Show changes in circulating neutrophil and white blood cells, respectively, over time following sc administration of the proteins. Data are means ± SD (a) or SEM (b and c) for 3 rats per group
Following sc administration, muGM-CSF plasma levels peaked 2 h post-administration (at 20.6 ± 0.9 ng/mL) and were undetectable by 10 h post-administration. The protein’s terminal half-life was 1.1 h. By comparison, plasma levels of 20 kDa-PEG T3C did not peak until 10 h post-administration (at 15.8 ± 2.8 ng/mL) and were detectable for 72 h post-administration. Plasma levels of 40 kDa-PEG T3C did not peak until 24 h post-administration (at 12.3 ± 1.7 ng/mL) and were detectable for 120 h post-administration. Plasma levels of the 20 kDa-and 40 kDa-PEG-T3C proteins were significantly (p ≤ 0.05) lower than plasma levels of muGM-CSF from 0.25 to 2 h, not significantly different at 4 h, and significantly higher from 10 to 72 h (20 kDa-PEG-T3C) and to 96 h (40 kDa-PEG-T3C). Plasma levels of the 40 kDa-PEG protein were higher than plasma levels of the 20 kDa-PEG protein between 48 and 120 h post-administration of the proteins. The terminal half-life of the 20 kDa- PEG T3C protein was 10.9 h (measured between 48 and 96 h); the half-life was 7.5 h when measured from 24 to 96 h. The terminal half-life of the 40 kDa-PEG T3C protein was 17.2 h (measured from 48 to 144 h); the half-life was 13.6 h when measured between 24 and 144 h.
3.3.2 Neutrophil Effects (Fig. 5b)
Neutrophils showed rapid and significant (p ≤ 0.05) increases following sc administration of muGM-CSF or the PEG-T3C proteins. Neutrophils increased significantly from 864 ± 220 cells/μL at baseline to a peak of 5355 ± 1019 cells/μL at 4 h following administration of muGM-CSF, but returned to baseline levels by 24 h post-administration (Fig. 5b). By contrast, neutrophil levels peaked at 24 h post-administration of both the 20 kDa- and 40 kDa-PEG proteins, and remained elevated for 48–72 h (20 kDa-PEG-T3C) or 72–96 h (40 kDa-PEG-T3C) post-administration of the proteins (Fig. 5b). For the 20 kDa-PEG protein, neutrophils increased significantly from 933 ± 119 cells/μL at baseline to a peak of 9076 ± 1682 cells/μL at 24 h. For the 40 kDa-PEG protein, neutrophils increased significantly from 999 ± 235 cells/μL at baseline to a peak of 7530 ± 418 cells/μL at 24 h. Neutrophils were significantly higher in rats treated with 20 kDa-PEG T3C compared to muGM-CSF at 24, 48, 96, and 120 h post dosing. Neutrophils were significantly higher in rats treated with 40 kDa-PEG T3C compared to muGM-CSF at 24, 48, 72, and 120 h post dosing.
3.3.3 WBC Effects (Fig. 5c)
SC administration of muGM-CSF caused WBC levels to increase (not significantly) from 8.0 ± 0.9 cells at baseline to a peak of 10.5 ± 0.7 × 103 cells/μL at 10 h post-dosing; WBC levels decreased to baseline levels by 24 h post-administration. By contrast, WBC levels increased significantly and peaked at 24 h and 48 h post-administration of the 20 kDa- and 40 kDa-PEG proteins, respectively, and remained elevated for 48–72 h (20 kDa-PEG-T3C) or 72–96 h (40 kDa-PEG-T3C) post-administration of the proteins (Fig. 5c). The 20 kDa-PEG protein caused WBCs to increase significantly from 7.6 ± 0.9 × 103 cells/μL at baseline to a peak of 14.1 ± 1.7 × 103 cells/μL at 24 h. The 40 kDa-PEG protein caused WBCs to increase significantly from 7.9 ± 1.2 × 103 cells/μL at baseline to a peak of 15.1 ± 2.1 × 103 cells/μL at 48 h. WBCs were significantly higher in rats treated with 20 kDa-PEG T3C compared to muGM-CSF at 24 and 96 h post-dosing, and significantly higher in rats treated with 40 kDa-PEG T3C compared to muGM-CSF at 24 and 120 h post-dosing.
3.3.4 Other Blood Cell Effects
No significant effects on red blood cell or monocyte levels were observed following sc administration of any of the proteins, but the PEG-T3C proteins caused mild, but significant, 16–29% decreases in platelet levels at 72–96 h post-administration (data not shown).
3.4 Hematopoietic Properties of muGM-CSF and PEG-T3C Proteins in Neutropenic Rats
In vivo bioactivities (acceleration of hematopoietic recovery) of muGM-CSF and the PEG-T3C proteins were also studied in neutropenic rats. Rats were randomized to different test groups based upon their neutrophil counts and then treated with CPA (day 0) to induce neutropenia. Beginning one day later different groups of rats received sc injections of vehicle solution, muGM-CSF, or the PEG-T3C proteins using different dosing regimens (daily dosing on days 1–5, single dose on day 1, or variable dosing using multiple regimens during days 1–5). The proteins were dosed at 100 μg protein/kg/d. Some experiments included a control group of rats that did not receive CPA but did receive injections of vehicle solution using the same dosing regimen as the test proteins. Temporal changes in neutrophil and WBC counts in the different treatment groups were followed over time.
3.4.1 Daily Dosing Study (Fig. 6)
Effects of every day administration for 5 days of muGM-CSF, PEG-T3C proteins and vehicle solution on circulating neutrophil and white blood cell levels in rats treated with CPA. Rats received a single injection of CPA (100 mg/kg) on day 0 and daily sc injections (on days 1–5) of vehicle solution (PBS), muGM-CSF (100 μg/kg), 20 kDa-PEG-T3C (100 μg protein/kg), or 40 kDa-PEG-T3C (100 μg protein/kg). One group of control rats did not receive any CPA but did receive daily sc injections of vehicle solution. Blood samples were obtained from the rats on the indicated days for determining neutrophil (a) and white blood cell (b) counts in the animals. Data are means ± SEM for 5 rats per group
In the daily dosing experiment CPA-treated rats received 5 consecutive daily sc injections of vehicle solution, muGM-CSF, 20 kDa-PEG-T3C or 40 kDa-PEG-T3C on days 1–5 following CPA treatment. CPA treatment caused rapid decreases in neutrophil and WBC counts that reached nadirs about day 4 and did not recover to baseline levels until day 9 or 10 in vehicle treated animals (Fig. 6a and b). Daily injection of muGM-CSF for 5 days showed no benefit versus the vehicle solution for accelerating the time for neutrophil recovery from nadir to baseline values. In contrast, daily administration of the 20 kDa- or 40 kDa-PEG-T3C proteins for 5 days (days 1–5) caused neutrophil levels to recover to baseline levels by day 5, approximately 4 days earlier than the vehicle controls. Neutrophil recovery was followed by a transient neutrophilia that lasted several days. Similar results were obtained for WBCs, although WBCs never completely returned to baseline values during the course of the study, largely due to failure of the lymphocyte populations to recover to normal levels. Baseline (pre-CPA dosing), day 4 nadir, and day 5 recovery values for neutrophils and WBCs for the different test groups are presented in Table 2. The PEG-T3C proteins caused significant, transient increases in circulating neutrophils and WBCs on day 3; these increases were not observed in rats treated with vehicle solution or muGM-CSF (Table 2). Rats treated daily for 5 days with the PEG-T3C proteins, but not muGM-CSF, gained significantly less weight than vehicle treated rats during the 12 day study period (31.8 ± 3.5 g for the 20 kDa-PEG T3C group, 29.5 ± 2.1 g for the 40 kDa-PEG T3C group, 45.7 ± 2.3 g for the muGM-CSF group, and 45.5 ± 2.1 g for the vehicle group). No other adverse clinical signs were noted.
3.4.2 Single Dose Study (Fig. 7)
Effects of a single sc administration of muGM-CSF, PEG-T3C proteins, and vehicle solution on circulating neutrophil and white blood cell levels in rats treated with CPA. Rats received a single injection of CPA (100 mg/kg) on day 0 and a single sc injection of vehicle solution (PBS), muGM-CSF (100 μg/kg or 250 μg/kg), 20 kDa-PEG-T3C (100 or 250 μg protein/kg), or 40 kDa-PEG-T3C (100 or 250 μg protein/kg) on day 1. One group of control rats did not receive any CPA but did receive a sc injection of vehicle solution on day 1. Blood samples were obtained from the rats on the indicated days for determining neutrophil (a) and white blood cell (b) counts in the animals. Data are means ± SEM for 5 rats per group
In a second experiment CPA-treated rats received only a single sc injection of vehicle solution or 100 μg/kg or 250 μg/kg of muGM-CSF, 20 kDa-PEG-T3C or 40 kDa-PEG-T3C proteins on day 1. None of the PEG-T3C or muGM-CSF treatment groups showed an acceleration of time to neutrophil recovery to baseline values compared to vehicle-treated rats; neutrophil and WBC levels in all test groups returned to baseline values between days 8 and 9 (Fig. 7). The PEG-T3C proteins, but not vehicle solution or muGM-CSF, caused significant, transient increases in neutrophils and WBCs on day 2 (Fig. 7b), confirming that the rats had received the PEG-proteins, and that the PEG-proteins were biologically active in the rats. One rat treated with a single injection of 250 μg/kg of 40 kDa-PEG T3C died on day 6 during bleeding. All other rats survived to study end and no adverse clinical signs were noted.
3.4.3 Variable Dosing Study (Fig. 8)
Effects of every other day (EOD) administration of vehicle solution, muGM-CSF and PEG-T3C proteins on circulating neutrophil and white blood cell levels in rats treated with CPA. Rats received a single injection of CPA (100 mg/kg) on day 0. Different groups of rats received every day (days 1–5) or every other day (days 1, 3 and 5) injections of 20 kDa-PEG-T3C (100 μg protein/kg). Additional test groups received sc injections of 20 kDa-PEG T3C on days 1 and 3 only, days 3 and 5 only, day 3 only, or on day 5 only. Control rats received daily (days 1–5) injections of vehicle solution (PBS) or muGM-CSF (100 μg/kg). Blood samples were obtained from the rats on the indicated days for determining neutrophil (a) and white blood cell (b) counts in the animals. Data are means ± SEM for 5 rats per group
In a third experiment CPA-treated rats were given sc injections of the 20 kDa-PEG-T3C protein every day (on days 1–5), every other day (on days 1, 3 and 5), on days 1 and 3 only, or as a single injection on day 3 only or on day 5 only. Control CPA-treated rats received daily (days 1–5) injections of vehicle or muGM-CSF. Only the 20 kDa-PEG-T3C protein was tested in this experiment because of the large number of test groups and similar hematopoietic responses of neutropenic rats to the 20 kDa- and 40 kDa-PEG-T3C proteins seen in previous studies. Daily administration and every other day administration of 20 kDa-PEG-T3C shortened the time for neutrophil recovery to baseline levels from 9 days (vehicle group) to 6 days, and accelerated WBC recovery, but not to pretreatment levels (Fig. 8). Baseline (pre-CPA dosing), day 4 nadir, and day 6 recovery values for neutrophils and WBCs for the different test groups are shown in Table 3. Administration of the 20 kDa-PEG protein as a single injection on day 3 or on day 5, or as 2 injections on days 1 and 3 showed no benefit versus vehicle at shortening the time to neutrophil recovery (Fig. 8, Table 3). Daily administration of muGM-CSF also showed no benefit versus vehicle. All test groups receiving the 20 kDa-PEG T3C protein on day 1 showed significant, transient increases in neutrophils and WBCs on day 2; this increase was not seen with any of the other treatment groups (Fig. 8, Table 3). As was observed in the first daily dosing experiment, rats treated daily for 5 days with the 20 kDa-PEG T3C protein gained significantly less weight over the 12 day study period than vehicle-treated rats (20.5 ± 3.5 g versus 33.5 ± 2.1 g; p < 0.05). Weight gains for the other treatment groups were not significantly different than for the vehicle group. One rat treated daily for 5 days with PEG-T3C was found dead on day 12. This rat was noted to have lost about 50 g between days 8 and 10. The cause of death was not determined. All other rats survived to study end and no adverse clinical signs were noted.
4 Discussion
The data presented here show that site-specific PEGylation of muGM-CSF (T3C) yields PEG-protein conjugates that display minimal loss of in vitro bioactivity, even when modified with a large 40 kDa-PEG. Similar results were reported for the huGM-CSF (A3C) protein [17]. These data provide further evidence the N-terminal region preceding the first alpha helix in human and mouse GM-CSF is non-essential for receptor binding and in vitro bioactivity of the protein.
The availability of highly active, PEGylated muGM-CSF analogs allowed us to investigate the in vivo pharmacokinetic and hematological properties of long-acting muGM-CSF proteins in normal and neutropenic rats. The PEG-T3C proteins had 10- to 25-fold longer circulating half-lives than unmodified murine GM-CSF following iv or sc administration. Half-lives and circulating plasma levels correlated with the sizes of the attached PEG molecule. A single injection of unmodified muGM-CSF stimulated significant but short lived increases in peripheral neutrophil and WBC counts that peaked 4–10 h post-administration and returned to baseline values within 24 h. These increases likely represent release of preformed, mature neutrophils from the bone marrow into the circulation, as rat bone marrow typically requires 2 to 3 days for maturation and release of newly formed neutrophils [23]. In contrast, a single injection of the PEG-T3C proteins stimulated significant increases in circulating neutrophils and WBCs that peaked 10- to 48 h post administration and persisted for 72–96 h before returning to baseline values. The 40 kDa-PEG-T3C protein stimulated longer-lasting increases in neutrophil and WBC counts than the 20 kDa-PEG-T3C protein, consistent with its longer half-life and higher circulating levels. The prolonged increases in circulating neutrophils observed with the PEG-T3C proteins likely represent both early release of preformed mature neutrophils from the bone marrow as well as maturation and release of newly formed neutrophils during the following few days. These results suggest that the PEG-T3C proteins stimulate a broader range of processes affecting neutrophil kinetics than does muGM-CSF, at least following a single injection. Repeated injections of G-CSF in mice decreased the cell cycle time for neutrophil progenitors by 25–40% and decreased the time required for neutrophil maturation and release from the bone marrow from 4 days to 1 day [24]. These processes were not affected by repeated injections of muGM-CSF [24]. Whether the increased in vivo potencies of the PEG-T3C proteins allows them to affect these aspects of neutrophil kinetics remains to be determined.
Clearance of the PEG-T3C proteins from the blood stream accelerated when neutrophil levels reached their peak levels 24 to 48 h post-administration. This pattern is similar to what is observed following administration of granulocyte-colony-stimulating factor (G-CSF) and PEGylated G-CSF analogs [22, 25] to animals and has been attributed to receptor-mediated endocytosis of G-CSF by neutrophils, bone marrow cells, and other cell types that express G-CSF receptors [25]. A similar mechanism may be operating to promote clearance of PEGylated GM-CSF proteins in rats. The terminal half-lives of the PEGylated muGM-CSF (T3C) proteins were shorter than those of the corresponding PEGylated huGM-CSF (A3C) analogs (23 h and 47 h, respectively, for human 20 kDa- and 40 kDa-PEG-A3C proteins versus 1 h for huGM-CSF following sc administration to rats; [17]). This difference is consistent with the fact that huGM-CSF does not bind muGM-CSF receptors efficiently, and thus would not be expected to be cleared by receptor-mediated endocytosis. The slower clearance of the PEG-huGM-CSF analogs in rats likely reflects primarily clearance by kidney and liver filtration mechanisms.
The PEG-T3C proteins were significantly more potent hematopoietic growth factors than muGM-CSF in neutropenic rats. No significant benefit of muGM-CSF versus vehicle was detected in accelerating the time for neutrophil or WBC recovery to baseline levels in neutropenic rats when the protein was administered daily for 5 days following chemotherapy treatment. muGM-CSF also did not cause the transient increases in neutrophils and WBCs that was observed with the PEG-T3C proteins on day 2 or 3 post-injection. The lack of an effect of unmodified GM-CSF may be due to its short half-life and short-lived effect on circulating neutrophil levels. Other researchers reported that daily administration of muGM-CSF produced minimal or no physiological effects in rodents [26,27,28]. In contrast, both PEG-T3C proteins shortened the time required for neutrophil recovery to normal levels from 9–10 days to 5–6 days when administered daily for 5 days beginning 1 day after chemotherapy treatment. The 20 kDa-PEG T3C protein also shortened the time required for neutrophil recovery to normal levels in neutropenic rats when administered every other day for 5 days (3 injections). Neither PEG-T3C protein shortened the time required for neutrophil recovery to normal levels when administered as only a single injection 1 day after CPA treatment. Although the PEG-T3C proteins were not effective at shortening the time for neutrophil recovery as a single injection in CPA-treated rats, they still stimulated transient increases in circulating neutrophils on days 2 or 3 post-administration, indicating that they were biologically active in the rats. These data indicate that the cellular processes responsible for the early transient increase in neutrophils seen on days 2 to 3 in the CPA-treated rats and the cellular processes responsible for the acceleration of neutrophil recovery seen on days 5–6 following CPA administration are distinct. Three every other day sc administrations of the 20 kDa-PEG-T3C protein was the minimum number of treatments required to accelerate neutrophil recovery in this model because single injections of the protein on day 1, day 3 or day 5, or two injections on days 1 and 3 showed no benefit versus vehicle solution. At least two, and possibly three, injections of the 40 kDa-PEG-T3C protein also are required to accelerate the time of neutrophil recovery to normal levels in this model since a single injection of the 40 kDa-PEG-T3C protein on Day 1 following CPA treatment was ineffective. These data suggest that GM-CSF must be present continuously during the 5 day period following CPA administration to accelerate the time to neutrophil recovery in this model. The 10 to 17 h sc half-lives of the PEG-T3C proteins are consistent with this finding and the need for multiple administrations over 5 days. In contrast to these results with PEG-GM-CSF proteins, a single sc injection of the same dose (100 µg protein/kg) of a 20 kDa-PEG G-CSF protein on day 1 following CPA treatment is able to accelerate the time for neutrophil recovery to baseline levels from 9 to 6 days in this model [29]. Why more administrations of the 20 kDa-PEG-GM-CSF (T3C) protein are required for efficacy compared to a 20 kDa-PEG-G-CSF protein will require further investigation.
GM-CSF’s proliferative effects on granulocyte and myeloid cell lineages led to it being developed as a treatment for neutropenia in cancer patients receiving chemotherapy [1, 2]. The data presented here suggest that PEG-GM-CSF proteins will be significantly more effective than GM-CSF for treating neutropenia in humans, and potentially other disease states. GM-CSF exhibits a broad spectrum of biological activities and is now under investigation as a treatment for a wide variety of medical disorders, including autoimmune diseases, central nervous system diseases, acute radiation exposure, wound-healing, gastrointestinal diseases, cancer immunotherapy, and as a vaccine adjuvant, among others [3,4,5,6,7,8,9,10,11,12,13,14,15,16, 30]. The availability of a highly potent, PEGylated muGM-CSF analog such as PEG-T3C will facilitate evaluation of the therapeutic utility of GM-CSF and long-acting GM-CSF analogs in rodent models of these disease states. The short half-life of muGM-CSF limits its efficacy in rodents when administered using daily or less frequent dosing regimens. The longer half-lives of the PEG-muGM-CSF proteins allows less frequent dosing and the proteins stimulate more robust physiological responses; thus, in addition to convenience, PEG-muGM-CSF proteins may provide better insights into the potential utility of GM-CSF proteins to treat diseases when tested in rodents. The efficacious doses and dosing regimens of the PEG-T3C proteins identified here for rats provide a useful starting point for choosing appropriate doses and dosing regimens for future studies in other rodent disease models.
Abbreviations
- CBC:
-
Complete blood cell count
- CPA:
-
Cyclophosphamide
- EC50 :
-
Concentration causing half maximal response
- ELISA:
-
Enzyme-linked immunosorbent assay
- FBS:
-
Fetal bovine serum
- G-CSF:
-
Granulocyte colony-stimulating factor
- GM-CSF:
-
Granulocyte-macrophage colony-stimulating factor
- Hu:
-
Human
- IV:
-
Intravenous
- Mu:
-
Murine
- PEG:
-
Polyethylene glycol
- RT-PCR:
-
Reverse transcriptase-polymerase chain reaction
- SC:
-
Subcutaneous
- SD:
-
Standard deviation
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEM:
-
Standard error of the mean
- TCEP:
-
Tris[2-carboxyethylphosphine]hydrochloride
- WBC:
-
White blood cell (total of lymphocytes, neutrophils, monocytes, eosinophils, and basophils)
References
Cebon JS, Lieschke GJ (1994) Granulocyte-macrophage colony-stimulating factor for cancer treatment. Oncology 51:177–188
Armitage JO (1998) Emerging applications of recombinant human granulocyte-macrophage colony-stimulating factor. Blood 92:4491–4507
Hodi FS, Lee S, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, Leming P, Puzanov I, Shin D, Kirkwood JM (2014) Sargramostim plus ipilimumab vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 312:1744–1753
Spitler LE, Weber RW, Allen RE, Meyer J, Cruickshank S, Garbe E, Lin H-Y, Soong S-J (2009) Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF, Sargramostim) administered for 3 years as adjuvant therapy of stages II (T4), IIIand IV melanoma. J Immunother 32:632–637
Dranoff G (2002) GM-CSF-based cancer vaccines. Immunol Rev 188:147–154
Mittendorf EA, Clifton GT, Holmes JP, Clive KS, Patil R, Benavides LC, Gates JD, Sears AK, Stojadinovic A, Ponniah S, Peoples GE (2012) Clinical trial results of the HER-2/neu (E75) vaccine to prevent breast cancer recurrence in high-risk patients: from US Military Cancer Institute Clinical Trials Group Study I-01 and I-02. Cancer 118:2594–2602
Kim NK, Choi BH, Huang X, Snyder BJ, Bukhari S, Kong T-H, Park H, Park HC, Park SR, Ha Y (2009) Granulocyte-macrophage colony-stimulating factor promotes survival of dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced murine Parkinson’s disease model. Eur J Neurosci 29:891–900
Kong TH, Choi J-K, Park H, Choi BH, Snyder BJ, Bukjari S, Kim N-K, Huang X, Park SR, Park HC, Ha Y (2009) Reduction in programmed cell death and improvement in functional outcome of transient focal cerebral ischemia after administration of granulocyte-macrophage colony-stimulating factor in rats. J Neurosci 111:155–163
Boyd TD, Bennett SP, Mori T, Governatori N, Runfeldt M, Norden M, Padmanabhan J, Neame P, Wefes I, Sanchez-Ramos J, Arendash GW, Potter H (2010) GM-CSF upregulated in rheumatoid arthritis reverses cognitive impairment and amyloidosis in Alzheimer mice. J Alzheimer’s Dis 21:507–518
Dieckgraefe BK, Korzenik JR (2002) Treatment of active Crohn's disease with recombinant human granulocyte-macrophage colony-stimulating factor. Lancet 360:1478–1480
Gaudreau S, Guindi C, Ménard M, Besin G, Dupuis G, Amrani A (2007) Granulocyte-macrophage colony-stimulating factor prevents diabetes development in NOD mice by inducing tolerogenic dendritic cells that sustain the suppressive function of CD4+CD25+ regulatory T cells. J Immunol 179:3638–3647
Sheng JR, Muthusamy T, Prabhakar BS, Meriggioli MN (2011) GM-CSF-induced regulatory T cells selectively inhibit anti-acetylcholine receptor-specific immune responses in experimental myasthenia gravis. J Neuroimmunol 240–241:65–73
Cheatem D, Ganesh BB, Gangia E, Vasu C, Prabhakar BS (2009) Modulation of dendritic cells using granulocyte-macrophage colony-stimulating factor (GM-CSF) delays type 1 diabetes by enhancing CD4+CD25+ regulatory T cell function. Clin Immunol 131:260–270
Ganesh BB, Cheatem DM, Sheng JR, Vasu C, Prabhakar BS (2009) GM-CSF-induced CD11c1CD8a—dendritic cells facilitate Foxp31 and IL-101 regulatory T cell expansion resulting in suppression of autoimmune thyroiditis. Int Immunol 21:269–282
Fang Y, Shen J, Yao M, Beagley KW, Hambly BD, Bao S (2009) Granulocyte-macrophage colony-stimulating factor enhances wound healing in diabetes via upregulation of proinflammatory cytokines. Br J Dermatol 162:478–486
Zhang L, Chen J, Han C (2009) A multicenter clinical trial of recombinant human GM-CSF hydrogel for the treatment of deep second-degree burns. Wound Repair Regen 17:685–689
Doherty DH, Rosendahl MS, Smith DJ, Hughes JM, Chlipala EA, Cox GN (2005) Site-specific PEGylation of engineered cysteine analogues of recombinant human granulocyte-macrophage colony-stimulating factor. Bioconjug Chem 16:1291–1298
Gough NM, Gough J, Metcalf D, Kelso A, Grail D, Nicola NA, Burgess AW, Dunn AR (1984) Molecular cloning of a cDNA encoding a murine haematopoietic growth regulator, granulocyte-macrophage colony-stimulating factor. Nature 309:763–767
Lee F, Yokota T, Otsuka T, Giemmell L, Larson N, Luh J, Arai K-I, Rennick D (1985) Isolation of cDNA for a human granulocyte-macrophage colony-stimulating factor by functional expression in mammalian cells. Proc Natl Acad Sci USA 82:4360–4364
Kawasaki ES (1990) In: Innis MA, Gelfand DH, Sninsky JJ, White TJ (eds) PCR Protocols: a guide to methods and applications. Academic Press, San Diego
Picken RN, Mazaitis AJ, Maas WK, Rey M, Heyneker H (1983) Nucleotide sequence of the gene for heat-stable enterotoxin II of Escherichia coli. Infect Immun 42:269–275
Rosendahl MS, Doherty DH, Smith DJ, Bendele AM, Cox GN (2005) Site-specific protein PEGylation: application to cysteine analogs of recombinant human granulocyte colony-stimulating factor. BioProcess Int 3:52–62
Smeaton TC (1984) Migration of polymorphonuclear neutrophils and macrophages from bone marrow to the peritoneal cavity after (3H)-thymidene labeling of rat tibial bone marrow in vivo. Aust J Exp Biol Med Sci 62:453–463
Lord BI, Molineux G, Pojda Z, Souza LM, Mermod J-J, Dexter TM (1991) Myeloid cell kinetics in mice treated with recombinant interleukin-3, granulocyte-colony-stimulating factor (CSF), or granulocyte-macrophage CSF in vivo. Blood 77:2154–2159
Kuwabara T, Kato Y, Kobayashi S, Suzuki H, Sugiyama Y (1994) Nonlinear pharmacokinetics of a recombinant human granulocyte colony-stimulating factor derivative (nartograstim): species differences among rats, monkeys and humans. JPET 271:1535–1543
Pulendran B, Smith JL, Caspary G, Brasel K, Pettit D, Maraskovsky E, Maliszewski CR (1999) Distinct dendritic cell subsets differentially regulate the class of immune response in vivo. Proc Natl Acad Sci USA 96:1036–1041
Daro E, Pulendran B, Brasel K, Teepe M, Pettit D, Lynch DH, Vremec D, Robb L, Shortman K, McKenna HJ, Maliszewski CR, Maraskovsky E (2000) Polyethylene glycol-modified GM-CSF expands CD11bhigh CD11chigh but not CD11blow CD11chigh murine dendritic cells in vivo: a comparative analysis with Flt3 ligand. J Immunol 165:49–58
Sainathan KS, Tu L, Bishnupuri KS, Han M, Li A, Newberry RD, McDonald KG, Crimmins DL, Houchen C, Anant S, Dieckgraefe BK (2005) PEGylated murine granulocyte-macrophage colony-stimulating factor: production, purification and characterization. Protein Expr Purif 44:94–103
Cox GN, Chlipala EA, Smith DJ, Carlson SJ, Bell SJ, Doherty DH (2014) Hematopoietic properties of granulocyte colony-stimulating factor/immunoglobulin fusion proteins (G-CSF/IgG-Fc) in normal and neutropenic rodents. PLoS ONE 9(3):e91990. https://doi.org/10.1371/journal.pone.0091990
Plett PA, Chua HL, Sampson CH, Katz BP, Fam CM, Anderson LA, Cox GN, Orschell CM (2014) PEGylated G-CSF (BBT-015), GM-CSF (BBT-007), and IL-11 (BBT-059) analogs enhance survival and hematopoietic cell recovery in a mouse model of the hematopoietic syndrome of the acute radiation syndrome. Health Phys J 106:7–20
Acknowledgements
We thank Jennifer Hughes and Sharon Carlson for performing the ELISA and WinNonlin pharmacokinetic analyses.
Funding
This research was supported by Grant R44CA084850 from the National Cancer Institute to DHD. The publication’s contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or The National Institutes of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
GNC, DHD, MSR, and JIL are employees or former employees of Bolder BioTechnology. GNC and DHD have a financial interest in Bolder BioTechnology. GNC, DHD, and MSR are inventors on patents and patent applications owned by Bolder BioTechnology related to GM-CSF and other proteins and methods. EAC has a financial interest in Premier Laboratory, which was paid by Bolder BioTechnology to perform the animal studies described in the manuscript, as well as other animal studies.
Ethical Approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of Premier Laboratory and the University of Colorado, Boulder, where the studies were conducted.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cox, G.N., Lee, J.I., Rosendahl, M.S. et al. Characterization of a Long-Acting Site-Specific PEGylated Murine GM-CSF Analog and Analysis of Its Hematopoietic Properties in Normal and Cyclophosphamide-Treated Neutropenic Rats. Protein J 39, 160–173 (2020). https://doi.org/10.1007/s10930-020-09894-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-020-09894-0